Somatic Embryogenesis in Centaurium erythraea Rafn—Current Status and Perspectives: A Review
Abstract
:1. Somatic Embryogenesis: Biotechnological Exploitation of Plant Cells’ Totipotency
2. Centuries of Centaury Research
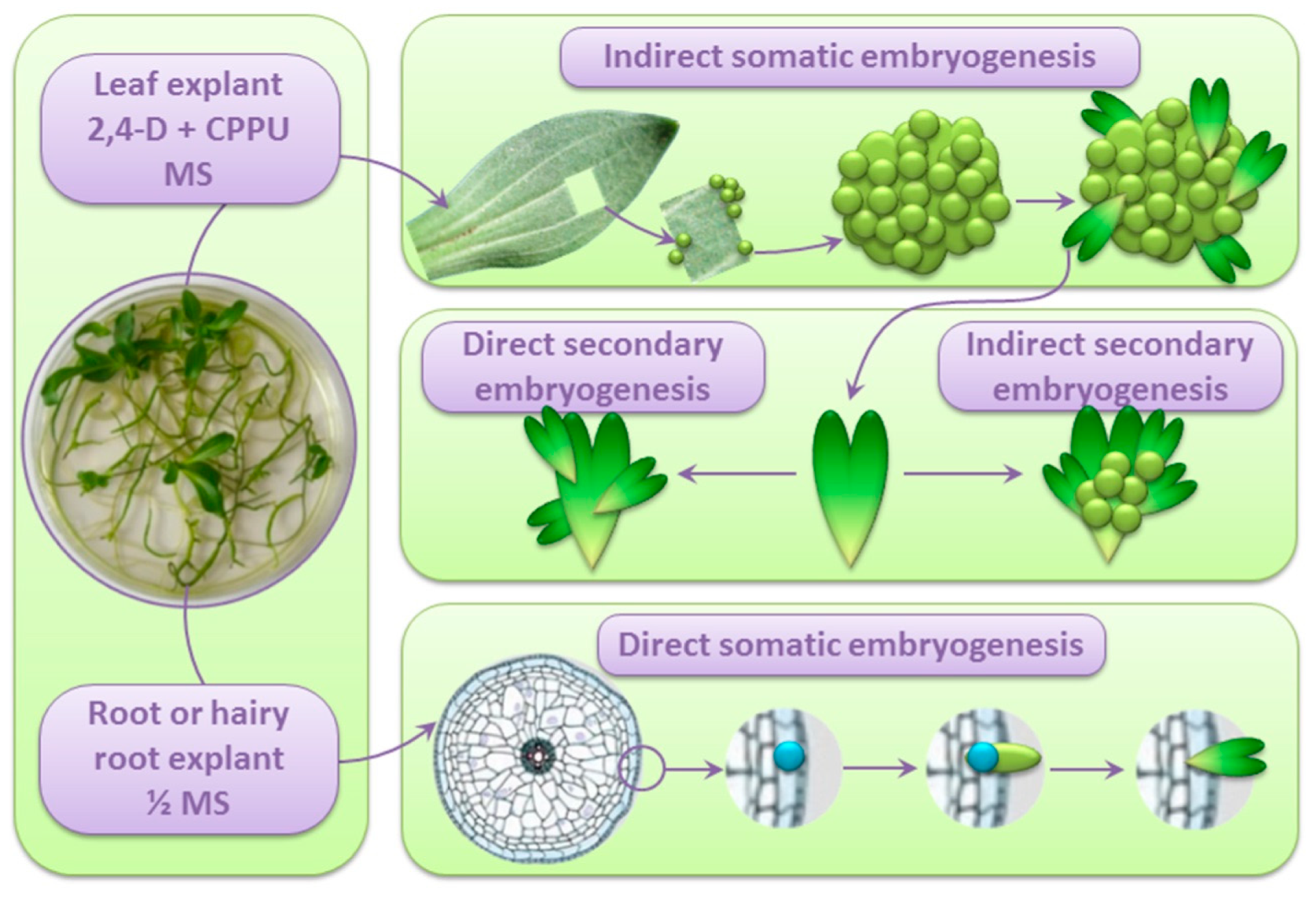
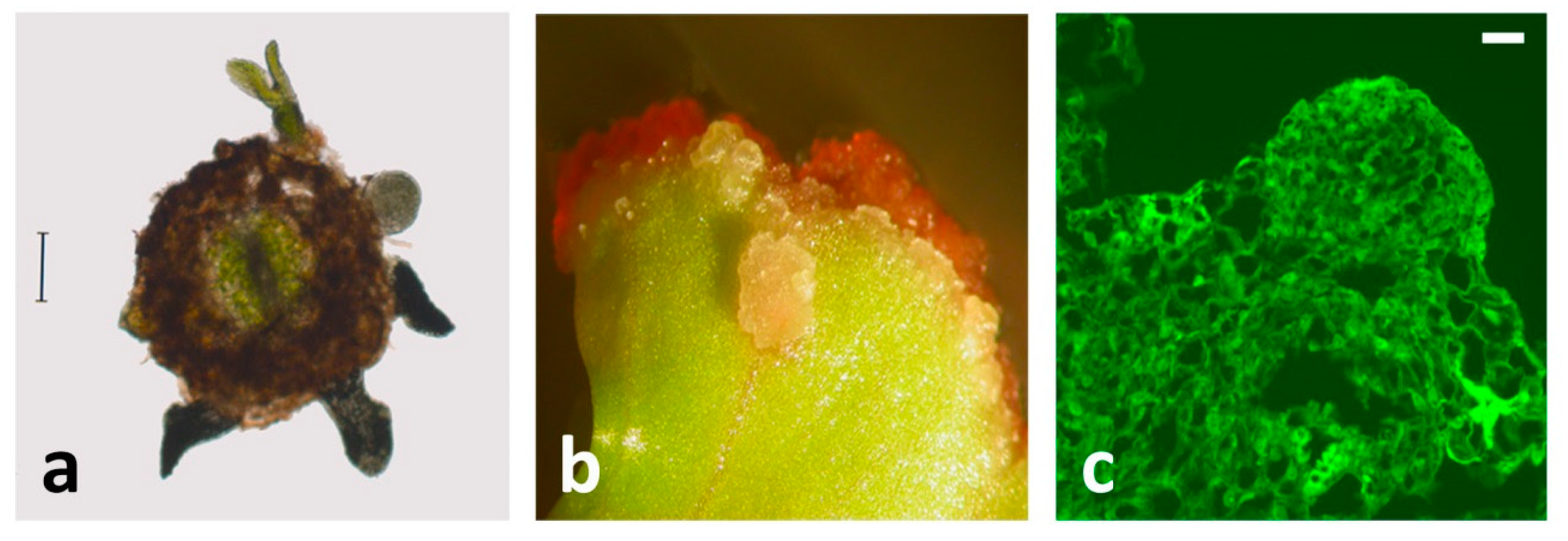
3. SE from Centaury Root Explants Is Spontaneous and Direct
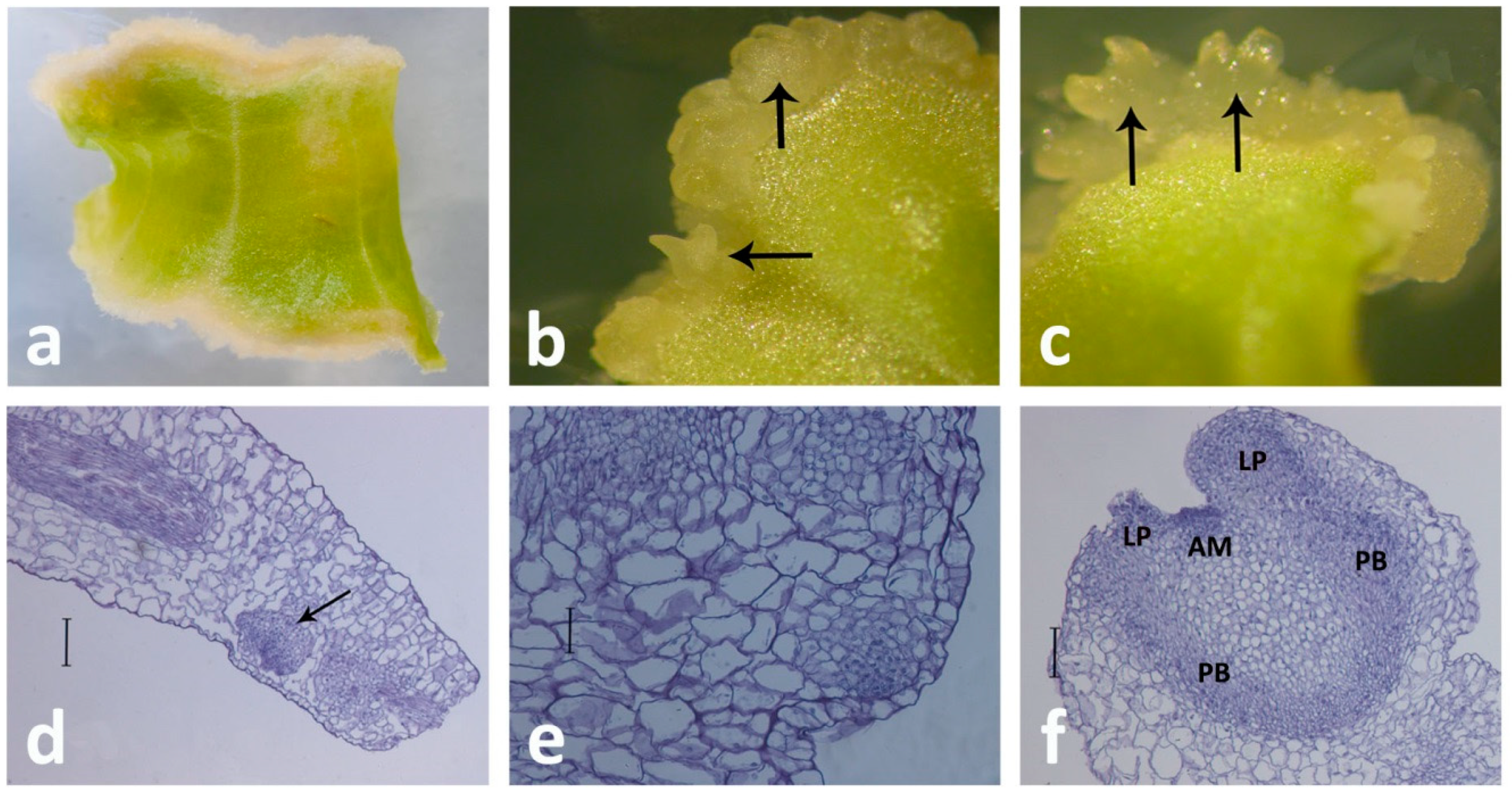
4. Indirect SE from Centaury Leaf Explants
5. Maintaining Reactive Oxygen Species Homeostasis during SE in Centaury: The Role of Antioxidative Enzymes
6. Studies on the Role of AGPs during SE in Centaury Using β-D-glucosyl Yariv Reagent
7. Dynamic Changes of AGPs Distribution and Expression during SE in Centaury
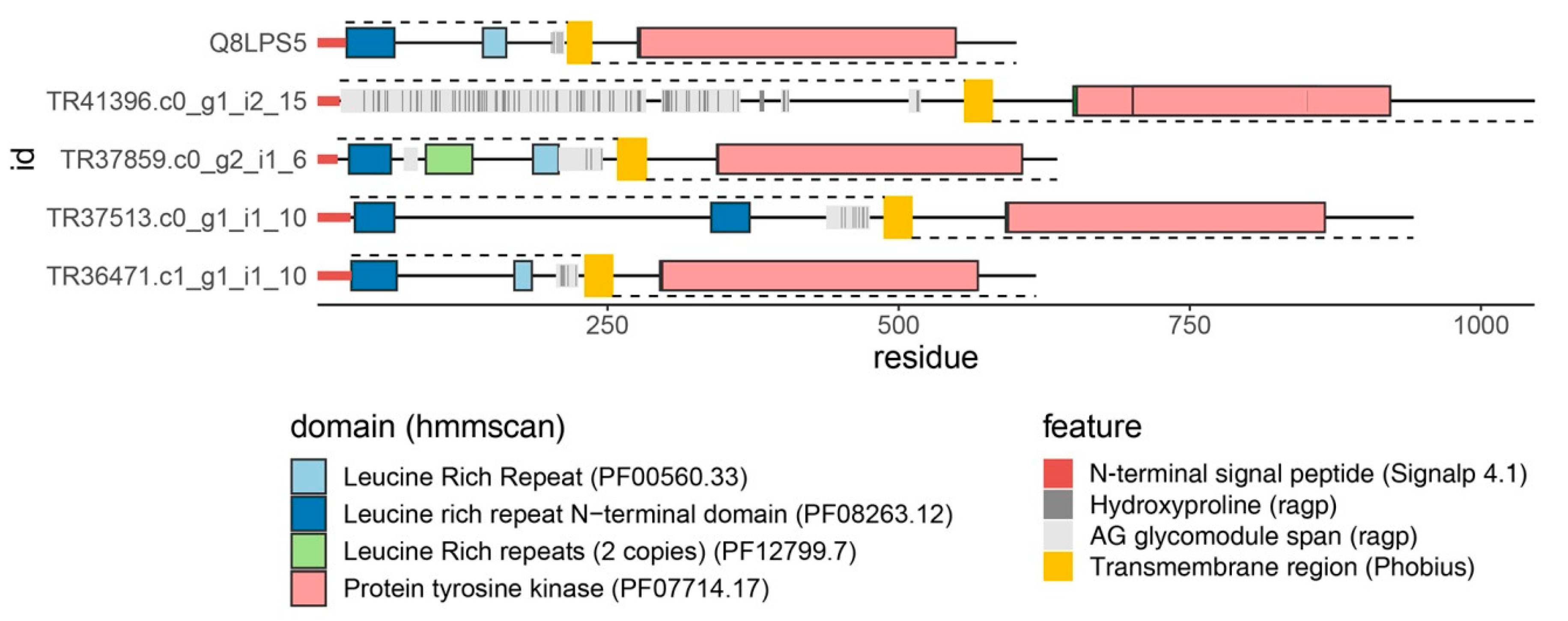
8. Perspectives: Novel SE Markers, “AGP-Tyr Kinases”, and Time-Laps Embryogenesis
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Somleva, M.; Alexieva, V.; Sergiev, I.; Karanov, E. Alterations in the activities of some hydrogen peroxide scavenging enzymes during induction of somatic embryogenesis in leaf explants from Dactylis glomerata L. Dokl. Bolg. Akad. Nauk. 2000, 53, 91–94. [Google Scholar]
- Quiroz-Figueroa, F.; Méndez-Zeel, M.; Larqué-Saavedra, A.; Loyola-Vargas, V. Picomolar concentrations of salicylates induce cellular growth and enhance somatic embryogenesis in Coffea arabica tissue culture. Plant Cell Rep. 2001, 20, 679–684. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Kareem, F.; El-Mahrouk, M.E.; Fuller, M.P. Artificial seeds (principle, aspects and applications). Agronomy 2017, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Hortsman, A.; Bemer, M.; Boutilier, K. A transcriptional view on somatic embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef]
- Phillips, G.C. In vitro morphogenesis in plants-recent advances. In Vitro Cell. Dev. Biol. Plant 2004, 40, 342–345. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.R.; Rafael, R.H.; Galaz-Avalos, R.M.; Loyola-Vargas, V.M. Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Organ Cult. 2006, 86, 258–301. [Google Scholar] [CrossRef]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, V.M. Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul. 2005, 47, 91–110. [Google Scholar] [CrossRef]
- Fehér, A.; Pasternak, T.P.; Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Smertenko, A.; Bozhkov, P.V. Somatic embryogenesis: Life and death processes during apical-basal patterning. J. Exp. Bot. 2014, 65, 1343–1360. [Google Scholar] [CrossRef] [Green Version]
- Karami, O.; Saidi, A. The molecular basis for stress-induced acquisition of somatic embryogenesis. Mol. Biol. Rep. 2010, 37, 2493–2507. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, B.; Mujib, A.; Moien Qadir, M.; Sayeed, R.; Mamgain, J.; Ejaz, B. Genes, proteins and other networks regulating somatic embryogenesis in plants. J. Genet. Eng. Biotechnol. 2020, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Tchorbadjieva, M.I. Protein markers for somatic embryogenesis. In Plant Cell Monographs; Mujib, A., Šamaj, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 215–233. [Google Scholar] [CrossRef]
- Haloui, M.; Louedec, L.; Michel, J.B.; Lyoussi, B. Experimental diuretic effects of Rosmarinus officinalis and Centaurium erythraea. J. Ethnopharmacol. 2000, 71, 465–472. [Google Scholar] [CrossRef]
- Stefkov, G.; Miova, B.; Dinevska-Kjovkarovska, S.; Stanoeva, J.P.; Stefova, M.; Petrusevska, G.; Kulevanova, S. Chemical characterization of Centaurium erythrea L. and its effects on carbohydrate and lipid metabolism in experimental diabetes. J. Ethnopharmacol. 2014, 152, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Guedes, L.; Reis, P.B.P.S.; Machuqueiro, M.; Ressaissi, A.; Pacheco, R.; Serralheiro, M.L. Bioactivities of Centaurium erythraea (Gentianaceae) decoctions: Antioxidant activity, enzyme inhibition and docking studies. Molecules 2019, 24, 3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkan, T.; Ustünes, L.; Lermioglu, F.; Ozer, A. Antiinflammatory, analgesic, and antipyretic effects of an aqueous extract of Erythraea centaurium. Planta Med. 1991, 57, 34–37. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidant activity of Centaurium erythraeainfusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. J. Agric. Food Chem. 2001, 49, 3476–3479. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Naha, L.; Cox, P.J.; Jaspars, M.; Sarker, S.D. Bioactivity of secoiridoid glycosides from Centaurium erythraea. Phytomedicine 2003, 10, 344–347. [Google Scholar] [CrossRef]
- Tuluce, Y.; Ozkol, H.; Koyuncu, I.; Ine, H. Gastroprotective effect of small centaury (Centaurium erythraea L.) on aspirin-induced gastric damage in rats. Toxicol. Ind. Health 2011, 27, 760–768. [Google Scholar] [CrossRef]
- Trifunović-Momčilov, M.; Krstić-Milošević, D.; Trifunović, S.; Podolski-Renić, A.; Pešić, M.; Subotić, A. Secondary metabolite profile of transgenic centaury (Centaurium erythraea Rafn) plants, potential producers of anticancer compounds. In Transgenesis and Secondary Metabolism, Reference Series in Phytochemistry; Jha, S., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Trifunović-Momčilov, M.; Krstić-Milošević, D.; Trifunović, S.; Ćirić, A.; Glamočlija, J.; Jevremović, S.; Subotić, A. Antimicrobial activity, antioxidant potential and total phenolic content of transgenic AtCKX1 centaury (Centaurium erythraea Rafn) plants grown in vitro. Environ. Eng. Manag. J. 2019, 18, 2063–2072. [Google Scholar] [CrossRef]
- Đorđević, M.; Grdović, N.; Mihailović, M.; Arambašić-Jovanović, J.; Uskoković, A.; Rajić, J.; Đordjević, M.; Tolić, A.; Mišić, D.; Šiler, B.; et al. Centaurium erythraea methanol extract protects red blood cells from oxidative damage in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2017, 202, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Šiler, B.; Avramov, S.; Banjanac, T.; Cvetković, J.; Nestorović-Živković, J.; Patenković, M.; Mišić, D. Secoiridoid glycosides as a marker system in chemical variability estimation and chemotype assignment of Centaurium erythraea Rafn from the Balkan Peninsula. Ind. Crop. Prod. 2012, 40, 336–344. [Google Scholar] [CrossRef]
- Šiler, B.; Živković, S.; Banjanac, T.; Cvetković, J.; Nestorović-Živković, J.; Ćirić, A.; Soković, M.; Mišić, D. Centauries as underestimated food additives: Antioxidant and antimicrobial potential. Food Chem. 2014, 147, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, O.; Radulović, N.; Stojanović, G.; Palić, R.; Zlatković, B.; Gudžić, B. Chemical composition of the essential oil of Centaurium erythraea Rafn (Gentianaceae) from Serbia. J. Essent. Oil Res. 2009, 21, 317–322. [Google Scholar] [CrossRef]
- Filipović, B.K.; Simonović, A.D.; Trifunović, M.M.; Dmitrović, S.S.; Savić, J.M.; Jevremović, S.B.; Subotić, A.R. Plant regeneration in leaf culture of Centaurium erythraea Rafn Part 1: The role of antioxidant enzymes. Plant Cell Tissue Organ Cult. 2015, 121, 703–719. [Google Scholar] [CrossRef]
- Subotić, A.; Budimir, S.; Grubišić, D.; Momčilović, I. Direct regeneration of shoots from hairy root cultures of Centaurium erythraea inoculated with Agrobacterium rhizogenes. Biol. Plantarum 2003, 47, 617–619. [Google Scholar] [CrossRef]
- Trifunović, M.; Cingel, A.; Simonović, A.; Jevremović, S.; Petrić, M.; Dragićević, I.Č.; Motyka, V.; Dobrev, P.I.; Zahajská, L.; Subotić, A. Overexpression of Arabidopsis cytokinin oxidase/dehydrogenase genes AtCKX1 and AtCKX2 in transgenic Centaurium erythraea Rafn. Plant Cell Tissue Organ Cult. 2013, 115, 139–150. [Google Scholar] [CrossRef]
- Trifunović, M.; Motyka, V.; Cingel, A.; Subotić, A.; Jevremović, S.; Petrić, M.; Holík, J.; Malbeck, J.; Dobrev, P.I.; Dragićević, I.Č. Changes in cytokinin content and altered cytokinin homeostasis in AtCKX1 and AtCKX2-overexpressing centaury (Centaurium erythraea Rafn) plants grown in vitro. Plant Cell Tissue Organ Cult. 2015, 120, 767–777. [Google Scholar] [CrossRef]
- Banjanac, T.; Šiler, B.; Skorić, M.; Ghalawenji, N.; Milutinović, M.; Božić, D.; Mišić, D. Interspecific in vitro hybridization in genus Centaurium and identification of hybrids via flow cytometry, RAPD, and secondary metabolite profiles. Turk. J. Bot. 2014, 38, 68–79. [Google Scholar] [CrossRef]
- Banjanac, T.; Dragićević, M.; Šiler, B.; Gašić, U.; Bohanec, B.; Nestorović-Živković, J.; Trifunović, S.; Mišić, D. Chemodiversity of two closely related tetraploid Centaurium species and their hexaploid hybrid: Metabolomic search for high-resolution taxonomic classifiers. Phytochemistry 2017, 140, 27–44. [Google Scholar] [CrossRef]
- Banjanac, T.; Đurović, S.; Jelić, M.; Dragićević, M.; Mišić, D.; Skorić, M.; Nestorović-Živković, J.; Šiler, B. Phenotypic and genetic variation of an interspecific Centaurium hybrid (Gentianaceae) and its parental species. Plants 2019, 8, 224. [Google Scholar] [CrossRef] [Green Version]
- Subotiċ, A.; Jankoviċ, T.; Jevremoviċ, S.; Grubišiċ, D. Plant Tissue Culture and Secondary Metabolites Productions of Centaurium erythraea Rafn, a Medical plant. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Science Books: London, UK, 2006; pp. 564–570. [Google Scholar]
- Subotić, A.; Jevremović, S.; Grubišić, D.; Janković, T. Spontaneous plant regeneration and production of secondary metabolites from hairy root cultures of Centaurium erythraea Rafn. In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, Methods in Molecular Biology; Jain, S.M., Saxena, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 205–215. [Google Scholar] [CrossRef]
- Božunović, J.; Živković, S.; Gašić, U.; Glamočlija, J.; Ćirić, A.; Matekalo, D.; Šiler, B.; Soković, M.; Tešić, Ž.; Mišić, D. In Vitro and In Vivo transformations of Centaurium erythraea secoiridoid glucosides alternate their antioxidant and antimicrobial capacity. Ind. Crop. Prod. 2018, 111, 705–721. [Google Scholar] [CrossRef]
- Šiler, B.; Mišić, D.; Filipović, B.; Popović, Z.; Cvetić, T.; Mijović, A. Effects of salinity on in vitro growth and photosynthesis of common centaury (Centaurium erythraea Rafn). Arch. Biol. Sci. 2007, 59, 129–134. [Google Scholar] [CrossRef]
- Trifunović-Momčilov, M.; Paunović, D.; Milošević, S.; Marković, M.; Jevremović, S.; Dragićević, I.Č.; Subotić, A. Salinity stress response of non-transformed and AtCKX transgenic centaury (Centaurium erythraea Rafn) shoots and roots grown in vitro. Ann. Appl. Biol. 2020, 177, 74–89. [Google Scholar] [CrossRef]
- Bogdanović, M.; Ćuković, K.; Dragićević, M.; Simonović, A.; Subotić, A.; Todorović, S. Secondary somatic embryogenesis in Centaurium erythraea. In Proceedings of the 3rd International Conference on Plant biology, Belgrade, Serbia, 9–12 June 2018; p. 34. [Google Scholar]
- Gutiérrez-Mora, A.; González-Gutiérrez, A.G.; Rodriguez-Garay, B.; Ascencio-Cabral, A.; Li-Wei, L. Plant Somatic Embryogenesis: Some useful considerations. In Embryogenesis; Ken-Ichi, S., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Fiuk, A.; Rybczyński, J.J. Morphogenic capability of Gentiana kuroo Royle seedling and leaf explants. Acta Physiol. Plant. 2008, 30, 157–166. [Google Scholar] [CrossRef]
- Fiuk, A.; Rybczyński, J.J. Genotype and plant growth regulator dependent response of somatic embryogenesis from Gentiana spp. leaf explants. In Vitro Cell. Dev. Biol. Plant 2008, 44, 90–99. [Google Scholar] [CrossRef]
- Ghanti, S.K.; Sujata, K.G.; Rao, S.; Udayakumar, M.; Kavi Kishor, P.B. Role of enzymes and identification of stage-specific proteins in developing somatic embryos of chickpea (Cicer arietinum L.). In Vitro Cell. Dev. Biol. Plant 2009, 45, 667–672. [Google Scholar] [CrossRef]
- Ma, G.; Xu, Q. Induction of somatic embryogenesis and adventitious shoots from immature leaves of cassava. Plant Cell Tissue Organ Cult. 2002, 70, 281–288. [Google Scholar] [CrossRef]
- Cantelmo, L.; Soares, B.O.; Rocha, L.P.; Pettinelli, J.A.; Callado, C.H.; Mansur, E.; Casteller, A.; Gagliardi, R.F. Repetitive somatic embryogenesis from leaves of the medicinal plant Petiveria alliacea L. Plant Cell Tissue Organ Cult. 2013, 115, 385–393. [Google Scholar] [CrossRef]
- Ma, G.; Lu, J.; Teixeira da Silva, J.A.; Zhang, X.; Zhao, J. Shoot organogenesis and somatic embryogenesis from leaf and shoot explants of Ochna integerrima (Lour). Plant Cell Tissue Organ Cult. 2011, 104, 157–162. [Google Scholar] [CrossRef]
- Yang, X.; Lu, J.; Teixeira da Silva, J.M.; Ma, G. Somatic embryogenesis and shoot organogenesis from leaf explants of Primulina tabacum. Plant Cell Tissue Organ Cult. 2012, 109, 213–221. [Google Scholar] [CrossRef]
- Barešová, H.; Kamínek, M. Light induce embryogenesis in suspension culture of Centaurium erythraea Rafn. In Plant Tissue and Cell Culture Propagation to Crop Improvement; Novák, F.J., Havel, L., Doležel, J., Eds.; Czechoslovak Academy of Sciences: Prague, Czechoslovakia, 1984; pp. 163–164. [Google Scholar]
- Subotić, A.; Grubišić, D. Histological analysis of somatic embryogenesis and adventitious formation from root explants of Centaurium erythreae Gillib. Biol. Plantarum 2007, 51, 514–516. [Google Scholar] [CrossRef]
- Subotić, A.; Jevremović, S.; Trifunović, M.; Petrić, M.; Milošević, S.; Grubišić, D. The influence of gibberelic acid and paclobutrazol on induction of somatic embryogenesis in wild type and hairy root cultures of Centaurium erythraea Gillib. Afr. J. Biotechnol. 2009, 8, 3223–3228. [Google Scholar]
- Tomiczak, K.; Mikuła, A.; Niedziela, A.; Wójcik-Lewandowska, A.; Domżalska, L.; Rybczyński, J.J. Somatic embryogenesis in the family Gentianaceae and its biotechnological application. Front. Plant Sci. 2019, 10, 762. [Google Scholar] [CrossRef]
- Mikuła, A.; Rybczyńsky, J.J. Somatic embryogenesis of Gentiana genus I. The effect of the preculture treatment and primary explant origin on somatic embryogenesis of Gentiana cruciata (L.), G. pannonica (Scop.), and G. tibetica (King). Acta Physiol. Plant 2001, 23, 15–25. [Google Scholar] [CrossRef]
- Mikuła, A.; Tykarska, T.; Rybczyńsk, J.J.; Kuraś, M. Ultrastructural analysis of initial stages of dedifferentiation of root explants of Gentianaseedlings. Acta Soc. Bot. Pol. 2002, 71, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Holobiuc, I. Somatic embryogenesis in long-term cultures of Gentiana lutea L. in the presence of osmotic stress. In The Gentianaceae—Volume 2: Biotechnology and Application; Rybczyński, J.J., Davey, M.R., Mikuła, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 139–161. [Google Scholar] [CrossRef]
- Yumbla-Orbes, M.; Ferreira da Cruz, A.C.; Marques Pinheiro, M.V.; Rocha, D.I.; Batista, D.S.; Koehler, A.D.; Barbosa, J.G.; Otoni, W.C. Somatic embryogenesis and de novo shoot organogenesis can be alternatively induced by reactivating pericycle cells in Lisianthus (Eustoma grandiflorum (Raf.) Shinners) root explants. In Vitro Cell. Dev. Biol. Plant 2017, 53, 209–218. [Google Scholar] [CrossRef]
- Gaj, D.M. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004, 43, 27–47. [Google Scholar] [CrossRef]
- Thu, H.T.M.; Naing, A.H.; Jeong, H.Y.; Kim, C.K. Regeneration of genetically stable plants from in vitro vitrified leaves of different carnation cultivars. Plants 2020, 9, 950. [Google Scholar] [CrossRef]
- Čellárová, E.; Repčáková, K.; Repčák, M.; Hončariv, R. Morphogenesis in tissue cultures of some medicinal plants. Acta Hortic. 1983, 132, 249–256. [Google Scholar] [CrossRef]
- Barešová, H.; Herben, T.; Kamínek, M.; Krekule, J. Hormonal control of morphogenesis in leaf segments of Centaurium erythraea. Biol. Plantarum 1985, 27, 286–291. [Google Scholar] [CrossRef]
- Laureová, D.; Čellárová, E.; Hončariv, R. Tollerance of plant tissue of Centaurium erythraea to increased concentrations of ions present in soils Eastern Slovakian lowlands. In Dni Rastlinnej Fyziológie IV; Repčák, M., Ed.; Slovenska Botaničká Spoločnost Pri Sav: Bratislava, Slovakia, 1986; pp. 221–222. [Google Scholar]
- Piatczak, E.; Wysokinska, H. In vitro regeneration of Centaurium erythraea Rafn from shoot tips and other seedling explants. Acta Soc. Bot. Pol. 2003, 72, 283–288. [Google Scholar] [CrossRef]
- Subotić, A.; Jevremović, S.; Grubišić, D. Influence of cytokinins on in vitro morphogenesis in root cultures of Centaurium erythraea—valuable medicinal plant. Sci. Hortic. 2009, 120, 386–390. [Google Scholar] [CrossRef]
- Chung, H.H.; Chen, J.T.; Chang, W.C. Plant regeneration through direct somatic embryogenesis from leaf explants of Dendrobium. Biol. Plantarum 2007, 51, 346–350. [Google Scholar] [CrossRef]
- Bach, A.; Pawłowska, B. Somatic embryogenesis in Gentiana pneumonanthe L. Acta Biol. Cracov. Bot. 2003, 45, 79–86. [Google Scholar]
- Cai, Y.; Liu, Y.; Liu, Z.; Zhang, F.; Xiang, F.; Xia, G. High-frequency embryogenesis and regeneration of plants with high content of gentiopicroside from Chinese medicinal plant Gentiana straminea Maxim. In Vitro Cell. Dev. Biol. Plant 2009, 45, 730–739. [Google Scholar] [CrossRef]
- Vinterhalter, B.; Mitić, N.; Vinterhalter, D.; Uzelac, B.; Krstić-Milošević, D. Somatic embryogenesis and in vitro shoot propagation of Gentiana utriculosa. Biologia 2016, 71, 139–148. [Google Scholar] [CrossRef]
- He, T.; Yang, L.; Zhao, Z. Embryogenesis of Gentiana straminea and assessment of genetic stability of regenerated plants using inter simple sequence repeat (ISSR) marker. Afr. J. Biotechnol. 2011, 10, 7604–7610. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chen, Q.L.; Xu, D.; Hao, J.G.; Schläppi, M.; Xu, Z.Q. Changes of gentiopicroside synthesis during somatic embryogenesis in Gentiana macrophylla. Planta Med. 2009, 75, 1618–1624. [Google Scholar] [CrossRef]
- Jha, B.T.; Dafadar, A.; Chaudhur, R.K. Somatic embryogenesis in Swertiachirata Buch. Ham. Ex Wall—A multipotent medicinal plant. Asian J. Biotechnol. 2011, 3, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Trifunović-Momčilov, M.; Motyka, V.; Dragićević, I.Č.; Petrić, M.; Jevremović, S.; Malbeck, J.; Holík, J.; Dobrev, P.I.; Subotić, A. Endogenous phytohormones in spontaneously regenerated Centaurium erythraea Rafn plants grown in vitro. J. Plant Growth Regul. 2016, 35, 543–552. [Google Scholar] [CrossRef]
- Dudits, D.; Bogre, L.; Gyorgyey, J. Molecular and cellular approaches to the analysis of plant embryo development from somatic cells in vitro. J. Cell Sci. 1991, 99, 473–482. [Google Scholar]
- Karami, O.; Aghavaisi, B.; Pour, A.M. Molecular aspects of somatic-to-embryogenic transition in plants. J. Chem. Biol. 2009, 2, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavattieri, M.A.; Frederico, A.M.; Lima, M.; Sabino, R.; Amhold-Schmidt, B. Induction of somatic embryogenesis as an example of stress-related plant reaction. Electron. J. Biotechnol. 2010, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Tchorbadjieva, M.I. Advances in proteomics of somatic embryogenesis. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 67–90. [Google Scholar] [CrossRef]
- Prudente, D.O.; de Souza, L.; Paiva, R. Plant somatic embryogenesis: Modulatory role of oxidative stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 483–487. [Google Scholar] [CrossRef]
- Libik, M.; Konieczny, R.; Pater, B.; Slesak, I.; Miszalski, Z. Differences in the activities of some antioxidant enzymes and H2O2 content during rhizogenesis and somatic embryogenesis in callus cultures of the ice plant. Plant Cell Rep. 2005, 2, 834–841. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Benson, E.E. Special Symposium: In vitro plant recalcitrance do free radicals have a role in plant tissue culture recalcitrance? In Vitro Cell. Dev. Biol. Plant 2000, 36, 163–170. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Reactive oxygen species:metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Liszkay, A.; Kenk, B.; Schopfer, P. Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 2003, 217, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Penel, C.; Dunand, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar] [CrossRef]
- Wang, X.D.; Nolan, K.E.; Irwanto, R.R.; Sheahan, M.B.; Rose, R.J. Ontogeny of embryogenic callus in Medicago truncatula: The fate of the pluripotent and totipotent stem cells. Ann. Bot. 2011, 107, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Slesak, I.; Slesak, A.; Libik, M.; Libik, M. Antioxidant response system in the short-term post-wounding effect in Mesembryanthemum crystallinum leaves. J. Plant Physiol. 2008, 165, 127–137. [Google Scholar] [CrossRef]
- Lup, S.D.; Tian, X.; Xu, J.; Pérez-Pérez, J.M. Wound signaling of regenerative cell reprogramming. Plant Sci. 2016, 250, 178–187. [Google Scholar] [CrossRef]
- Rose, R.J. Somatic embryogenesis in the Medicago truncatula model: Cellular and molecular mechanisms. Front. Plant. Sci. 2019, 10, 267. [Google Scholar] [CrossRef]
- Showalter, A.M.; Keppler, B.; Lichtenberg, J.; Gu, D.; Welch, L.R. A bioinformatics approach to the identification, classification, and analysis of hydroxyproline-rich glycoproteins. Plant Phys. 2010, 153, 485–513. [Google Scholar] [CrossRef] [Green Version]
- Dragićević, M.B.; Paunović, D.M.; Bogdanović, M.D.; Todorović, S.I.; Simonović, A.D. ragp: Pipeline for mining of plant hydroxyproline-rich glycoproteins with implementation in R. Glycobiology 2020, 30, 19–35. [Google Scholar] [CrossRef]
- Schultz, C.J.; Johnson, K.L.; Currie, G.; Bačić, A. The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell 2000, 12, 1751–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijazi, M.; Durand, J.; Pichereaux, C.; Pont, F.; Jamet, E.; Albenne, C. Characterization of the Arabinogalactan Protein 31 (AGP31) of Arabidopsis thaliana new advances on the hyp-o-glycosylation of the pro-rich domain. J. Biol. Chem. 2012, 287, 9623–9632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bačić, A. Arabinogalactan-proteins: Key regulators at the cell surface? Plant Phys. 2010, 153, 403–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguema-Ona, E.; Coimbra, S.; Vicré-Gibouin, M.; Mollet, J.C.; Driouich, A. Arabinogalactan proteins in root and pollen-tube cells: Distribution and functional aspects. Ann. Bot. 2012, 110, 383–404. [Google Scholar] [CrossRef] [Green Version]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef]
- Serpe, M.D.; Nothnagel, E.A. Effects of Yariv phenylglycosides on Rosa cell suspensions: Evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta 1994, 193, 542–550. [Google Scholar] [CrossRef]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef]
- Šamaj, J.; Baluška, F.; Bobák, M.; Volkmann, D. Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep. 1999, 18, 369–374. [Google Scholar] [CrossRef]
- Chapman, A.; Blervacq, A.S.; Vasseur, J.; Hilbert, J.L. Arabinogalactan-proteins in Cichorium somatic embryogenesis: Effect of β-glucosyl Yariv reagent and epitope localisation during embryo development. Planta 2000, 211, 305–314. [Google Scholar] [CrossRef]
- Pilarska, M.; Knox, J.P.; Konieczny, R. Arabinogalactan-protein and pectin epitopes in relation to an extracellular matrix surface network and somatic embryogenesis and callogenesis in Trifolium nigrescens Viv. Plant Cell Tissue Organ Cult. 2013, 115, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Trifunović, M.; Tadić, V.; Petrić, M.; Jontulović, D.; Jevremović, S.; Subotić, A. Quantification of arabinogalactan proteins during in vitro morphogenesis induced by β-D glucosyl Yariv reagent in Centaurim erythraea root culture. Acta Physiol. Plant. 2014, 36, 1187–1195. [Google Scholar] [CrossRef]
- Trifunović, M.; Subotić, A.; Petrić, M.; Jevremović, S. The Role of Arabinogalactan Proteins in Morphogenesis of Centaurium erythraea Rafn In Vitro. In The Gentianaceae—Volume 2: Biotechnology and Applications; Rybczyński, J.J., Davey, M.R., Mikuła, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 113–138. [Google Scholar] [CrossRef]
- Simonović, A.D.; Filipović, B.K.; Trifunović, M.M.; Malkov, S.N.; Milinković, V.P.; Jevremović, S.B.; Subotić, A.R. Plant regeneration in leaf culture of Centaurium erythraea Rafn Part 2: The role of arabinogalactan proteins. Plant Cell Tiss Organ Cult. 2015, 121, 721–739. [Google Scholar] [CrossRef]
- Filipović, B.K.; Trifunović-Momčilov, M.M.; Simonović, A.D.; Jevremović, S.B.; Milošević, S.M.; Subotić, A.R. Immunolocalization of some arabinogalactan protein epitopes during indirect somatic embryogenesis and shoot organogenesis in leaf culture of centaury (Centaurium erythraea Rafn). In Vitro Cell. Dev. Biol. Plant 2021, in press. [Google Scholar] [CrossRef]
- Yariv, J.; Lis, H.; Katchalski, E. Precipitation of arabic acid and some seed polysaccharides by glycosylphenylazo dyes. Biochem. J. 1967, 105, 1C–2C. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitazawa, K.; Tryfona, T.; Yoshimi, Y.; Hayashi, Y.; Kawauchi, S.; Antonov, L.; Tanaka, H.; Takahashi, T.; Kaneko, S.; Depree, P.; et al. β-Galactosyl Yariv reagent binds to the β-1, 3-galactan of arabinogalactan proteins. Plant Phys. 2013, 161, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.L.; Clarke, A.E.; Jermyn, M.A.; Knox, R.B.; Stone, B.A. A carbohydrate-binding arabinogalactan-protein from liquid suspension cultures of endosperm from Lolium multiflorum. Aust. J. Plant Phys. 1977, 4, 143–158. [Google Scholar] [CrossRef]
- Thompson, H.J.; Knox, J.P. Stage-specific responses of embryogenic carrot cell suspension cultures to arabinogalactan protein-binding β-glucosyl Yariv reagent. Planta 1998, 205, 32–38. [Google Scholar] [CrossRef]
- Steinmacher, D.A.; Saare-Surminski, K.; Lieberei, R. Arabinogalactan proteins and the extracellular matrix surface network during peach palm somatic embryogenesis. Phys. Plant. 2012, 146, 336–349. [Google Scholar] [CrossRef]
- Van Holst, G.J.; Clarke, A.E. Quantification of arabinogalactan-protein in plant extracts by single radial gel diffusion. Anal. Biochem. 1985, 148, 446–450. [Google Scholar] [CrossRef]
- Guan, Y.; Nothnagel, E.A. Binding of arabinogalactan proteins by Yariv phenylglycoside triggers wound-like responses in Arabidopsis cell cultures. Plant. Phys. 2004, 135, 1346–1366. [Google Scholar] [CrossRef] [Green Version]
- Van Holst, G.J.; Clarke, A.E. Organ-specific arabinogalactan-proteins of Lycopersiconperuvianum (Mill) demonstrated by crossed electrophoresis. Plant Physiol. 1986, 80, 786–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malkov, S.; Simonović, A. Shotgun Assembly of Centaurium erythraea Transcriptome. In Proceedings of the 19th Symposium of the Serbian Plant Physiology Society, Banja Vrujci, Serbia, 13–15 June 2011; p. 16. [Google Scholar]
- Johnson, K.L.; Jones, B.J.; Bačić, A.; Schultz, C.J. The fasciclin-like arabinogalactan proteins of Arabidopsis. A multigene family of putative cell adhesion molecules. Plant Physiol. 2003, 133, 1911–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonović, A.D.; Dragićević, M.B.; Bogdanović, M.D.; Trifunović-Momčilov, M.M.; Subotić, A.R.; Todorović, S.I. DUF1070 as a signature domain of a subclass of arabinogalactan peptides. Arch. Biol. Sci. 2016, 68, 737–746. [Google Scholar] [CrossRef]
- Ćuković, K.; Dragićević, M.; Bogdanović, M.; Paunović, D.; Giurato, G.; Filipović, B.; Subotić, A.; Todorović, S.; Simonović, A. Plant regeneration in leaf culture of Centaurium erythraea Rafn Part 3: De novo transcriptome assembly and validation of housekeeping genes for studies of in vitro morphogenesis. Plant Cell Tissue Organ Cult. 2020, 1–17. [Google Scholar] [CrossRef]
- Ćuković, K.; Dragićević, M.; Todorović, S.; Bogdanović, M.B.; Simonović, A. Selection of differentially expressed genes in Centaurium erythraea Rafn during in vitro somatic embryogenesis. In Proceedings of the X International Scientific Agriculture Symposium AgroSym 2019, Jahorina, Republika Srpska, 3–6 October 2019; p. 226. [Google Scholar]
- Paunović, D.; Bogdanović, M.; Trifunović-Momčilov, M.; Todorović, S.; Simonović, A.; Subotić, A.; Dragićević, M. Are receptor tyrosine kinases chimeric AGP’s? In Proceedings of the 3rd International Conference on Plant Biology, Belgrade, Serbia, 9–12 June 2018; p. 17. [Google Scholar]
- Bogdanović, M.; Ćuković, K.; Dragićević, M.; Simonović, A.; Todorović, S. Somatic embryogenesis of Centaurium erythraea Rafn time-lapse documentation of in vitro development. In Proceedings of the IX International Scientific Agriculture Symposium AgroSym 2018, Jahorina, Bosnia and Herzegovina, 4–7 October 2018; p. 347. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonović, A.D.; M. Trifunović-Momčilov, M.; Filipović, B.K.; Marković, M.P.; Bogdanović, M.D.; Subotić, A.R. Somatic Embryogenesis in Centaurium erythraea Rafn—Current Status and Perspectives: A Review. Plants 2021, 10, 70. https://doi.org/10.3390/plants10010070
Simonović AD, M. Trifunović-Momčilov M, Filipović BK, Marković MP, Bogdanović MD, Subotić AR. Somatic Embryogenesis in Centaurium erythraea Rafn—Current Status and Perspectives: A Review. Plants. 2021; 10(1):70. https://doi.org/10.3390/plants10010070
Chicago/Turabian StyleSimonović, Ana D., Milana M. Trifunović-Momčilov, Biljana K. Filipović, Marija P. Marković, Milica D. Bogdanović, and Angelina R. Subotić. 2021. "Somatic Embryogenesis in Centaurium erythraea Rafn—Current Status and Perspectives: A Review" Plants 10, no. 1: 70. https://doi.org/10.3390/plants10010070







