Understanding a Mechanistic Basis of ABA Involvement in Plant Adaptation to Soil Flooding: The Current Standing
Abstract
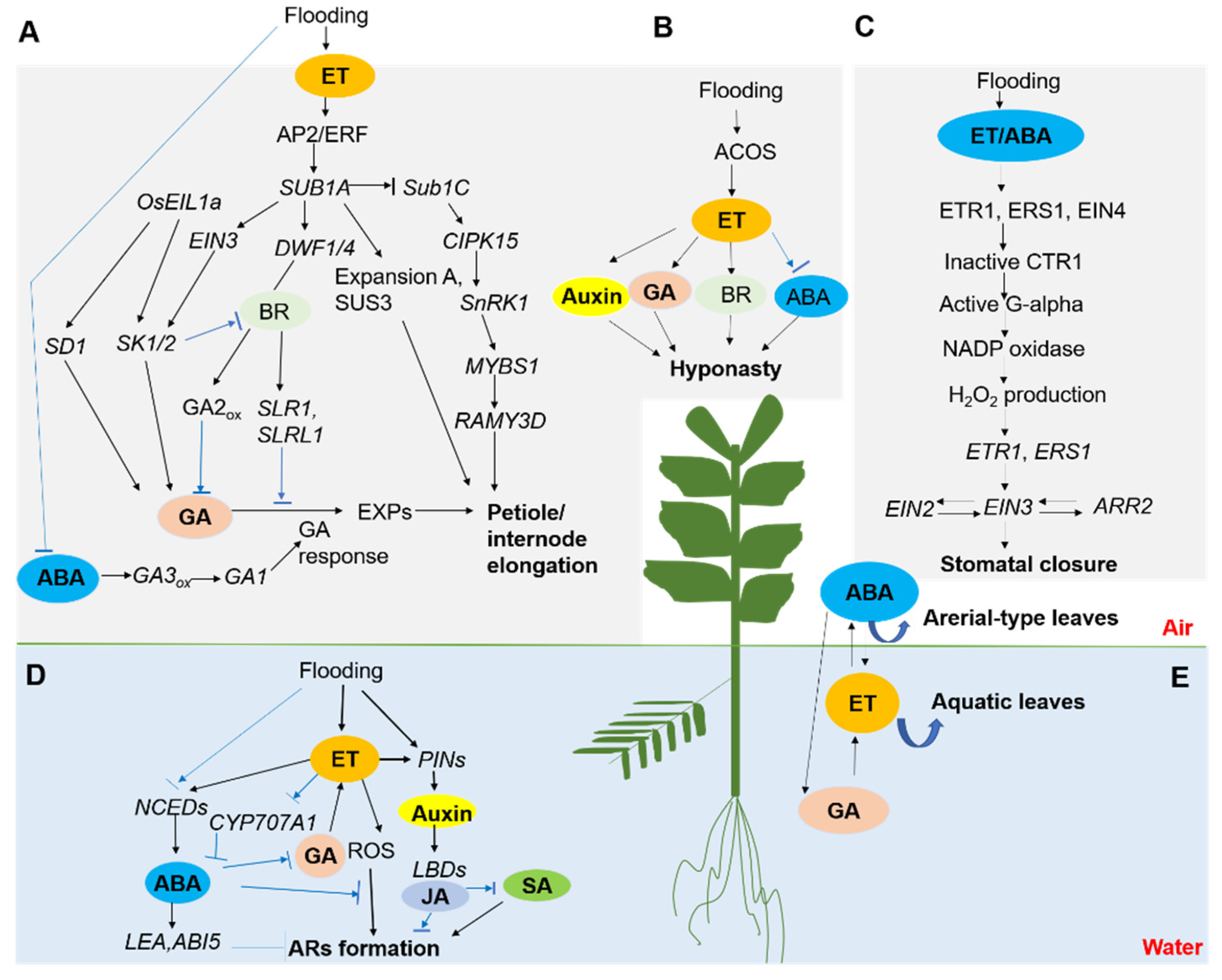
:1. Introduction
2. Changes in ABA Content and ABA-Regulated Responses during Submergence
| Stress | Species | Tissue | Response | Reference |
|---|---|---|---|---|
| flooding | Solanum dulcamara | stems, AR primordia | decreased | [11,33] |
| flooding | Rumex palustris | petioles | sharply decreased | [36] |
| submergence | Lolium perenne | leaf | decreased | [5] |
| submergence | Oryza sativa, deepwater | internodes | decreased | [25] |
| submergence | Oryza sativa, lowland | shoot | decreased | [28] |
| submergence | Scirpus micronatus | shoots | decreased | [34] |
| flooding | citrus | root | decrease | [37] |
| submergence | Rumex palustris | petioles | decrease significantly | [11] |
| submergence | Nasturtium officinale | petioles, stem | decrease | [38] |
| waterlogging | Carrizo citrange | roots | decreased, back to control | [35] |
| flooding | citrus | leaf | sharp increase | [37] |
| flooding | Pilsum sativum L. | shoot | increase | [39] |
| flooding | Pisum sativum L. | root | early increase | [39] |
| flooding | Nicotiana tabacum L. | leaf | increase | [40] |
| waterlogging | Vigna radiata L. | leaf | increase | [41] |
| anoxia | Lactuca sativa L. | roots | unchanged | [42] |
| flooding | Glycine max | seedlings | unchanged | [43] |
| Species | Chemicals | Concentrations (uM) | Effect | Treatment | Reference |
|---|---|---|---|---|---|
| Lactuca sative L. | ABA | 1, 3, 10, 30, 100, 300 | increase survivability | anoxia 24 h | [42] |
| Glycine max | ABA | 5, 10, 50 | increase the survival | flooding | [43] |
| Oryza sativa | ABA | 0.1 uM, 24 h | improving seeds resistance | submergence | [53] |
| Arabidopsis | ABA | 10, 50, 100 | increase tolerance | anoxia | [54] |
| Zea mays. L. | ABA | 100 uM, 24 h | increase tolerance | anoxia | [55,56] |
| Nasturtium officinale | ABA | 0.5, 1, 5 | inhibits shoot elongation | submergence | [38] |
| Rumex palustris | ABA | - | inhibits petiole elongation | submergence | [57] |
| Oryza sativa | ABA | 1 | inhibits petiole elongation | submergence | [25] |
| Oryza sativa | fluridone (biosynthesis inhibitor) | - | induced AR emergence | submergence | [58,59,60] |
3. Stomatal Closure and Root-Shoot Response
4. Petioles Elongation and Hyponastic Growth
5. Heterophylly Initiation
6. Formation of Adventitious Roots (AR) and Aerenchyma
7. Unanswered Questions and an Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bubeck, P.; Otto, A.; Weichselgartner, J. Societal impacts of flood hazards. Nat. Hazards 2017. [Google Scholar] [CrossRef]
- Razzaq, A.; Wani, S.H.; Saleem, F.; Yu, M.; Zhou, M.; Shabala, S. Rewilding crops for climate resilience: Economic analysis and de novo domestication strategies. J. Exp. Bot. 2021, erab276. [Google Scholar] [CrossRef] [PubMed]
- Monre. Climate Change and Sea Level Rise Scenarios for Vietnam; Natural Resources-Environment and Mapping Publishing House: Ha Noi, Vietnam, 2011. [Google Scholar]
- Chakraborty, K.; Guru, A.; Jena, P.; Ray, S.; Guhey, A.; Chattopadhyay, K.; Sarkar, R.K. Rice with SUB1 QTL possesses greater initial leaf gas film thickness leading to delayed perception of submergence stress. Ann. Bot. 2020, 127, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Jannasch, A.H.; Jiang, Y. Submergence stress alters fructan and hormone metabolism and gene expression in perennial ryegrass with contrasting growth habits. Environ. Exp. Bot. 2020, 179, 104–202. [Google Scholar] [CrossRef]
- Armstrong, W. Aeration in higher plants. Adv. Bot. Res. 1980, 7, 255–332. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Shishova, M.F. The Role of Phytohormones in the Control of Plant Adaptation to Oxygen Depletion. In Phytohormones and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Plant responses to hypoxia—Is survival a aalancing act? Trends Plant Sci. 2014, 9, 449–456. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suits of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Parlanti, S.; Kudahettige, N.P.; Lombardi, L.; Mensuali-Sodi, A.; Alpi, A.; Perata, P. Distinct mechanisms for aerenchyma formation in leaf sheaths of rice genotypes displaying a quiescence or escape strategy for flooding tolerance. Ann. Bot. 2011, 107, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Van Veen, H.; Mustroph, A.; Barding, G.A.; Van Vergeer, E.M.; Welschen-Evertman, R.A.M.; Pedersen, O.; Visser, E.J.W.; Larive, C.K.; Pierik, R.; Bailey-Serres, J.; et al. Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell 2013, 25, 4691–4707. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, R.; Mustroph, A.; Boonman, A.; Akman, M.; Ammerlaan, A.M.H.; Breit, T.; Schranz, M.E.; Voesenek, L.A.C.J.; Van Tienderen, P.H. Root transcript profiling of two Rorippa species reveals gene clusters associated with extreme submergence tolerance. Plant Physiol. 2013, 163, 1277–1292. [Google Scholar] [CrossRef] [Green Version]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Hattori, Y.; Ashikari, M. Stunt or elongate? Two opposite strategies by which rice adapts to floods. J. Plant Res. 2010, 123, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Keisuke, N.; Yuma, K.; Takuya, K.; Tomonori, N.; Takeshi, K.; Angeles-Shim, R.B.; Hideshi, Y.; Atsushi, Y.; Motoyuki, A. QTL analysis of internode elongation in response to gibberellin in deepwater rice. AoB. Plants 2014, 6, plu028. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flooding tolerance: O2 sensing and survival strategies. Curr. Opin. Plant Biol. 2013, 16, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Grichko, V.P.; Glick, B.R. Flooding tolerance of transgenic tomato plants expressing the bacterial enzyme ACC deaminase controlled by the 35s, rold or prb-1b promoter. Plant Physiol. Biochem. 2001, 39, 19–25. [Google Scholar] [CrossRef]
- Armstrong, W.; Cousins, D.; Armstrong, J.; Turner, D.W.; Beckett, P.M. Distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: A microelectrode and modelling study with phragmites australis. Ann. Bot. 2000, 86, 687–703. [Google Scholar] [CrossRef] [Green Version]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Gao, J.; Im Kim, J.; Chen, K.; Bressan, R.A.; Zhu, J.K. Control of plant water use by ABA induction of senescence and dormancy: An overlooked lesson from evolution. Plant Cell Physiol. 2017, 58, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Xie, J.; Chen, C.; Cao, H.; Sun, J.; Kong, Q.; Shabala, S.; Shabal, L.; Huang, Y.; Bie, Z. An early ABA induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J. Exp. Bot. 2018, 69, 4945–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann-Benning, S.; Kende, H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol. 1992, 99, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Hirano, T.; Deki, Y.; Uchida, N.; Yamaguchi, T. Involvement of the decrease in levels of abscisic acid in the internodal elongation of submerged floating rice. J. Plant Physiol. 1995, 146, 323–328. [Google Scholar] [CrossRef]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, P.C.; Singh, B.B.; Singh, A.K.; Ram, P.; Singh, P.N.; Singh, H.P.; Boamfa, I.; Harren, F.; Santosa, E.; Jackson, M.B. Submergence tolerance in rainfed lowland rice: Physiological basis and prospects for cultivar improvement through marker-aided breeding. Field Crops Res. 2002, 76, 131–152. [Google Scholar] [CrossRef]
- Hiroaki, S.; Masanori, O.; Kentaro, M.; Tetsuo, K.; Shoko, S.; Yusuke, J.; Masaru, F.; Taku, A.; Hirokazu, T.; Miho, A. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8-hydroxylase in rice. Plant Cell Physiol. 2007, 48, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, A.; Piy, S.; Fernandez, J.C.; Chervin, C.; Hewezi, T.; Binder, B.M. Eethylene receptors signal via a noncanonical pathway to regulate abscisic acid responses. Plant Physiol. 2018, 176, 910–929. [Google Scholar] [CrossRef] [Green Version]
- Papdi, C.; Pérez-Salamó, I.; Joseph, M.P.; Giuntoli, B.; Szabados, L. The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor vii genes RAP2.12, RAP 2.2 and RAP 2.3. Plant J. 2015, 82, 772–784. [Google Scholar] [CrossRef] [Green Version]
- Benech-Arnold, R.L.; Gualano, N.; Leymarie, J.; Come, D.; Corbineau, F. Hypoxia interferes with ABA metabolism and increases ABA sensitivity in embryos of dormant barley grains. J. Exp. Bot. 2006, 57, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Jansen, M.J.; Zhang, Q.; Sergeeva, L.; Ligterink, W.; Mariani, C.; Rieu, I.; Visser, E.J.W. A disturbed auxin signaling affects adventitious root outgrowth in solanum dulcamara under complete submergence. J. Plant Physiol. 2018, 224–225, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Shieh, Y.-J.; Chou, C.-H. Abscisic acid inhibits shoot elongation of Scirpus mucronatus. Physiol. Plant. 1996, 97, 1. [Google Scholar] [CrossRef]
- Arbona, V.; Zandalinas, S.I.; Manzi, M.; Gonzalez-Guzman, M.; Rodriguez, P.L.; Gómez-Cadenas, A. Depletion of abscisic acid levels in roots of flooded carrizo citrange (Poncirus trifoliata L. raf. × Ctrus sinensis L. osb.) plants is a stress-specific response associated to the differential expression of pyr/pyl/rcar receptors. Plant Mol Biol. 2017, 93, 623–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benschop, J.J.; Jackson, M.B.; Gühl, K.; Vreeburg, R.; Voesenek, L. Contrasting interactions between ethylene and abscisic acid in rumex species differing in submergence tolerance. Plant J. 2010, 44, 756–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbona, V.; Cadenas, A.G. Hormonal modulation of citrus responses to flooding. Aust. Econ. Pap. 2008, 16, 194–210. [Google Scholar] [CrossRef]
- Müller, J.T.; Veen, H.V.; Bartylla, M.M.; Akman, M.; Pedersen, O.; Sun, P.; Schuurink, R.C.; Takeuchi, J.; Todoroki, Y.; Weig, A.R. Keeping the shoot above water—Submergence triggers antithetical growth responses in stems and petioles of watercress (Nasturtium officinale). New Phytol. 2019, 229, 140–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, X. Can early wilting of old leaves account for much of the ABA accumulation in flooded pea plants? J. Exp Bot. 1994, 45, 1335–1342. [Google Scholar] [CrossRef]
- Hurng, W.P.; Lur, H.S.; Liao, C.K.; Kao, C.H. Role of abscisic acid, ethylene and polyamines in flooding-promoted senescence of tobacco leaves. J. Plant Physiol. 1994, 143, 102–105. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Sakuratani, T. Changes of endogenous ABA and ACC, and their correlations to photosynthesis and water relations in mungbean (Vigna radiata L. Wilczak cv. KPS1) during waterlogging. Environ. Exp. Bot. 2006, 57, 278–284. [Google Scholar] [CrossRef]
- Noguchi, H.K. Abscisic acid and hypoxic induction of anoxia tolerance in roots of lettuce seedlings. J. Exp. Bot. 2000, 352, 1939–1944. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, S.; Han, C.; Nanjo, Y.; Altaf-Un-Nahar, M.; Yang, P. Label-free quantitative proteomic analysis of abscisic acid effect in early-stage soybean under flooding. J. Proteome Res. 2013, 12, 4769–4784. [Google Scholar] [CrossRef]
- Castonguay, Y.; Nadeau, P.; Simard, R.R. Effects of flooding on carbohydrate and aba levels in roots and shoots of alfalfa. Plant Cell Environ. 2010, 16, 695–702. [Google Scholar] [CrossRef]
- Jackson, M.B.; Young, S.F.; Hall, K.C. Are roots a source of abscisic acid for the shoots of flooded pea plants? J. Exp. Bot. 1988, 39, 1631–1637. [Google Scholar] [CrossRef]
- Olivella, C.; Biel, C.; Vendrell, M.; Save, R. Hormonal and physiological responses of Gerbera jamesonii to flooding stress. Hortic. Sci. 2000, 35, 222–225. [Google Scholar] [CrossRef]
- Hartung, W.; Sauter, A.; Turner, N.C.; Fillery, I.; Heilmeier, H. Abscisic acid in soils: What is its function and which factors and mechanisms influence its concentration? Plant Soil 1996, 184, 105–110. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. Int. 2021, 28, 30528–30550. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.D.; Bungau, S.G.; Tit, D.M.; Frunzulica, C.E.M.; Badea, G.E. Effects of long term application of organic and mineral fertilizers on soil enzymes. Rev. Chim. 2018, 69, 2608–2612. [Google Scholar] [CrossRef]
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Purza, L.; Samuel, A.D.; Bungău, S.; Badea, G.E.; Aleya, L. Orchard management under the effects of climate change: Implications for apple, plum, and almond growing. Environ. Sci. Pollut. Res. Int. 2019, 26, 9908–9915. [Google Scholar] [CrossRef]
- Liu, H.; Fang, Y.; Junyan, W.U.; Chen, Q.; Sun, W.; Liu, Z.; Fang, Y.; Chao, M.I.; Yuanyuan, P.U.; Zhao, Y. Response of endogenous ABA and GA to cold resistance of Brassica rapa L. and Brassica napus L. Chin. J. Eco-Agric. 2016, 24, 1529–1538. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zou, H.W. ABA improving rice seeds resistance to waterlogging stress during germinating period. J. Anhui Agric. Sci. 2013, 41, 593–594. [Google Scholar] [CrossRef]
- Ellis, M.H.; Dennis, E.S.; Peacock, W.J. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 1999, 119, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.Y.; VanToai, T.T. Abscisic acid induced anaerobiosis tolerance in corn. Plant Physiol. 1991, 97, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Vantoai, T.T. Field Performance of Abscisic Acid-Induced Flood-Tolerant Corn. Crop Sci. 1993, 33, 344–346. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Jackson, M.B.; Toebes, A.H.W.; Vriezen, W.H.; Colmer, T.D. De-submergence-induced ET production in Rumex palustris: Regulation and ecophysiological significance. Plant J. 2003, 33, 341–352. [Google Scholar] [CrossRef]
- Steffens, B.; Sauter, M. Epidermal cell death in rice (Oryza sativa L.) is regulated by ethylene, gibberellins and abscisic acid. Plant Physiol. 2005, 139, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef]
- Liu, P.; Sun, F.; Gao, R.; Dong, H. RAP2.6L Overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol. Biol. 2012, 79, 609–622. [Google Scholar] [CrossRef]
- Joshi, S.A.; Valon, C.; Leung, J.A. Brand new start: Abscisic acid perception and transduction in the guard cell. Sci. Signal. 2011, 4, re4. [Google Scholar] [CrossRef]
- Hsu, F.C.; Chou, M.Y.; Peng, H.P.; Chou, S.J.; Shih, M.C. Insights into hypoxic systemic responses based on analyses of transcriptional regulation in Arabidopsis. PLoS ONE 2011, 6, e28888. [Google Scholar] [CrossRef] [Green Version]
- Janowiak, F.; Else, M.A.; Jackson, M.B. Leaf-area-specific delivery rates of indole acetic acid and abscisic acid in the transpiration stream of flooded tomato plants in relation to stomatal closure. Zeszyty Problemowe Postępów Nauk Rolniczych 2010, 545, 151–159. [Google Scholar]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Jackson, M.B. Long-distance signalling from roots to shoots assessed: The flooding story. J. Exp. Bot. 2002, 53, 175–181. [Google Scholar] [CrossRef]
- Else, M.A.; Taylor, J.M.; Atkinson, C.J. Anti-transpirant activity in xylem sap from flooded tomato (Lycopersicon esculentum Mill.) plants is not due to pH-mediated redistributions of root- or shoot-sourced ABA. J. Exp. Bot. 2006, 57, 3349–3357. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, R.; Voesenek, L.A.C.J. Ethylene-Mediated Acclimations to Flooding Stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; van Dongen, J.T.V. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef]
- Dongen, J.T.V.; Licausi, F. Low-oxygen stress in plants. Plant Cell Monogr. 2014, 21. [Google Scholar] [CrossRef]
- Bashar, K.; Tareq, M.; Amin, M.; Honi, U.; Tahjib-Ul-Arif, M.; Sadat, M.; Hossen, Q. Phytohormone-mediated stomatal response, escape and quiescence strategies in plants under flooding stress. Agronomy 2019, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Wanke, D. The ABA-mediated switch between submersed and emersed life-styles in aquatic macrophytes. J. Plant Res. 2011, 124, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, G.M.; Cadenas, G.A.; Arbona, V. Abscisic acid as an emerging modulator of the responses of plants to low oxygen conditions. Front. Plant Sci. 2021, 12, 661–789. [Google Scholar] [CrossRef]
- De Ollas, C.; Guzmán, G.M.; Pitarch, Z.; Matus, J.T.; Candela, H.; Rambla, J.L.; Granell, A.; Cadenas, G.A.; Arbona, V. Identification of ABA-Mediated Genetic and Metabolic Responses to Soil Flooding in Tomato (Solanum lycopersicum L. Mill). Front. Plant Sci. 2021, 12, 613059. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.C.H.; Benschop, J.J.; Vreeburg, R.A.M.; Wagemaker, C.A.M.; Moritz, T.; Peeters, A.J.M.; Voesenek, L.A.C.J. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol. 2004, 136, 2948–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Straeten, D.; Zhou, Z.; Prinsen, E.; Van Onckelen, H.; Van Montagu, M.C. A comparative molecular-physiological study of submergence response in lowland and deepwater rice. Plant Physiol. 2001, 125, 955–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Q.; Wang, Y.; Li, X.; Wu, S.; Wang, Y.; Luo, J.; Mattson, N.; Xu, Y. Interactions between ethylene, gibberellin and abscisic acid in regulating submergence induced petiole elongation in Nelumbo nucifera. Aquat. Bot. 2016, 137, 9–15. [Google Scholar] [CrossRef]
- Kende, H. Deepwater Rice: A Model Plant to Study Stem Elongation. Plant Physiol. 1998, 118, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Raskin, I.; Kende, H. Regulation of growth in stem sections of deep-water rice. Planta 1984, 160, 66–72. [Google Scholar] [CrossRef]
- Chen, X.; Pierik, R.; Peeters, A.J.M.; Poorter, H.; Visser, E.J.W.; Huber, H.; Kroon, H.D.; Voesenek, L.A.C.J. Endogenous abscisic acid as a key switch for natural variation in flooding-induced shoot elongation. Plant Physiol. 2010, 154, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.; Hedrich, R. Channeling auxin action: Modulation of ion transport by indole-3-acetic acid. Plant Mol. Biol. 2002, 49, 349–356. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef]
- Rijnders, J.G.H.M.; Yang, Y.Y.; Kamiya, Y.; Takahashi, N.; Barendse, G.W.M.; Blom, C.W.P.M.; Voesenek, L.A.C.J. Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta 1997, 203, 20–25. [Google Scholar] [CrossRef]
- Cox, M.C.H.; Peeters, A.J.M.; Voesenek, L.A.C.J. The stimulating effects of ethylene and auxin on petiole elongation and on hyponastic curvature are independent processes in submerged Rumex palustris. Plant Cell Environ. 2010, 29, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.J.; O’Neill, D.P.; Smith, J.J.; Kerckhoffs, L.H.J.; Elliott, R.C. Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J. 2000, 21, 547–552. [Google Scholar] [CrossRef]
- Rauf, M.; Arif, M.; Fisahn, J.; Xue, G.P.; Balazadeh, S.; Mueller-Roeber, B. NAC transcription factor speedy hyponastic growth regulates flooding induced leaf movement in Arabidopsis. Plant Cell 2013, 25, 4941–4955. [Google Scholar] [CrossRef] [Green Version]
- Phukan, U.J.; Mishra, S.; Timbre, K.; Luqman, S.; Shukla, R.K. Mentha arvensis exhibits better adaptive characters in contrast to Mentha piperita when subjugated to sustained waterlogging stress. Protoplasma 2014, 251, 603–614. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Blom, C.W.P.M. Growth responses of Rumex species in relation to submergence and ethylene. Plant Cell Environ. 2010, 12, 433–439. [Google Scholar] [CrossRef]
- Pierik, R.; Cuppens, M.L.C.; Voesenek, L.A.C.J.; Visser, E.J.W. Interactions between ethylene and gibberellins in phytochrome mediated shade avoidance responses in tobacco. Plant Physiol. 2004, 136, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Polko, J.K.; Pierik, R.; van Zanten, M.; Tarkowská, D.; Strnad, M.; Voesenek, L.A.C.J.; Peeters, A.J.M. Ethylene promotes hyponastic growth through interaction with ROTUNDIFOLIA3/CYP90C1 in Arabidopsis. J. Exp. Bot. 2013, 64, 613–624. [Google Scholar] [CrossRef]
- Zotz, G.; Wilhelm, K.; Becker, A. Heteroblasty-A Review. Bot. Rev. 2011, 77, 109–151. [Google Scholar] [CrossRef]
- Kuwabara, A.; Ikegami, K.; Koshiba, T.; Nagata, T. Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae). Planta 2003, 217, 880–887. [Google Scholar] [CrossRef]
- Kuwabara, A.; Tsukaya, H.; Nagata, T. Identification of factors that cause heterophylly in Ludwigia arcuate walt. (Onagraceae). Plant Biol. 2000, 3, 98–105. [Google Scholar] [CrossRef]
- Kuwabara, A.; Nagata, T. Cellular basis of developmental plasticity observed in heterophyllous leaf formation of Ludwigia arcuata (Onagraceae). Planta 2006, 224, 761–770. [Google Scholar] [CrossRef]
- Ling, B.L.; Wang, H.J.; Wang, J.S.; Irina, Z.L.; Abrams, S.R. Abscisic acid regulation of heterophylly in Marsilea quadrifolia L.: Effects of R-(−) and S-(+) isomers. J. Exp. Bot. 2005, 56, 2935–2948. [Google Scholar] [CrossRef] [Green Version]
- Juhyun, K.; Youngsun, J.; Jinseul, K.; Myeongjune, J.; Yoon, P.J.; Gyun, L.H.; Soo, C.D.; Eunju, L.; Ilha, L.; Hao, Y. A molecular basis behind heterophylly in an amphibious plant, Ranunculus trichophyllus. PLoS Genet. 2018, 14, e1007208. [Google Scholar] [CrossRef] [Green Version]
- Walbot, V. Abscisic acid induces pink pigmentation in maize aleurone tissue in the absence of bronze-2. Maydica 1994, 1, 19–28. [Google Scholar]
- Yanai, O.; Shani, E.; Russ, D.; Ori, N. Gibberellin partly mediates LANCEOLATE activity in tomato. Plant J. 2011, 68, 571–582. [Google Scholar] [CrossRef]
- Farquharson, K.L. Examining the molecular basis of heterophylly in NorthAmerican Lake cress. Plant Cell 2014, 26, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, H.; Nakayama, N.; Seiki, S.; Kojima, M.; Sakakibara, H.; Sinha, N.; Kimura, S. Regulation of the KNOX-GA gene module induces heterophyllic alteration in North American Lake Cress. Plant Cell 2014, 26, 4733–4748. [Google Scholar] [CrossRef] [Green Version]
- Deschamp, P.A.; Cooke, T.J. Leaf dimorphism in the aquatic angiosperm Callitriche heterophylla. Am. J. Bot. 1985, 72, 1377–1387. [Google Scholar] [CrossRef]
- Townsley, B.T.; Sinha, N.R. A new development: Evolving concepts in leaf ontogeny. Annu. Rev. Plant Biol. 2012, 63, 535–562. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.E. Making leaves. Curr. Opin. Plant Biol. 2011, 15, 24–30. [Google Scholar] [CrossRef]
- Mommer, L.; Pons, T.L.; Visser, E.J. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. J. Exp. Bot. 2006, 57, 283–290. [Google Scholar] [CrossRef]
- Mommer, L.; Pons, T.L.; Wolters-Arts, M.; Venema, J.K.; Visser, E.J.W. Submergence induced morphological, anatomical, and biochemical responses in a terrestrial species affects gas diffusion resistance and photosynthetic performance. Plant Physiol. 2005, 139, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Visser, E.J.W.; Cohen, J.D.; Barendse, G.W.M.; Blom, C.W.P.M.; Voesenek, L.A. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol. 1996, 112, 1687–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, W.T. The role of adventitious roots in recovery of shoots following flooding of the original root systems. Am. J. Bot. 1955, 42, 816–819. [Google Scholar] [CrossRef]
- Yamauchi, T.; Abe, F.; Kawaguchi, K.; Oyanagi, A.; Nakazono, M. Adventitious roots of wheat seedlings that emerge in oxygen-deficient conditions have increased root diameters with highly developed lysigenous aerenchyma. Plant Signal Behav. 2014, 9, e28506-1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorbiecke, R.; Sauter, M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999, 119, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Ecker, J.R. The ethylene signal transduction pathway in plants. Science 1995, 268, 667–675. [Google Scholar] [CrossRef]
- Xue, Z.H.; Kou, X.H.; Luo, Y.B.; Zhu, B.Z.; Xu, W.T. Effect of ethylene on polygalacturonase, lipoxygenase and expansin in ripening of tomato fruits. Trans. Tianjin Univ. 2009, 15, 173–177. [Google Scholar] [CrossRef]
- Dawood, T.; Yang, X.; Visser, E.J.; Te Beek, T.A.; Kensche, P.R.; Cristescu, S.M.; Rieu, I. A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiol. 2016, 170, 2351–2364. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Pacurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Pacurar, M.; Demailly, H.; Geiss, G. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [Green Version]
- Lischweski, S.; Muchow, A.; Guthörl, D.; Hause, B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef] [Green Version]
- Le Nguyen Huynh, N.L.; VanToai, T.; Streeter, J.; Banowetz, G. Regulation of flooding tolerance of SAG12:ipt Arabidopsis plants by cytokinin. J. Exp. Bot. 2005, 56, 1397–1407. [Google Scholar] [CrossRef] [Green Version]
- Shtratnikova, V.Y.; Kudryakova, N.V.; Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Danilova, M.N.; Kusnetsov, V.V.; Kulaeva, O.N. Effect of the ipt gene expression on wheat tolerance to root flooding. Russ. J. Plant Physiol. 2011, 58, 799–807. [Google Scholar] [CrossRef]
- Yoon-Ha, K.; Sun-Joo, H.; Muhammad, W.; Khan, A.L.; Joon-Hee, L.; Jeong-Dong, L.; Nguyen, H.T.; In-Jung, L. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- Dengler, N.G. Anisophylly and dorsiventral shoot symmetry. Int. J. Plant Sci. 1999, 160, S67–S80. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.L.; Guerreiro, S.M.C.; Sodek, L. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Ann. Bot. 2005, 96, 1191–1198. [Google Scholar] [CrossRef]
- Shimamura, S.; Mochizuki, T.; Nada, Y.; Fukuyama, M. Secondary aerenchyma formation and its relation to nitrogen fixation in root nodules of soybean plants (Glycine max) grown under flooded conditions. Plant Prod. Sci. 2002, 5, 294–300. [Google Scholar] [CrossRef]
- Shimamura, S.; Mochizuki, T.; Nada, Y.; Fukuyama, M. Formation andfunction of secondary aerenchyma in hypocotyl, roots, and nodules of soybean (Glycine max) under flooded conditions. Plant Soil. 2003, 251, 351–359. [Google Scholar] [CrossRef]
- Shimamura, S.; Yamamoto, R.; Nakamura, T.; Shimada, S.; Komatsu, S. Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Ann. Bot. 2010, 106, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Shimamura, S.; Yoshioka, T.; Yamamoto, R.; Hiraga, S.; Nakamura, T.; Shimada, S.; Komatsu, S. Role of abscisic acid in flood-induced secondary aerenchyma formation in soybean (Glycine max) hypocotyls. Plant Prod. Sci. 2014, 17, 131–137. [Google Scholar] [CrossRef]
- Larré, C.F.; Fernando, J.A.; Marini, P.; Bacarin, M.A.; Peters, J.A. Growth and chlorophyll a fluorescence in Erythrina crista-galli L. plants under flooding conditions. Acta Physiol. Plant. 2013, 35, 1463–1471. [Google Scholar] [CrossRef]
- Larson, K.D.; Schaffer, B.; Davies, F.S. Floodwater oxygen content, ethylene production and lenticel hypertrophy in flooded mango (Mangifera indica L) trees. J. Exp. Bot. 1993, 44, 665–671. [Google Scholar] [CrossRef]
- Syed, N.H.; Prince, S.J.; Mutava, R.N.; Patil, G.; Song, L.; Chen, W.; Babu, V.; Joshi, T.; Khan, S.; Nguyen, H.T. Core clock, SUB1, and ABAR genes mediate flooding and drought responses via alternative splicing in soybean. J. Exp. Bot. 2015, 66, 7129. [Google Scholar] [CrossRef] [Green Version]
- Covington, M.F.; Harmer, S.L. The circadian clock regulates auxin signaling and responses in Arabidopsis. PloS Biol. 2007, 5, 1773–1784. [Google Scholar] [CrossRef] [Green Version]
- Fukao, T.; Yeung, E.; Bailey-Serres, J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 2011, 23, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Setter, T.L.; Bhekasut, P.; Greenway, H. Desiccation of leaves after desubmergence is one cause for intolerance to complete submergence of the rice cultivar IR42. Funct. Plant Biol. 2010, 37, 1096–1104. [Google Scholar] [CrossRef]
- Tamang, B.G.; Magliozzi, J.O.; Maroof, M.A.S.; Fukao, T. Physiological and transcriptomic characterization of submergence and reoxygenation responses in soybean seedlings. Plant Cell Environ. 2014, 37, 2350–2365. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.J.; Chou, S.J.; Shih, M.C. ET plays an essential role in the recovery of Arabidopsis during post-anaerobiosis reoxygenation. Plant Cell Environ. 2014, 37, 2391–2405. [Google Scholar] [CrossRef]
- Yuan, L.B.; Dai, Y.S.; Xie, L.J.; Yu, L.J.; Zhou, Y.; Lai, Y.X.; Yang, Y.C.; Xu, L.; Fang, Q.; Chen, S.X. Jasmonate regulates plant responses to postsubmergence reoxygenation through transcriptional activation of antioxidant synthesis. Plant Physiol. 2017, 173, 1864–1880. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.J.; Lin, C.Y.; Ting, C.Y.; Shih, M.C. ET-regulated glutamate dehydrogenase fine-tunes metabolism during anoxia-reoxygenation. Plant Physiol. 2016, 172, 1548–1562. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, D.J.; Lee, S.C.; Isa, N.M.; Gramuglia, S.; Fukao, T.; Bassel, G.W.; Correia, C.S.; Corbineau, F.; Theodoulou, F.L.; Bailey-Serres, J. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011, 479, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.C.J.; Perata, P.; van Dongen, J.T.V. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef]
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J. Crosstalk between nitric oxide (NO) and abscisic acid (ABA) signalling molecules in higher plants. Environ. Exp. Bot. 2018, 161, 41–49. [Google Scholar] [CrossRef]
- León, J.; Costa-Broseta, Á.; Castillo, M.C.; Spoel, S. RAP2.3 negatively regulates nitric oxide biosynthesis and related responses through a rheostat-like mechanism in Arabidopsis. J. Exp. Bot. 2020, 71, 3157–3171. [Google Scholar] [CrossRef] [Green Version]
- Hartman, S.; Liu, Z.; Van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; Dongen, N.V.; Bosman, F.; Bassel, G.W. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef] [Green Version]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Hancock, J.T. A forty year journey: The generation and roles of NO in plants. Nitric Oxide Biol. Chem. 2019, 93, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.; Lozano-Juste, J.; Gonzalez-Guzmán, M.; Rodriguez, L.; Rodriguez, P.L.; León, J. Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci. Signal. 2015, 8, ra89. [Google Scholar] [CrossRef]
- Gibbs, D.J.; Md Isa, N.; Movahedi, M.; Lozano-Juste, J.; Mendiondo, G.M.; Berckhan, S.; Rosa, N.M.; Conde, J.V.; Correia, C.S.P.; Pearce, S. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol. Cell 2014, 53, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Gannon, L.; Jones, P.D.; Rundle, C.A.; Hassall, K.L.; Gibbs, D.J.; Holdsworth, M.J.; Theodoulou, F.L. Genetic interactions between ABA signalling and the Arg/N-end rule pathway during Arabidopsis seedling establishment. Sci. Rep. 2018, 8, 15192. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, W.; Abou-Elwafa, S.F.; Shabala, S.; Xu, L. Understanding a Mechanistic Basis of ABA Involvement in Plant Adaptation to Soil Flooding: The Current Standing. Plants 2021, 10, 1982. https://doi.org/10.3390/plants10101982
Zhao Y, Zhang W, Abou-Elwafa SF, Shabala S, Xu L. Understanding a Mechanistic Basis of ABA Involvement in Plant Adaptation to Soil Flooding: The Current Standing. Plants. 2021; 10(10):1982. https://doi.org/10.3390/plants10101982
Chicago/Turabian StyleZhao, Yancui, Wenying Zhang, Salah Fatouh Abou-Elwafa, Sergey Shabala, and Le Xu. 2021. "Understanding a Mechanistic Basis of ABA Involvement in Plant Adaptation to Soil Flooding: The Current Standing" Plants 10, no. 10: 1982. https://doi.org/10.3390/plants10101982
APA StyleZhao, Y., Zhang, W., Abou-Elwafa, S. F., Shabala, S., & Xu, L. (2021). Understanding a Mechanistic Basis of ABA Involvement in Plant Adaptation to Soil Flooding: The Current Standing. Plants, 10(10), 1982. https://doi.org/10.3390/plants10101982








