Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects
Abstract
1. Introduction
2. Results
2.1. Far-Red Gradient Experiment
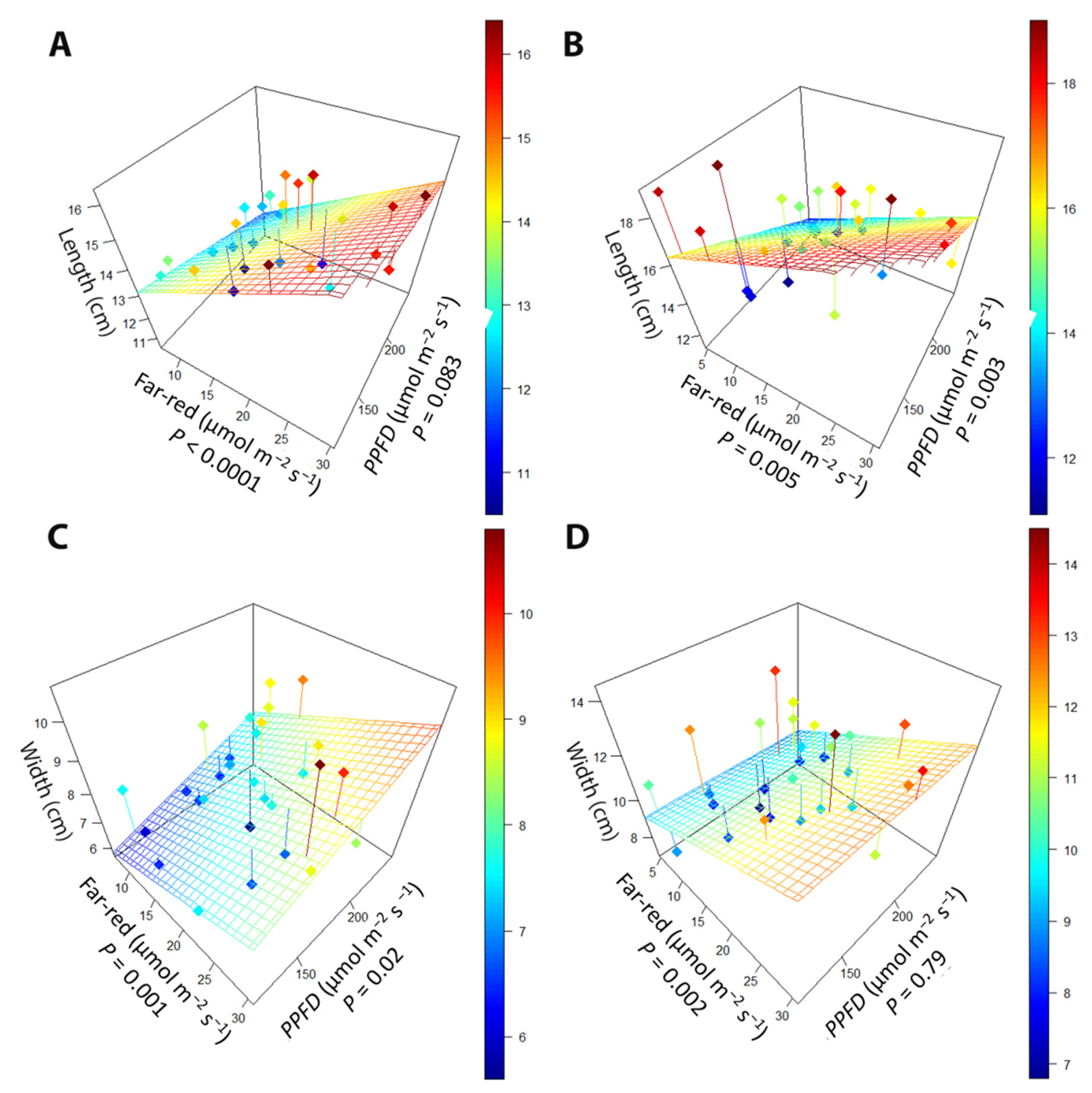
2.1.1. Leaf Morphology
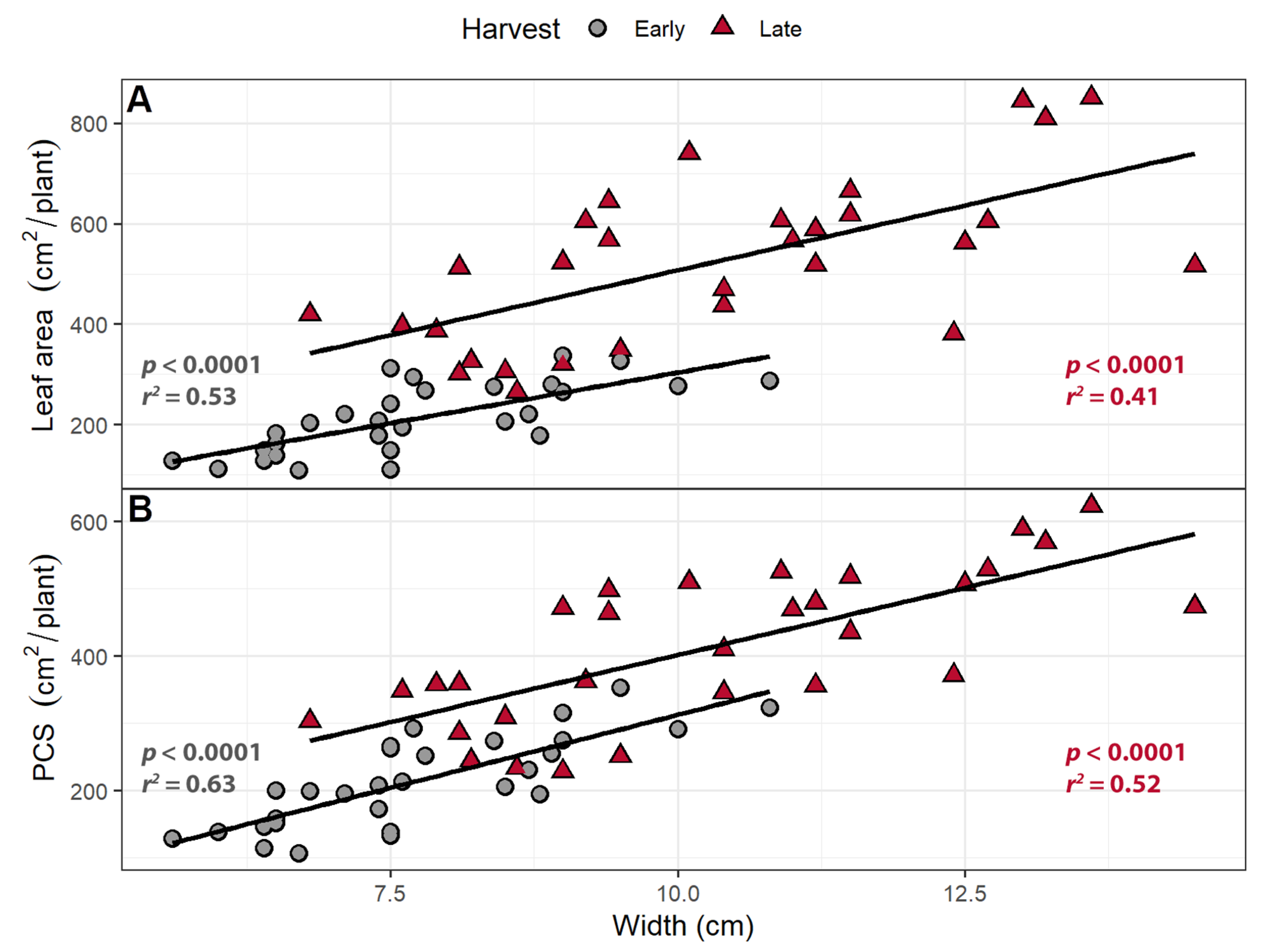
2.1.2. Canopy Traits
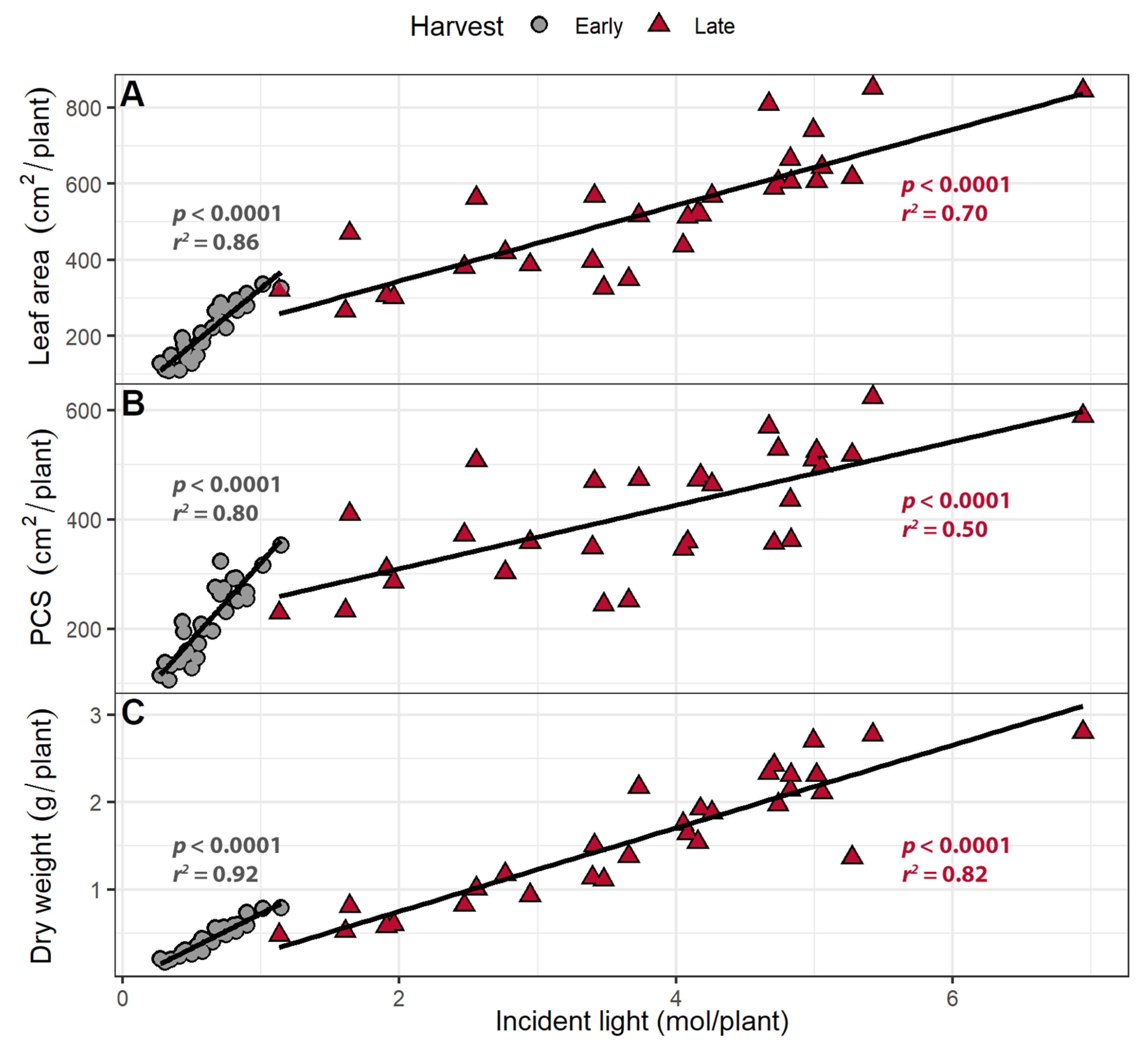
2.1.3. Incident Light
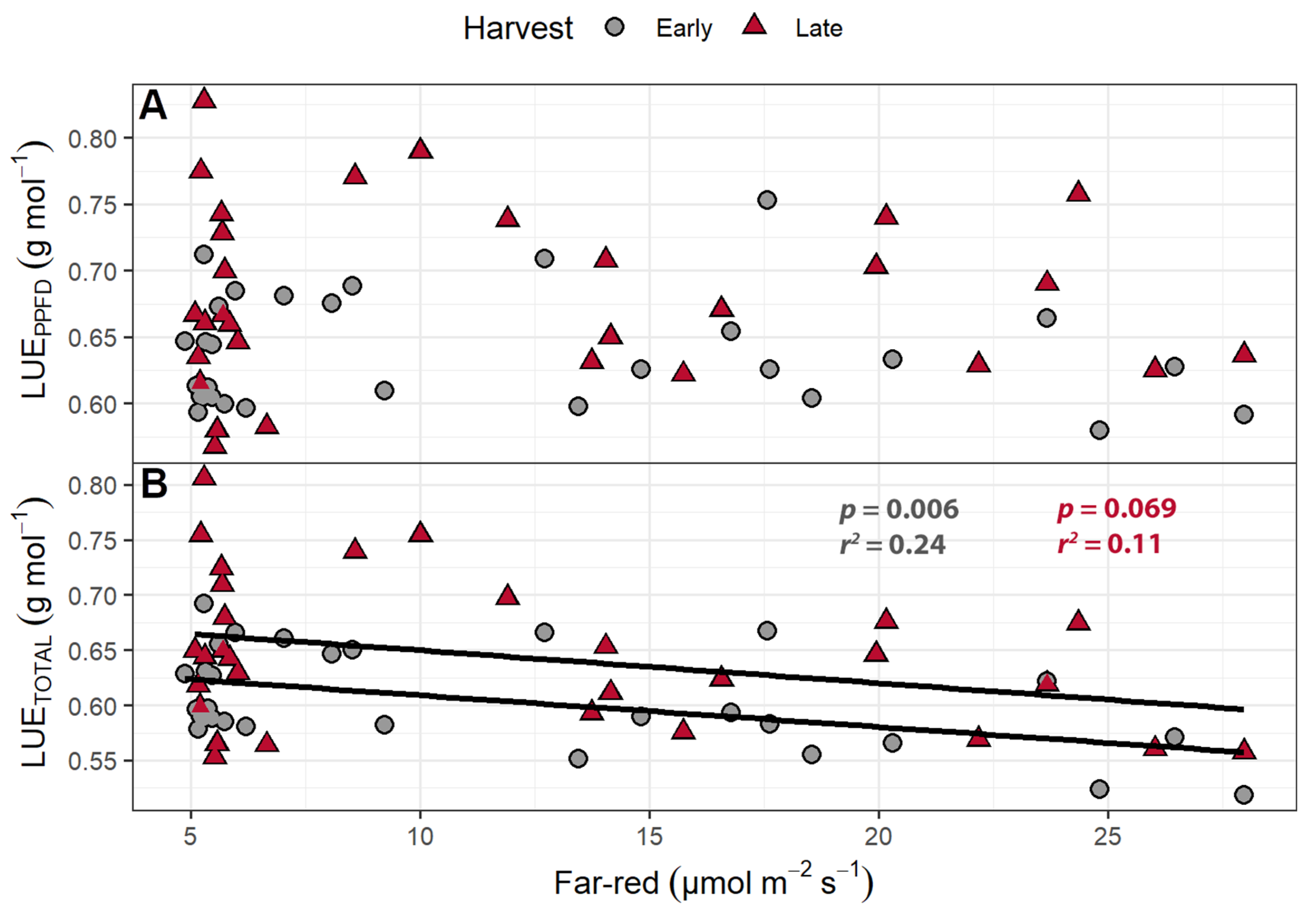
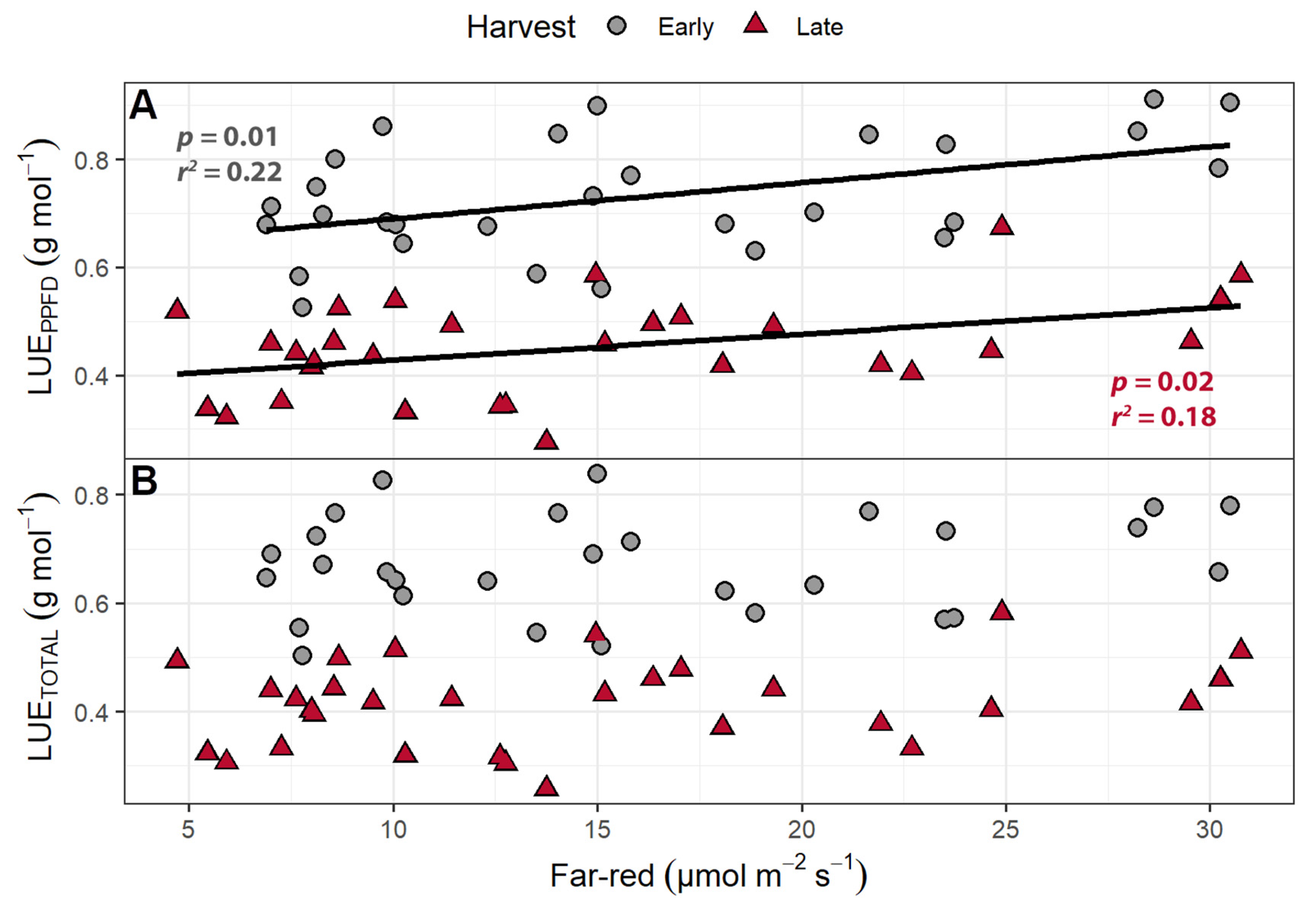
2.1.4. Light Use Efficiency
2.2. Perpendicular Light Gradient Experiment
2.2.1. Leaf Morphology
2.2.2. Canopy Traits
3. Discussion
3.1. Far-Red Light and PPFD Change Leaf Morphology
3.2. Larger Leaves Lead to Increased Canopy Size
3.3. Larger Canopies Intercept More Light
3.4. Far-Red Light Increases Light Interception and Plant Biomass More Efficiently Than PPFD
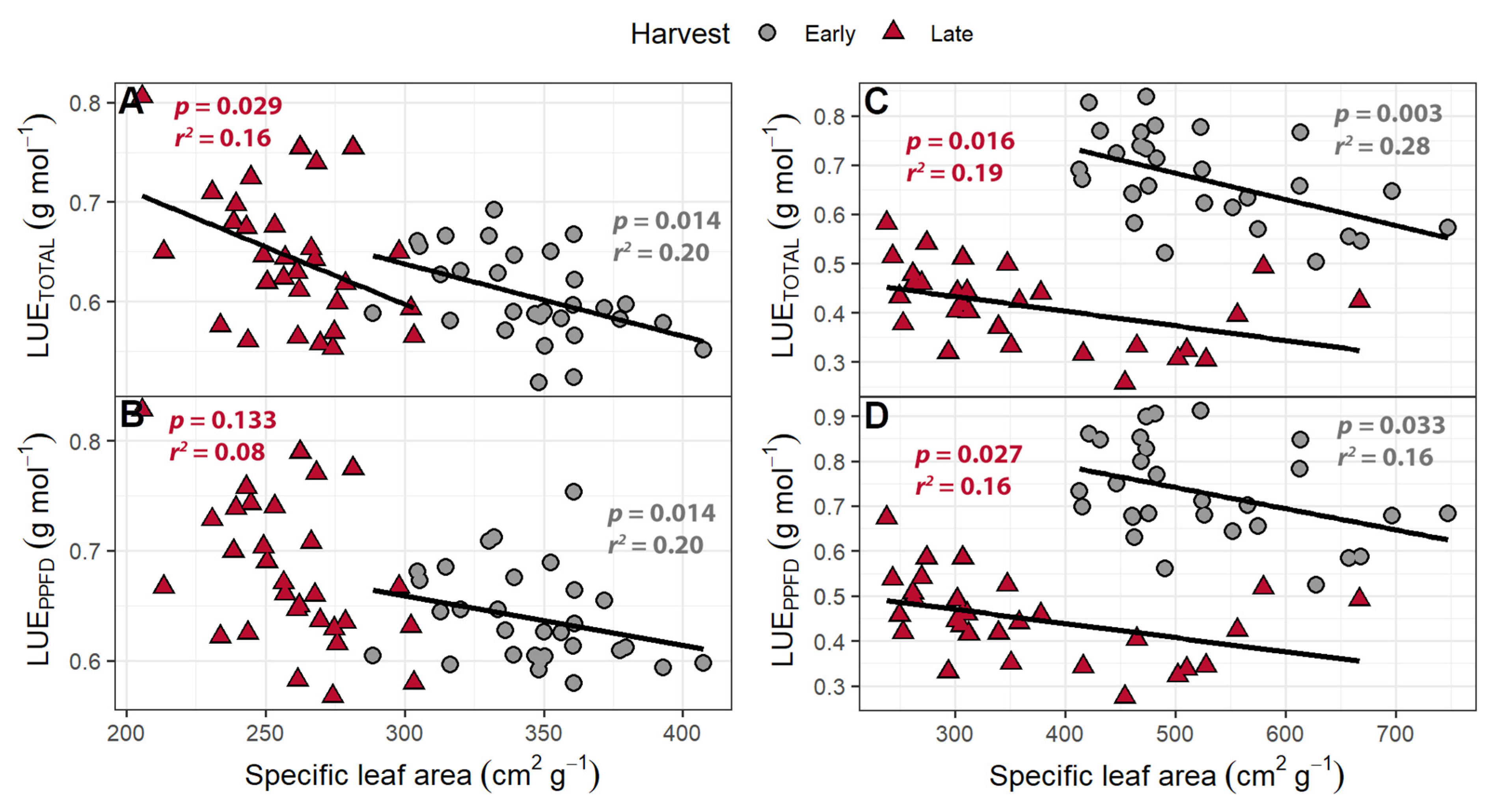
3.5. Light Use Efficiency
3.6. Implications
3.7. Conclusions
4. Materials and Methods
4.1. Growth Chamber Conditions
4.2. Plant Material
4.3. Digital Imaging and Image Analysis
4.4. Harvest
4.5. Modeling of Projected Canopy Size to Calculate Light Use Efficiency
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahbandeh, M. Forecasted Market Value of LED Grow Lights Used in Indoor Farming Worldwide from 2014 to 2021. Available online: https://www.statista.com/statistics/803331/global-led-grow-light-market-value/ (accessed on 4 June 2020).
- Schratz, M.; Gupta, C.; Struhs, T.; Gray, K. A new way to see the light: Improving light quality with cost-effective LED technology. IEEE Ind. Appl. Mag. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- van Iersel, M.W. Optimizing LED lighting in controlled environment agriculture. In Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 59–80. [Google Scholar]
- Gupta, S.D.; Agarwal, A. Artificial lighting system for plant growth and development: Chronological advancement, working principles, and comparative assessment. In Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–25. [Google Scholar]
- Liao, Y.; Suzuki, K.; Yu, W.; Zhuang, D.; Takai, Y.; Ogasawara, R.; Shimazu, T.; Fukui, H. Night-break effect of LED light with different wavelengths on shoot elongation of Chrysanthemum morifolium Ramat ‘Jimba’and ‘Iwa no hakusen’. Environ. Control Biol. 2014, 52, 51–55. [Google Scholar] [CrossRef]
- Giovanna, S.; Carabelli, M.; Possenti, M.; Morelli, G.; Ruberti, I. Multiple pathways in the control of the shade avoidance response. Plants 2018, 7, 102. [Google Scholar]
- Ballare, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Gianmaria, M.; Grassi, G.; Kotiranta, S. The effect of light spectrum on the morphology and cannabinoid content of Cannabis sativa L. Med Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar]
- Tao, H.; Vaganov, V.; Cao, S.; Li, Q.; Ling, L.; Cheng, X.; Peng, L.; Zhang, C.; Yakovlev, A.N.; Zhong, Y. Improving “color rendering” of LED lighting for the growth of lettuce. Sci. Rep. 2017, 7, 45944. [Google Scholar]
- Colquhoun, T.A.; Schwieterman, M.L.; Gilbert, J.L.; Jaworski, E.A.; Langer, K.M.; Jones, C.R.; Rushing, G.V.; Hunter, T.M.; Olmstead, J.; Clark, D.G. Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biol. Technol. 2013, 86, 37–44. [Google Scholar] [CrossRef]
- Lorraine, M. Shade-Tolerant Flowering Plants: Adaptations and Horticultural Implications; International Eucarpia Symposium, Section Ornamentals, Strategies for New Ornamentals-Part I 552: Melle, Belgium, 2001. [Google Scholar]
- Smith, H.; Whitelam, G. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- McCree, K.J. Photosynthetically active radiation. In Physiological Plant Ecology I.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 41–55. [Google Scholar]
- Emerson, R.; Chalmers, R.; Cederstrand, C. Some factors influencing the long-wave limit of photosynthesis. Proc. Natl. Acad. Sci. USA 1957, 43, 133. [Google Scholar] [CrossRef]
- Pettai, H.; Oja, V.; Freiberg, A.; Laisk, A. Photosynthetic activity of far-red light in green plants. Biochim. Biophys. Acta (BBA)-Bioenerg. 2005, 1708, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-red light enhances photochemical efficiency in a wavelength-dependent manner. Physiol. Plant. 2019, 167, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- USDA–NASS. Crop Production 2016 Summary; USDA Economics, Statistics and Market Information System. 2017. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/k3569432s/st74cs75f/nc580p978/CropProdSu-01-12-2017.pdf (accessed on 15 January 2017).
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Lee, M.; Park, S.; Oh, M. Growth and cell division of lettuce plants under various ratios of red to far-red light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 186–194. [Google Scholar] [CrossRef]
- Pinho, P.; Jokinen, K.; Halonen, L. The influence of the LED light spectrum on the growth and nutrient uptake of hydroponically grown lettuce. Lighting Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Lee, M.; Son, K.; Oh, M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E. Far red is the new red. Inside Grower 2017, 2017, 26–30. Available online: https://www.canr.msu.edu/floriculture/uploads/files/far-red-on-lettuce.pdf (accessed on 14 January 2021).
- Son, K.-H.; Jeon, Y.-M.; Oh, M.-M. Application of supplementary white and pulsed light-emitting diodes to lettuce grown in a plant factory with artificial lighting. Hortic. Environ. Biotechnol. 2016, 57, 560–572. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hort. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Klassen, S.; Ritchie, G.; Frantz, J.; Pinnock, D.; Bugbee, B. Real-time imaging of ground cover: Relationships with radiation capture, canopy photosynthesis, and daily growth rate. Digit. Imaging Spectr. Tech. Appl. Precis. Agric. Crop Physiol. 2003, 66, 3–14. [Google Scholar]
- Meng, Q.; Kelly, N.; Runkle, E. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Beall, F.D.; Yeung, E.C.; Pharis, R.P. Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Can. J. Bot. 1996, 74, 743–752. [Google Scholar] [CrossRef]
- Nakata, S.; Lockhart, J.A. Effects of red and far-red radiation on cell division and elongation in the stem of Pinto bean seedlings. Am. J. Bot. 1966, 53, 12–20. [Google Scholar] [CrossRef]
- Schmitt, J.; Wulff, R.D. Light spectral quality, phytochrome and plant competition. Trends Ecol. Evol. 1993, 8, 47–51. [Google Scholar] [CrossRef]
- Sager, J.; Smith, W.; Edwards, J.; Cyr, K. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nagatani, A. Nuclear localization activity of phytochrome B. Plant J. 1996, 10, 859–868. [Google Scholar] [CrossRef]
- Kircher, S.; Kozma-Bognar, L.; Kim, L.; Adam, E.; Harter, K.; Schäfer, E.; Nagy, F. Light quality–dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 1999, 11, 1445–1456. [Google Scholar]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Read, M.; Tibbits, T. Lettuce growth and tipburn incidence as influenced by CO2 concentration and light intensity. Hortscience 1970, 5, 297–309. [Google Scholar]
- Park, J.; Park, Y.; Jeong, B.; Hwang, S. Growth of lettuce in closed-type plant production system as affected by light intensity and photoperiod under influence of white LED light. Prot. Hortic. Plant Fact. 2013, 22, 228–233. [Google Scholar] [CrossRef]
- Kitaya, Y.; Niu, G.; Kozai, T.; Ohashi, M. Photosynthetic photon flux, photoperiod, and CO2 concentration affect growth and morphology of lettuce plug transplants. HortScience 1998, 33, 988–991. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhai, H. Evaluation of growth and quality of hydroponic lettuce at harvest as affected by the light intensity, photoperiod and light quality at seedling stage. Sci. Hortic. 2019, 248, 138–144. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Bensink, J. On morphogenesis of lettuce leaves in relation to light and temperature. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1971. [Google Scholar]
- Weaver, G.; van Iersel, M.W. Longer photoperiods with adaptive lighting control can improve growth of greenhouse-grown ‘Little Gem’ lettuce (Lactuca sativa). HortScience 2020, 55, 573–580. [Google Scholar] [CrossRef]
- Purcell, L.C. Soybean canopy coverage and light interception measurements using digital imagery. Crop Sci. 2000, 40, 834–837. [Google Scholar] [CrossRef]
- Campillo, C.; Prieto, M.; Daza, C.; Monino, M.; Garcia, M. Using digital images to characterize canopy coverage and light interception in a processing tomato crop. Hortscience 2008, 43, 1780–1786. [Google Scholar] [CrossRef]
- Cometti, N.N.; Frantz, J.; Bugbee, B. Imaging Lettuce Growth: A Comparison between %Ground Coverage and %PPF Absorption by Lettuce Grown in Hydroponics; Crop Physiology Laboratory, Utah State University: Logan, UT, USA, 2003. [Google Scholar]
- Jung, D.-H.; Park, S.H.; Han, H.; Kim, H.-J. Image processing methods for measurement of lettuce fresh weight. J. Biosyst. Eng. 2015, 40, 89–93. [Google Scholar] [CrossRef]
- Elkins, C.; van Iersel, M.W. Longer photoperiods with the same daily light integral increase daily electron transport through photosystem II in lettuce. Plants 2020, 9, 1172. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Lakso, A.N. The relationship between leaf area and light interception by spur and extension shoot leaves and apple orchard productivity. HortScience 2000, 35, 1202–1206. [Google Scholar] [CrossRef]
- Palmer, J. Canopy manipulation for optimum utilization of light. In Manipulation of Fruiting; Butterworth-Heinemann: Oxford, UK, 1989; pp. 245–262. [Google Scholar] [CrossRef]
- Wells, R. Soybean growth response to plant density: Relationships among canopy photosynthesis, leaf area, and light interception. Crop Sci. 1991, 31, 755–761. [Google Scholar] [CrossRef]
- Tei, F.; Scaife, A.; Aikman, D. Growth of lettuce, onion, and red beet. 1. Growth analysis, light interception, and radiation use efficiency. Ann. Bot. 1996, 78, 633–643. [Google Scholar] [CrossRef]
- Wurr, D.; Fellows, J.R. The influence of solar radiation and temperature on the head weight of crisp lettuce. J. Hortic. Sci. 1991, 66, 183–190. [Google Scholar] [CrossRef]
- Elkins, C.; van Iersel, M.W. Longer photoperiods with the same daily light integral improve growth of Rudbeckia seedlings in a greenhouse. HortScience 2020, 1, 1–7. [Google Scholar]
- Lee, R.J.; Bhandari, S.R.; Lee, G.; Lee, J.G. Optimization of temperature and light, and cultivar selection for the production of high-quality head lettuce in a closed-type plant factory. Hortic. Environ. Biotechnol. 2019, 60, 207–216. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-n.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Sago, Y. Effects of light intensity and growth rate on tipburn development and leaf calcium concentration in butterhead lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Wheeler, R.; Mackowiak, C.; Stutte, G.; Yorio, N.; Ruffe, L.; Sager, J.; Prince, R.; Knott, W. Crop productivities and radiation use efficiencies for bioregenerative life support. Adv. Space Res. 2008, 41, 706–713. [Google Scholar] [CrossRef]
- Holmes, M.; Smith, H. The function of phytochrome in plants growing in the natural environment. Nature 1975, 254, 512–514. [Google Scholar] [CrossRef]
- Kasperbauer, M. Spectral distribution of light in a tobacco canopy and effects of end-of-day light quality on growth and development. Plant Physiol. 1971, 47, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Kasperbauer, M.J. Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol. 1987, 85, 350–354. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Vogelmann, T.C. Do changes in light direction affect absorption profiles in leaves? Funct. Plant Biol. 2010, 37, 403–412. [Google Scholar] [CrossRef]
- Nageswara Rao, R.; Talwar, H.; Wright, G. Rapid assessment of specific leaf area and leaf nitrogen in peanut (Arachis hypogaea L.) using a chlorophyll meter. J. Agron. Crop Sci. 2001, 186, 175–182. [Google Scholar] [CrossRef]
- Sinclair, T.; Shiraiwa, T. Soybean radiation-use efficiency as influenced by nonuniform specific leaf nitrogen distribution and diffuse radiation. Crop Sci. 1993, 33, 808–812. [Google Scholar] [CrossRef]
- Bange, M.; Hammer, G.; Rickert, K. Effect of specific leaf nitrogen on radiation use efficiency and growth of sunflower. Crop Sci. 1997, 37, 1201–1208. [Google Scholar] [CrossRef]
- Sinclair, T.; Bennett, J.; Boote, K. Leaf nitrogen content, photosynthesis and radiation use efficiency in peanut. Peanut Sci. 1993, 20, 40–43. [Google Scholar] [CrossRef]
- Muchow, R.; Sinclair, T. Nitrogen response of leaf photosynthesis and canopy radiation use efficiency in field-grown maize and sorghum. Crop Sci. 1994, 34, 721–727. [Google Scholar] [CrossRef]
- Wang, H.; Deng, X.W. Phytochrome signaling mechanism. Arab. Book/Am. Soc. Plant Biol. 2004, 3. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Consortium, D. Available online: https://www.designlights.org/horticultural-lighting/technical-requirements/ (accessed on 15 October 2020).
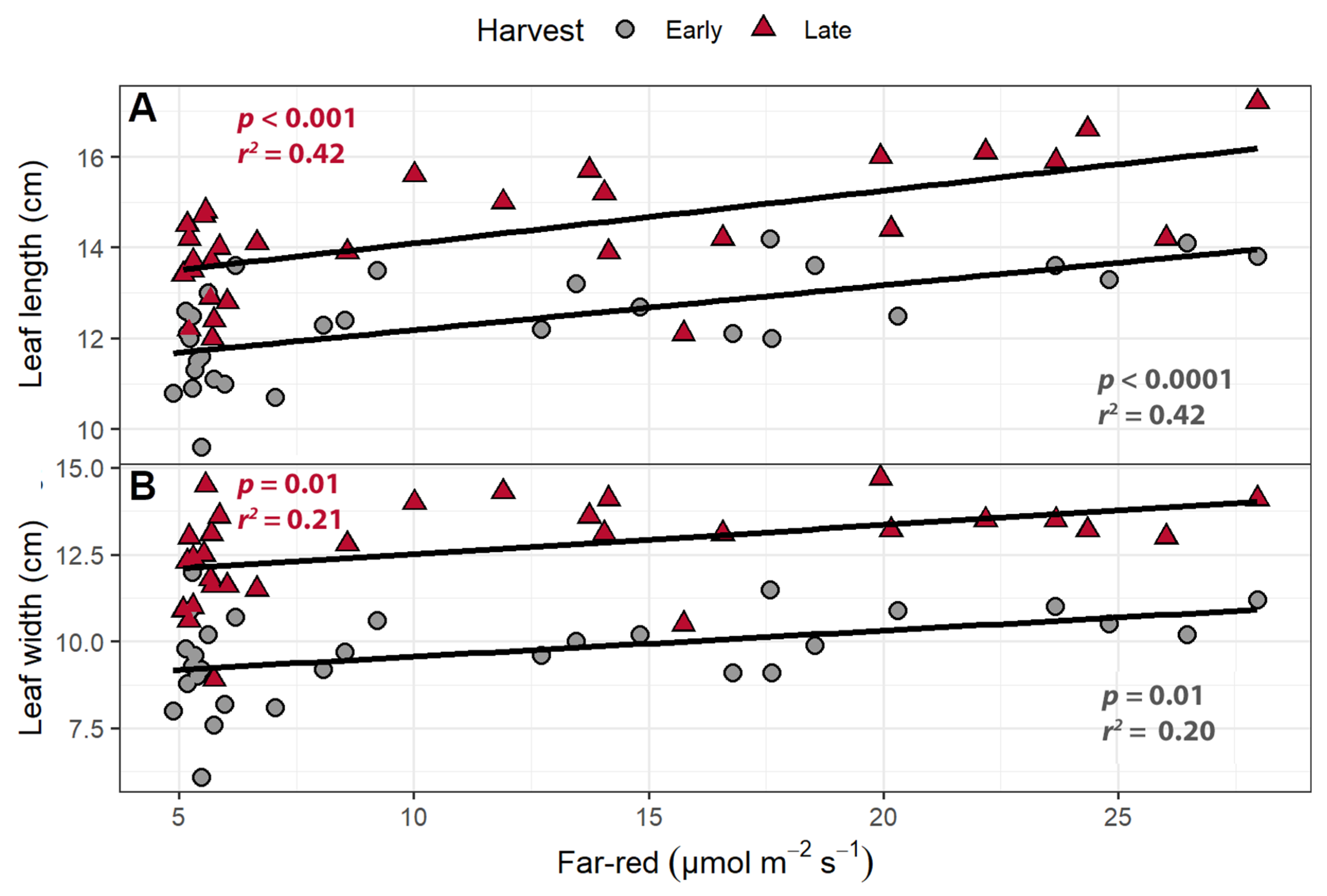
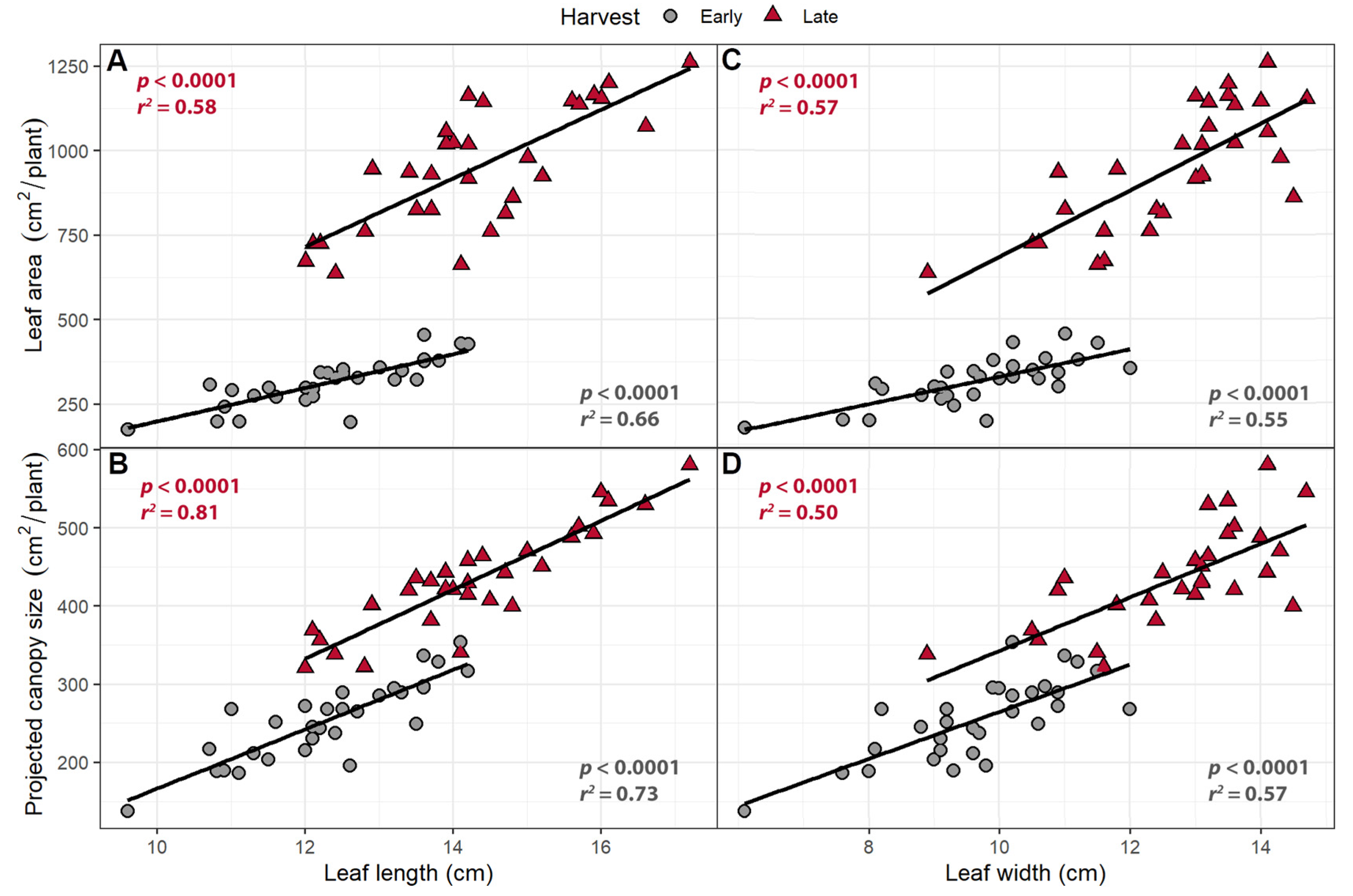
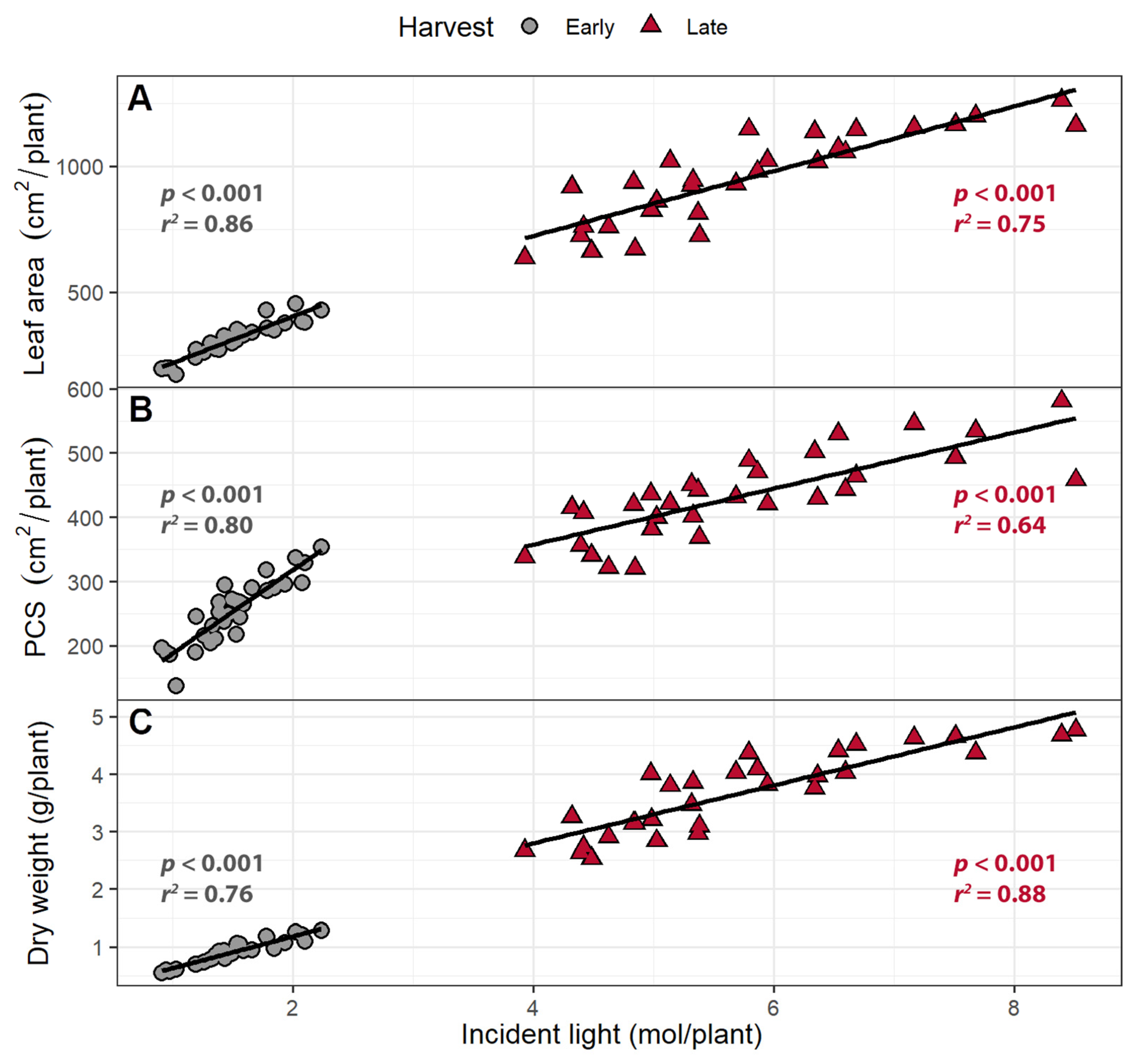
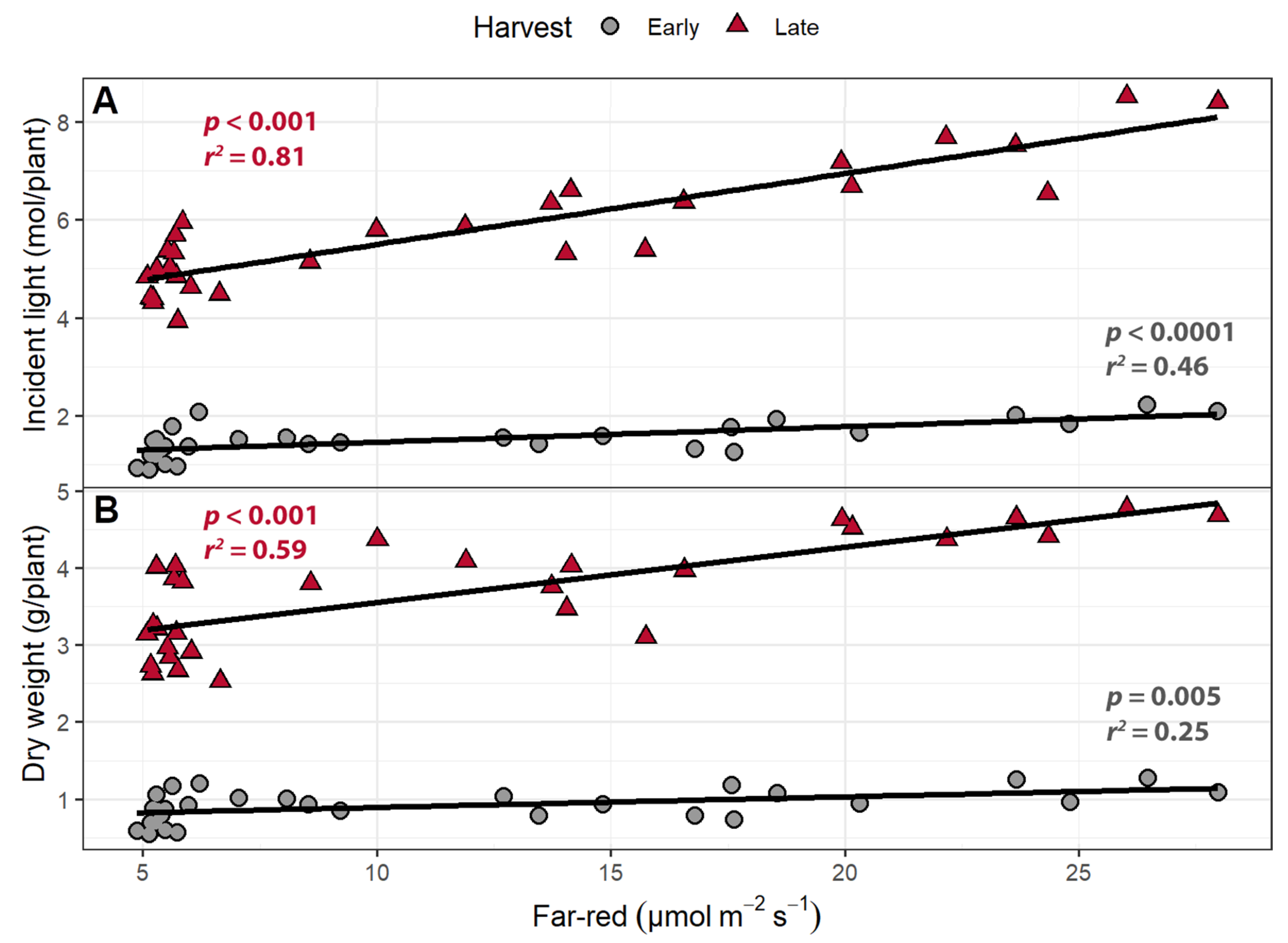

| Trait | Harvest | Intercept | FR | PPFD | R2 |
|---|---|---|---|---|---|
| Dry weight | Early | –0.49 | 0.00792 | 0.00414 | 0.53 |
| Dry weight | Late | –1.28 | 0.04276 | 0.01183 | 0.56 |
| Incident light | Early | –0.50 | 0.00815 | 0.00520 | 0.58 |
| Incident light | Late | –2.72 | 0.07946 | 0.02807 | 0.74 |
| Length | Early | 13.68 | 0.1478 | –0.0126 | 0.57 |
| Length | Late | 19.40 | 0.1305 | –0.0315 | 0.42 |
| Width | Early | 3.276 | 0.0966 | 0.0152 | 0.37 |
| Width | Late | 8.774 | 0.1385 | –0.0025 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legendre, R.; van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. https://doi.org/10.3390/plants10010166
Legendre R, van Iersel MW. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants. 2021; 10(1):166. https://doi.org/10.3390/plants10010166
Chicago/Turabian StyleLegendre, Reeve, and Marc W. van Iersel. 2021. "Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects" Plants 10, no. 1: 166. https://doi.org/10.3390/plants10010166
APA StyleLegendre, R., & van Iersel, M. W. (2021). Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants, 10(1), 166. https://doi.org/10.3390/plants10010166






