Light Quality Affected the Growth and Root Organic Carbon and Autotoxin Secretions of Hydroponic Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
2.2. Light Treatment
2.3. Sampling and Measurement Methods
2.3.1. Net Photosynthetic Rate
2.3.2. Root Morphology
2.3.3. Root Activity
2.3.4. Collection and Determination of Total Organic Carbon
2.3.5. Collection and Determination of Autotoxins in Deionized Water
2.4. Statistic Analysis
3. Results
3.1. Plant Morphology and Growth Characteristics
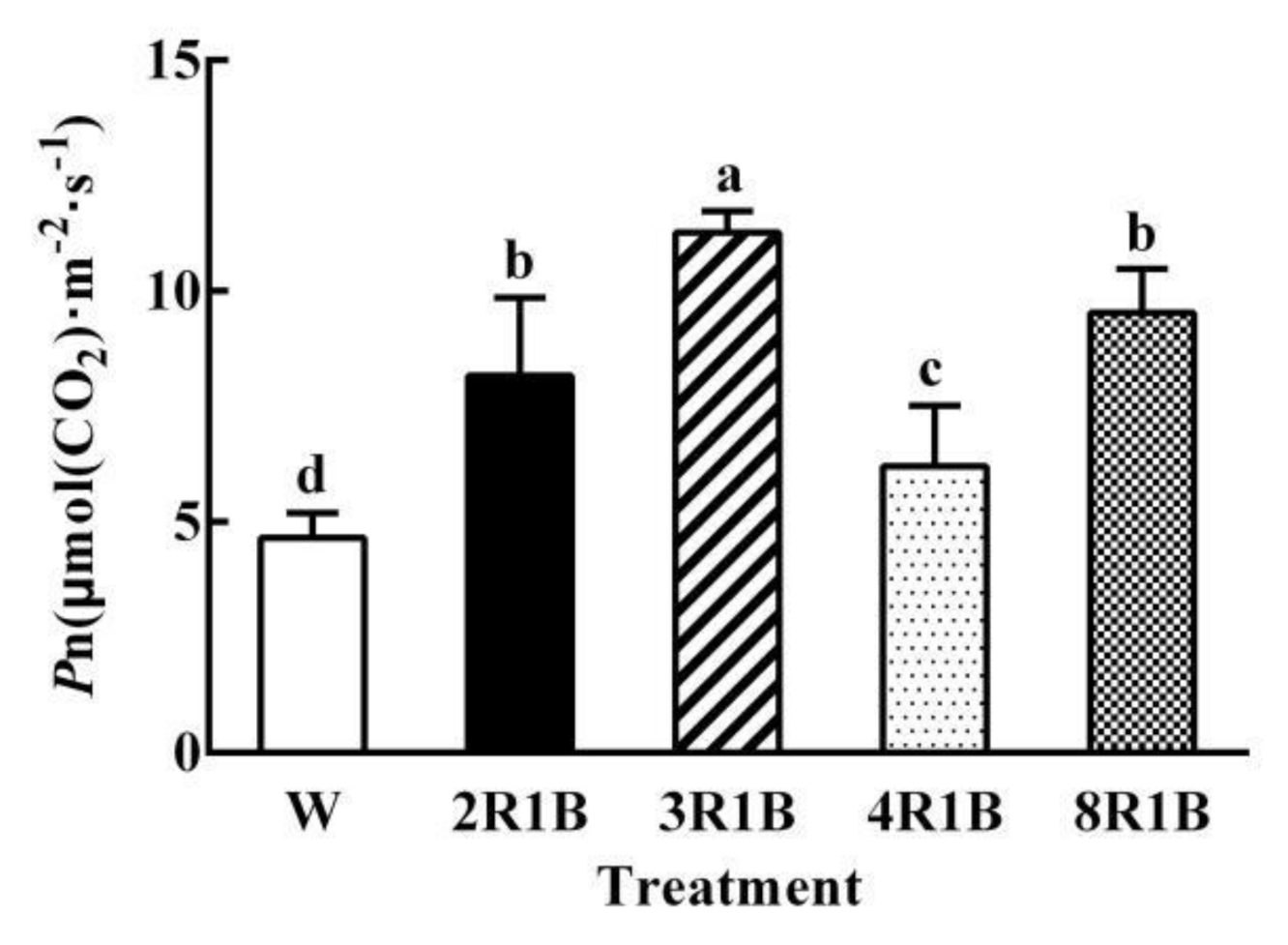
3.2. Leaf Photosynthesis
3.3. Root Morphology
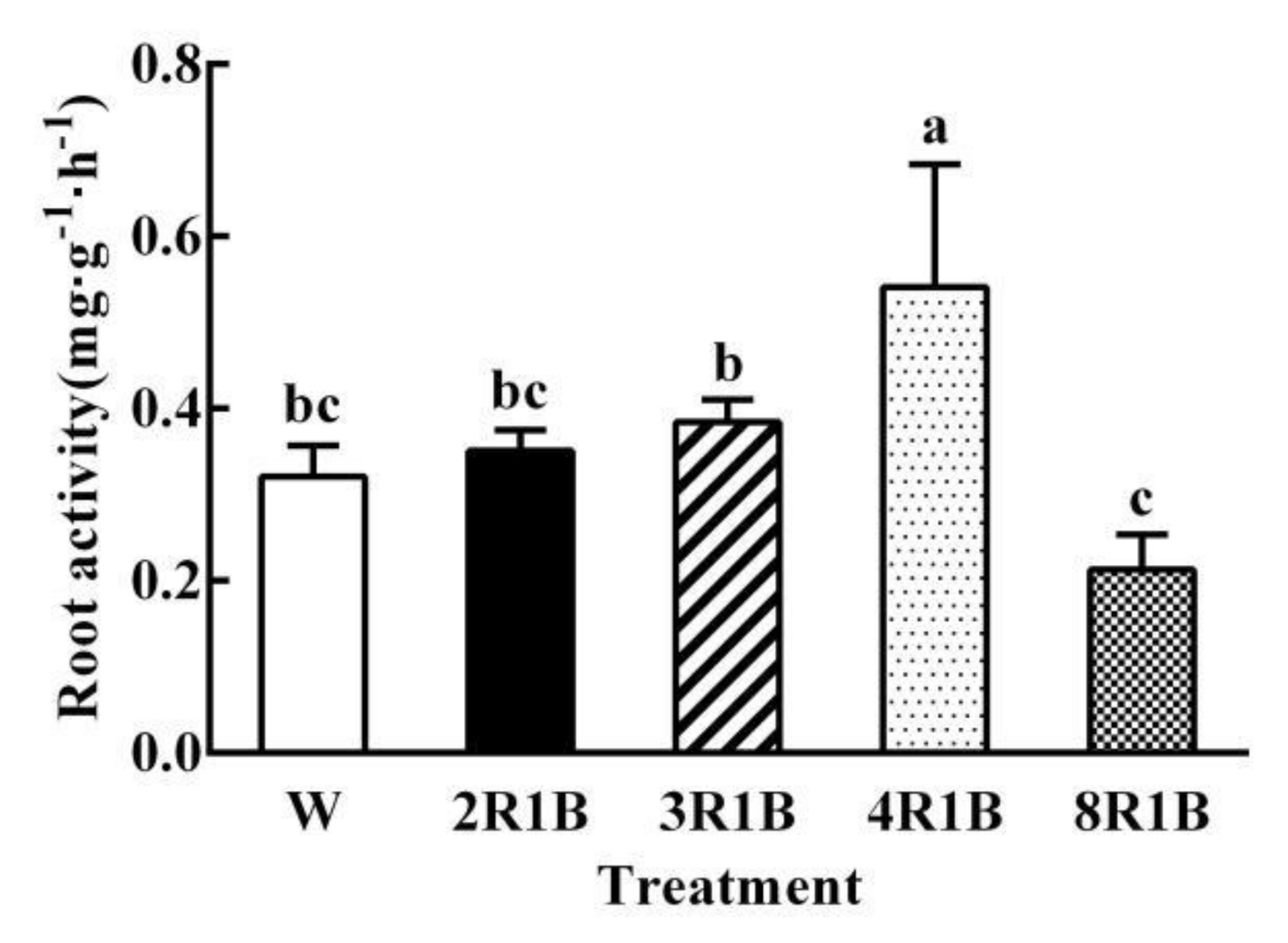
3.4. Root Activity
3.5. Organic Carbon Content
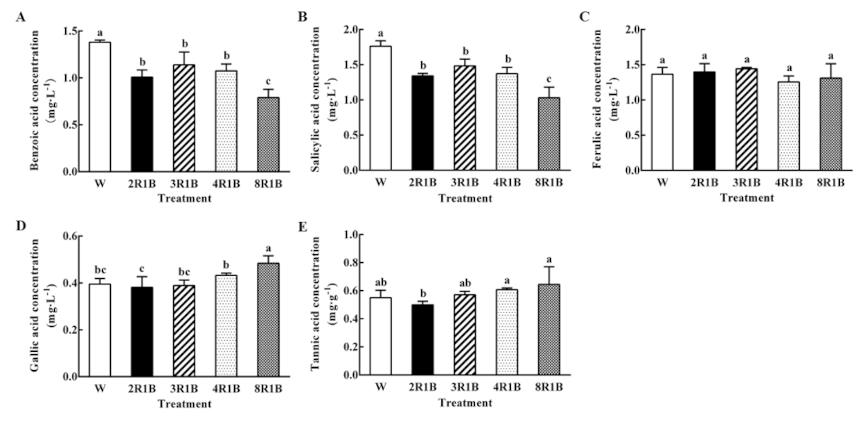
3.6. Autotoxin Contents
3.6.1. Effect of Light Quality on Five Autotoxins Concentration in Root Exudates of Hydroponic Lettuce
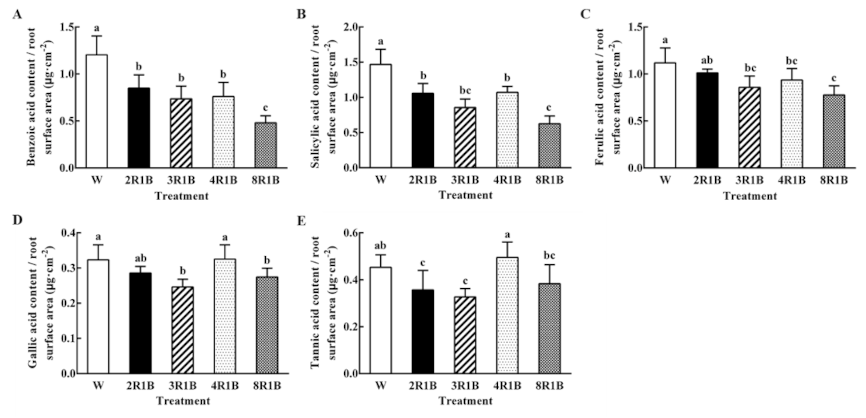
3.6.2. Autotoxin Content Based on Root Surface Area
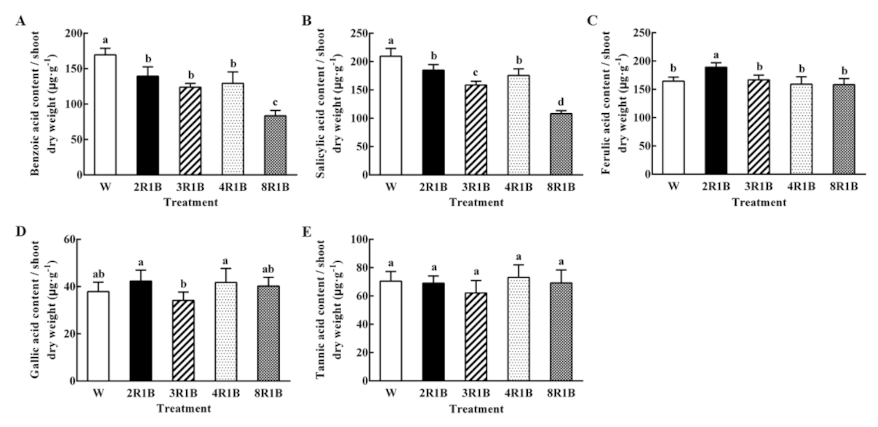
3.6.3. Autotoxin Content Based on Shoot Dry Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. JPN Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Verheust, Y.; Bonarrigo, G.; Van Hulle, S. Closed hydroponic systems: Operational parameters, root exudates occurrence and related water treatment. Rev. Environ. Sci. Biotechnol. 2017, 16, 59–79. [Google Scholar] [CrossRef]
- Yu, J.Q.; Matsui, Y. Phytotoxic substances in root exudates of cucumber (Cucumis sativus L.). J. Chem. Ecol. 1994, 20, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Ye, S.F.; Zhang, M.F.; Hu, W.H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 2003, 31, 129–139. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Dai, C. Advance in mechanism and countermeasures of peanut succession monocropping obstacles. Soils 2010, 42, 505–512. (In Chinese) [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Hao, W.; Ren, L.; Ran, W.; Shen, Q. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, B.Y.; Lee, H.J. Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce (Lactuca sativa L.). Sci. Hortic. 2006, 110, 119–128. [Google Scholar] [CrossRef]
- Sunada, K.; Ding, X.G.; Utami, M.S.; Kawashima, Y.; Miyama, Y.; Hashimoto, K. Detoxification of phytotoxic compounds by TiO2 photocatalysis in a recycling hydroponic cultivation system of asparagus. J. Agr. Food Chem. 2008, 56, 4819–4824. [Google Scholar] [CrossRef]
- Qiu, Z.; Yang, Q.; Liu, W. Photocatalytic degradation of phytotoxic substances in waste nutrient solution by various immobilized levels of nano-TiO2. Water Air Soil Poll. 2013, 224, 1461. [Google Scholar] [CrossRef]
- Qiu, Z.; Yang, Q.; Liu, W. Effect of nano-TiO2 photocatalysis on yield and quality of hydroponic lettuce. Acta Agric. Boreali Sin. 2013, 28, 103–107. (In Chinese) [Google Scholar]
- Talukder, M.R.; Asaduzzaman, M.; Tanaka, H.; Asao, T. Electro-degradation of culture solution improves growth, yield and quality of strawberry plants grown in closed hydroponics. Sci. Hortic. 2019, 243, 243–251. [Google Scholar] [CrossRef]
- Liu, W.K.; Yang, Q.C.; Wei, L.L. Light-Emitting Diodes (LEDs) and Their Applications in Protected Horticulture as Light Sources; China Agricultural Science and Technology Press: Beijing, China, 2012. [Google Scholar]
- Cao, G.; Zhang, G.; Yu, J.; Ma, Y. Effects of different LED light qualities on cucumber seedling growth and chlorophyll fluorescence parameters. Sci. Agric. Sin. 2013, 46, 1297–1304. (In Chinese) [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tiss. Org. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Li, S.S.; Pan, R.C. Effects of blue light on the growth of rice seedlings. Chin. J. Rice Sci. 1994, 8, 115–118. (In Chinese) [Google Scholar] [CrossRef]
- Urbonaviciute, A.; Pinho, P.; Samuoliene, G.; Duchovskis, P.; Vitta, P.; Stonkus, A.; Tamulaitis, G.; Zukauskas, A.; Halonen, L. Effect of short-wavelength light on lettuce growth and nutritional quality. Sodininkystė Ir Daržininkystė 2007, 26, 157–165. [Google Scholar]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue light dose-esponses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef]
- Yan, X.H.; Yu, J.H.; Xie, J.M. Effects of supplemental light quality and durations of illumination on growth and root activity of cucumber seedling. J. Nucl. Agric. Sci. 2016, 30, 1211–1217. (In Chinese) [Google Scholar] [CrossRef]
- Yang, J.; Pan, T.; Wang, J.; Bao, E.; Zou, Z. Effects of different light quality on tomato seedlings growth and root. North. Hortic. 2018, 42, 1–6. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, C.B.; Liu, W.K.; Zha, L.Y.; Li, Z.G.; Zhang, Y.B.; Shao, M.J.; Li, B.S. Effects of red and blue LED light intensities on the growth of hydroponic lettuce and secretions of organic carbon and autotoxins. Plant Physiol. J. 2019, 55, 466–474. (In Chinese) [Google Scholar]
- Zha, L.Y.; Liu, W.K. Effect of continuous light with red and blue LED lamps on growth and characteristics of photosynthesis and chlorophyll fluorescence of five lettuce cultivars. Plant Physiol. J. 2017, 53, 1735–1741. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Zhang, Y.; Liu, W. Dynamic responses of ascorbate pool and metabolism in lettuce to long-term continuous light provided by red and blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Pramanik, M.H.R.; Nagai, M.; Asao, T.; Matsui, Y. Effects of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J. Chem. Ecol. 2000, 26, 1953–1967. [Google Scholar] [CrossRef]
- Renella, G.; Egamberdiyeva, D.; Landi, L.; Mench, M.; Nannipieri, P. Microbial activity and hydrolase activities during decomposition of root exudates released by an artificial root surface in Cd-contaminated soils. Soil Biol. Biochem. 2006, 38, 702–708. [Google Scholar] [CrossRef]
- Meier, S.; Alvear, M.; Borie, F.; Aguilera, P.; Ginocchio, R.; Cornejo, P. Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotox. Environ. Saf. 2012, 75, 8–15. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant Soil Environ. 2013, 59, 80–88. [Google Scholar] [CrossRef]
- Duan, K.X. Experiments of Instrument Analysis; Chemical Industry Press: Beijing, China, 2009; pp. 26–28. (In Chinese) [Google Scholar]
- Fu, Y.R.; Zhang, W.M.; Chen, G.M.; Bai, X.M. Determination of gallic acid and total phenolic acid in Sedum Aizoon L. Chin. Tradit. Pat. Med. 2006, 28, 1016–1018. (In Chinese) [Google Scholar]
- Jiang, N.X.; Liu, Z.M.; Ren, H.Q.; Han, S.Y.; Zhu, C.Y. Changes of phenolic acids and total phenolic acids contents in cell walls of moso bamboos at different ages. J. Bamboo Res. 2010, 29, 24–28. (In Chinese) [Google Scholar]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biote. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate, a Quantitative Approach to Environmental Plant Physiology, 3rd ed.; University Press UK: Cambridge, UK, 2014. [Google Scholar]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Kim, H.H. Stomatal conductance of lettuce grown under or exposed to different light qualities. Ann. Bot. 2004, 94, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.H.; Cheng, R.F.; Yang, Q.C.; Wang, J.; Lu, C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Douwstra, P.; Trouwborst, G.; Van Ieperen, W.; Harbinson, J. An artificial solar spectrum substantially alters plant development compared with usual climate room irradiance spectra. J. Exp. Bot. 2010, 61, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Child, R.; Morgan, D.C.; Smith, H. Control of development in Chenopodium album L. by shadelight: The effect of light quality (red:far-red ratio) on morphogenesis. New Phytol. 1981, 89, 545–555. [Google Scholar] [CrossRef]
- Morgan, D.C.; Smith, H. Control of development in Chenopodium album L. by shadelight: The effect of light quantity (total fluence rate) and light quality (red:far-red ratio). New Phytol. 1981, 88, 239–248. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Liu, Q.; Cheng, B.; Feng, L.; Liu, J. Effects of different light qualities on growth and photosynthetic characteristics of pakchoi. North. Hortic. 2013, 22, 1–4. (In Chinese) [Google Scholar]
- Zhou, C.B.; Zhang, X.; Cui, Q.Q.; Li, M.; Zhang, W.D.; Ai, X.Z.; Bi, H.G.; Liu, B.B.; Li, Q.M. Effects of supplementary light quality on growth and photosynthesis of pakchoi (Brassica campestris). Plant Physiol. J. 2017, 53, 1030–1038. (In Chinese) [Google Scholar] [CrossRef]
- Satbhai, S.B.; Ristova, D.; Busch, W. Underground tuning: Quantitative regulation of root growth. J. Exp. Bot. 2015, 66, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.W.; Lv, J.; Wu, H.; Gou, C.; Xu, H.W.; Zhou, X.F. Research advances on plant roots. North. Hortic. 2012, 18, 206–209. (In Chinese) [Google Scholar]
- Liu, X.Y.; Xu, Z.G.; Chang, T.T.; Guo, S.R. Growth and photosynthesis of cherry tomato seedling exposed to different low light of led light quality. Acta Bot. Boreali. Occident. Sin. 2010, 30, 645–651. (In Chinese) [Google Scholar]
- Wu, F.Z.; Wang, W. Research on soil microflora of greenhouse tomato. North. Hortic. 1999, 3, 1–2. (In Chinese) [Google Scholar]
- Kil, B.S.; Lee, S.Y. Allelopathic effects of Chrysanthemum morifolium on germination and growth of several herbaceous plants. J. Chem. Ecol. 1987, 13, 299–308. [Google Scholar] [CrossRef]
- Yu, J.Q.; Lee, K.S.; Matsui, Y. Effect of the addition of activated charcoal to the nutrient solution on the growth of tomato in hydroponic culture. Soil Sci. Plant Nutr. 1993, 39, 13–22. [Google Scholar] [CrossRef][Green Version]
- Gallet, C. Allelopathic potential in bilberry-spruce forests: Influence of phenolic compounds on spruce seedlings. J. Chem. Ecol. 1994, 20, 1009–1024. [Google Scholar] [CrossRef]
- Li, F.M.; Hu, H.Y. Isolation and characterization of a novel antialgal allelochemical from Phragmites communis. Appl. Environ. Microb. 2005, 71, 6545–6553. [Google Scholar] [CrossRef]
- Yang, M.; Chuan, Y.; Guo, C.; Liao, J.; Xu, Y.; Mei, X.; Liu, Y.; Huang, H.; He, X.; Zhu, S. Panax notoginseng root cell death caused by the autotoxic ginsenoside Rg1 is due to over-accumulation of ROS, as revealed by transcriptomic and cellular approaches. Front. Plant Sci. 2018, 9, 264. [Google Scholar] [CrossRef]
- Bu, R.; Wang, R.; Wei, Q.; Hu, H.; Sun, H.; Song, P.; Yu, Y.; Liu, Q.; Zheng, Z.; Li, T.; et al. Silencing of glycerol-3-phosphate acyltransferase 6 (GPAT6) gene using a newly established virus induced gene silencing (VIGS) system in cucumber alleviates autotoxicity mimicked by cinnamic acid (CA). Plant Soil 2019, 438, 329–346. [Google Scholar] [CrossRef]
- Fankhauser, C.; Batschauer, A. Shadow on the plant: A strategy to exit. Cell 2016, 164, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Karayekov, E.; Galvão, V.C.; Ren, H.; Casal, J.J.; Fankhauser, C. Shade promotes phototropism through phytochrome B-controlled auxin production. Curr. Biol. 2016, 26, 3280–3287. [Google Scholar] [CrossRef]
- De Wit, M.; Keuskamp, D.H.; Bongers, F.J.; Hornitschek, P.; Gommers, C.M.; Reinen, E.; Martínez-Cerón, C.; Fankhauser, C.; Pierik, R. Integration of phytochrome and cryptochrome signals determines plant growth during competition for light. Curr. Biol. 2016, 26, 3320–3326. [Google Scholar] [CrossRef] [PubMed]
- Pomilio, A.B.; Leicach, S.R.; Grass, M.Y.; Ghersa, C.M.; Santoro, M.; Vitale, A.A. Constituents of the root exudate of Avena fatua grown under far-infrared-enriched light. Phytochem. Anal. 2000, 11, 304–308. [Google Scholar] [CrossRef]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.; Taulavuori, E.; Julkunen-Tiitto, R. Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Bian, Z.; Cheng, R.; Wang, Y.; Yang, Q.; Lu, C. Effect of green light on nitrate reduction and edible quality of hydroponically grown lettuce (Lactuca sativa L.) under short-term continuous light from red and blue light-emitting diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Taulavuori, K.; Julkunen-Tiitto, R.; Hyöky, V.; Taulavuori, E. Blue mood for superfood. Nat. Prod. Commun. 2013, 8, 791–794. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; Noga, G.; Hunsche, M. High blue light improves acclimation and photosynthetic recovery of pepper plants exposed to UV stress. Environ. Exp. Bot. 2014, 109, 254–263. [Google Scholar] [CrossRef]
- Suzuki, A.; Suriyagoda, L.; Shigeyama, T.; Tominaga, A.; Sasaki, M.; Hiratsuka, Y.; Yoshinaga, A.; Arima, S.; Agarie, S.; Sakai, T.; et al. Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 16837–16842. [Google Scholar] [CrossRef] [PubMed]
- Gundel, P.E.; Pierik, R.; Mommer, L.; Ballaré, C.L. Competing neighbors: Light perception and root function. Oecologia 2014, 176, 1–10. [Google Scholar] [CrossRef]
- Pierik, R.; Mommer, L.; Voesenek, L.A. Molecular mechanisms of plant competition: Neighbour detection and response strategies. Funct. Ecol. 2013, 27, 841–853. [Google Scholar] [CrossRef]
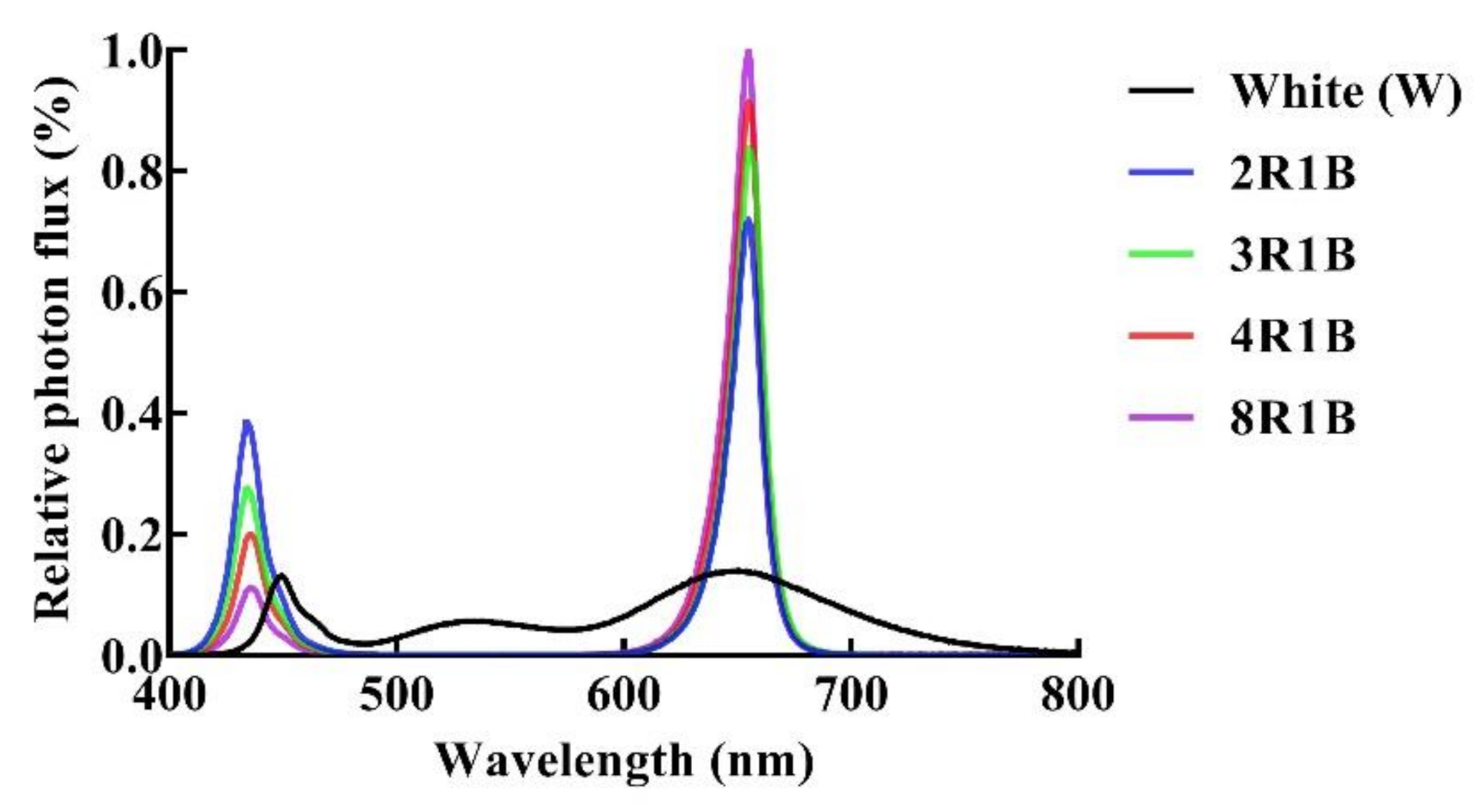

| Treatments | PFD | Blue (400–500 nm) | Green (500–600 nm) | Red (600–700 nm) | Far-Red (700–800 nm) |
|---|---|---|---|---|---|
| White (W) | 200 | 31.6 (15.8%) | 43.9 (22.0%) | 100.6 (50.3%) | 23.5 (11.8%) |
| 2R1B | 66.7 (33.3%) | - | 133.3 (66.7%) | - | |
| 3R1B | 50.0 (25.0%) | - | 150.0 (75.0%) | - | |
| 4R1B | 40.0 (20.0%) | - | 160.0 (80.0%) | - | |
| 8R1B | 22.2 (11.1%) | - | 177.8 (88.9%) | - |
| Treatments | Leaf Area (cm2) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|
| W | 287.8 ± 39.6 a | 14.5 ± 2.49 a | 2.36 ± 0.75 ab | 0.80 ± 0.11 a | 0.124 ± 0.03 a |
| 2R1B | 182.9 ± 24.0 c | 9.1 ± 1.25 c | 1.89 ± 0.42 ab | 0.59 ± 0.06 b | 0.093 ± 0.02 a |
| 3R1B | 200.8 ± 23.7b c | 10.0 ± 1.07 bc | 1.93 ± 0.75 ab | 0.58 ± 0.03 b | 0.094 ± 0.03 a |
| 4R1B | 184.5 ± 25.3 c | 9.2 ± 1.71 c | 1.75 ± 0.20 b | 0.53 ± 0.09 b | 0.093 ± 0.01 a |
| 8R1B | 235.6 ± 45.9 b | 12.4 ± 2.31 ab | 2.71 ± 0.44 a | 0.68 ± 0.11 ab | 0.125 ± 0.02 a |
| Treatments | Total Root Length (cm·plant−1) | Root Surface Area (cm2·plant−1) | Root Volume (cm3·plant−1) |
|---|---|---|---|
| W | 2578.0 ± 451.8 b | 220.6 ± 31.8 b | 1.47 ± 0.11 b |
| 2R1B | 2098.2 ± 54.3 bc | 169.6 ± 5.5 bc | 1.03 ± 0.09 c |
| 3R1B | 2240.9 ± 705.2 bc | 180.2 ± 52.2 bc | 1.16 ± 0.30 c |
| 4R1B | 1616.6 ± 151.5 c | 152.2 ± 1.7 c | 1.11 ± 0.15 c |
| 8R1B | 3345.9 ± 249.8 a | 283.6 ± 3.3 a | 2.00 ± 0.07 a |
| Treatments | TOC Content (mg) | TOC Content/Shoot Dry Weight (mg·g−1) | TOC Content/Root Dry Weight (mg·g−1) | TOC Content/Root Surface Area (mg·cm−2) |
|---|---|---|---|---|
| W | 27.49 ± 0.5 4b | 39.58 ± 2.56 c | 265.2 ± 60.5b | 0.157 ± 0.05 bc |
| 2R1B | 33.12 ± 0.99 ab | 53.57 ± 1.19 bc | 303.9 ± 63.6b | 0.193 ± 0.01 b |
| 3R1B | 37.45 ± 2.09 ab | 63.44 ± 2.48 b | 337.8 ± 26.8b | 0.150 ± 0.01 bc |
| 4R1B | 45.19 ± 6.73 a | 92.03 ± 9.34 a | 505.6 ± 80.9a | 0.335 ± 0.02 a |
| 8R1B | 29.53 ± 11.70 b | 62.01 ± 11.25 b | 279.1 ± 98.8b | 0.122 ± 0.04 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhang, Y.; Liu, W.; Zha, L.; Shao, M.; Li, B. Light Quality Affected the Growth and Root Organic Carbon and Autotoxin Secretions of Hydroponic Lettuce. Plants 2020, 9, 1542. https://doi.org/10.3390/plants9111542
Zhou C, Zhang Y, Liu W, Zha L, Shao M, Li B. Light Quality Affected the Growth and Root Organic Carbon and Autotoxin Secretions of Hydroponic Lettuce. Plants. 2020; 9(11):1542. https://doi.org/10.3390/plants9111542
Chicago/Turabian StyleZhou, Chengbo, Yubin Zhang, Wenke Liu, Lingyan Zha, Mingjie Shao, and Baoshi Li. 2020. "Light Quality Affected the Growth and Root Organic Carbon and Autotoxin Secretions of Hydroponic Lettuce" Plants 9, no. 11: 1542. https://doi.org/10.3390/plants9111542
APA StyleZhou, C., Zhang, Y., Liu, W., Zha, L., Shao, M., & Li, B. (2020). Light Quality Affected the Growth and Root Organic Carbon and Autotoxin Secretions of Hydroponic Lettuce. Plants, 9(11), 1542. https://doi.org/10.3390/plants9111542





