Anti-Aging Potential of Extracts from Washingtonia filifera Seeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Enzyme Inhibition
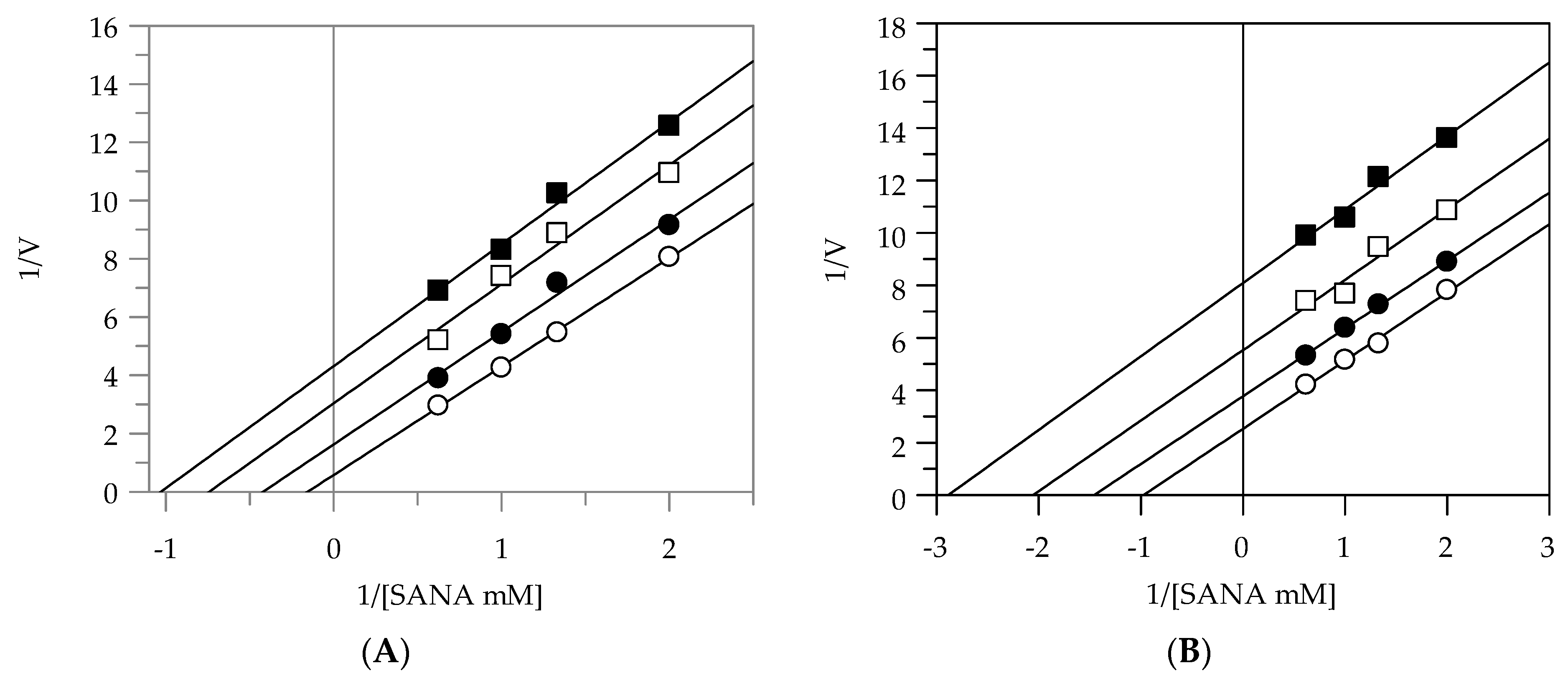
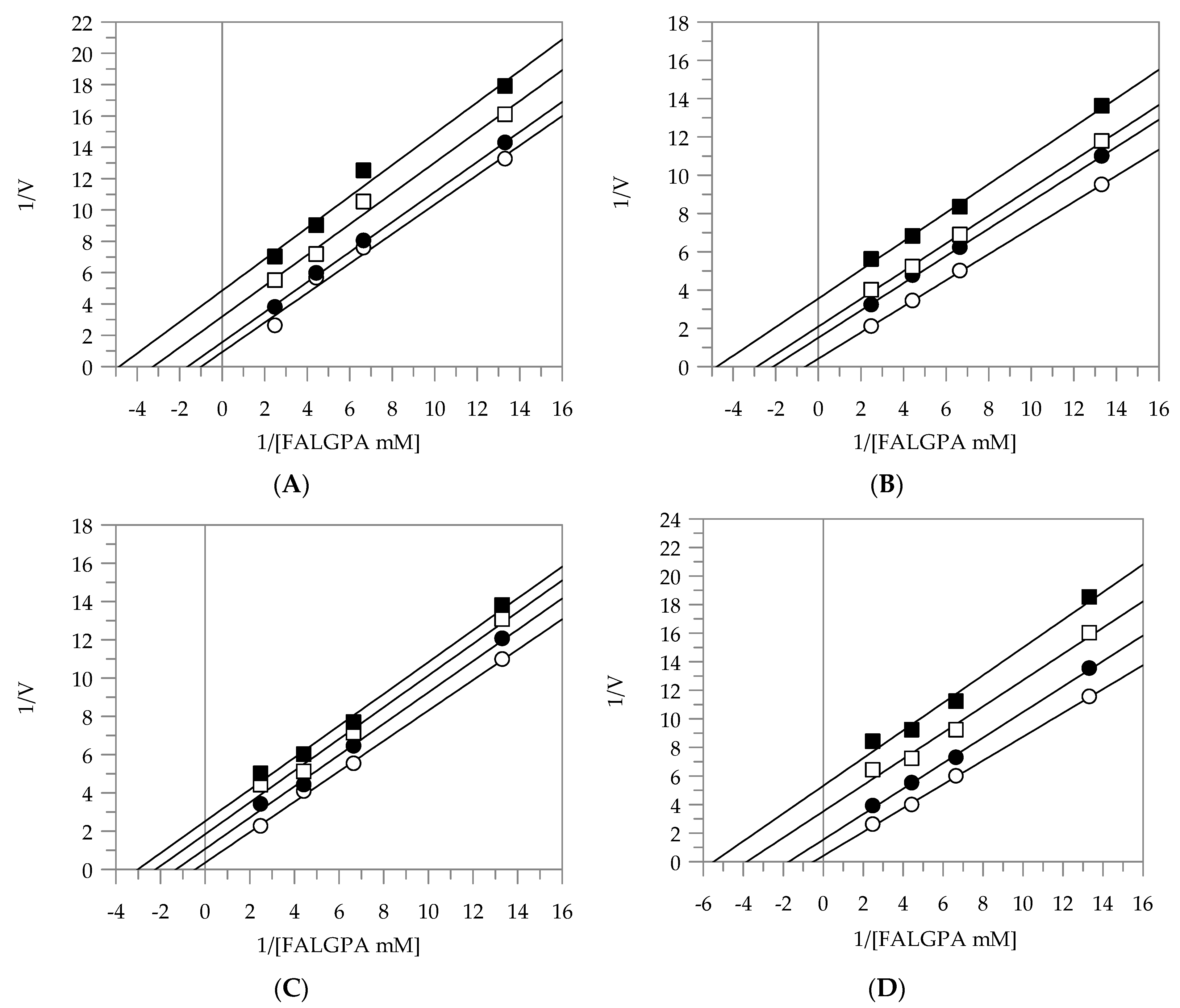
2.2. Kinetic Analysis by Lineweaver-Burk Plot
2.3. Sun Protection Factor
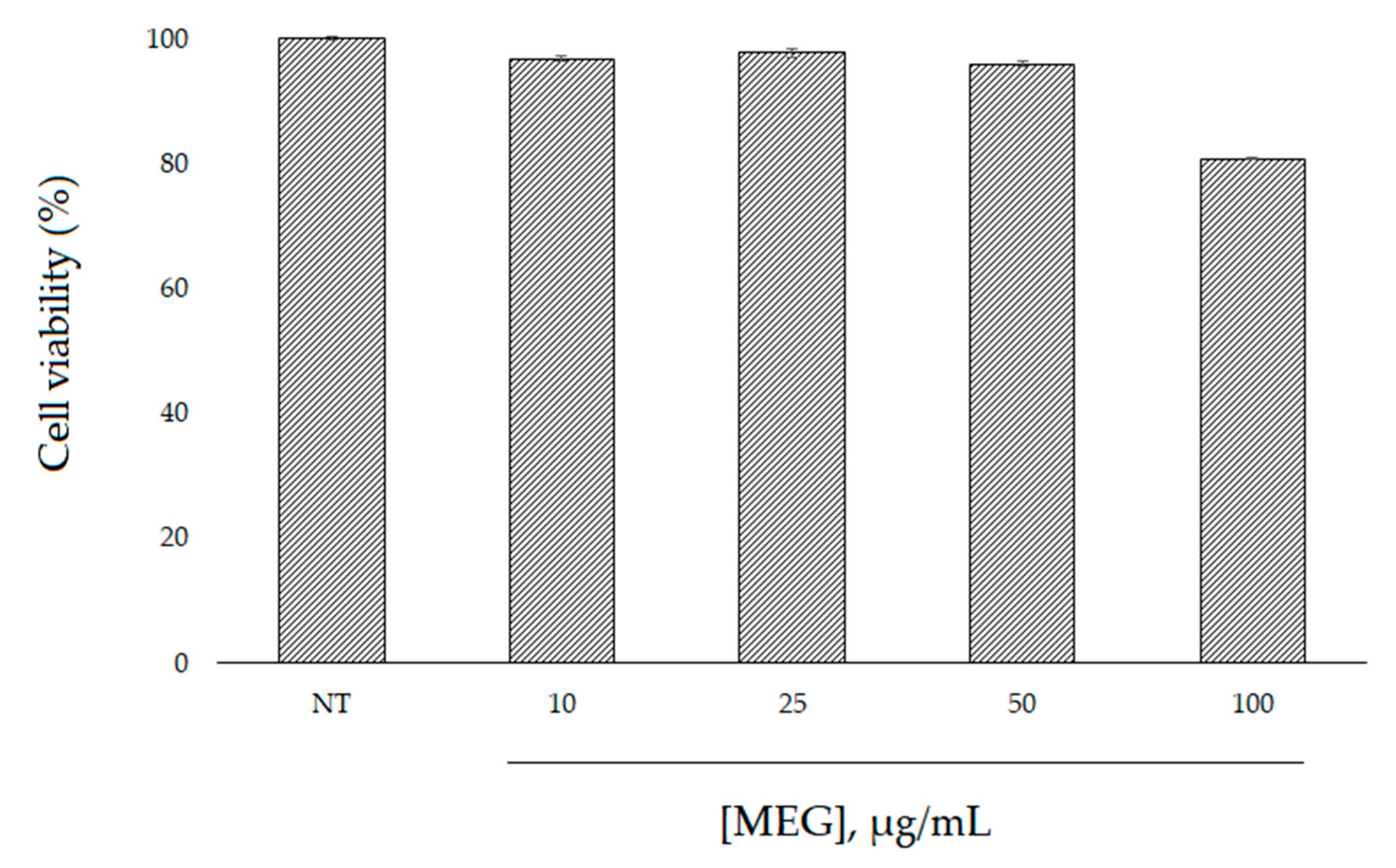
2.4. Cell Viability and Intracellular ROS Level
3. Materials and Methods
3.1. Chemicals
3.2. Plant Sample Preparation
3.3. Enzymatic Inhibition
3.3.1. Tyrosinase Inhibition Assay
3.3.2. Elastase Inhibition Assay
3.3.3. Collagenase Inhibition Assay
3.4. Determination of the In Vitro Sun Protection Factor
3.5. Cell Culture and Intracellular ROS Levels
3.6. Data Analysis
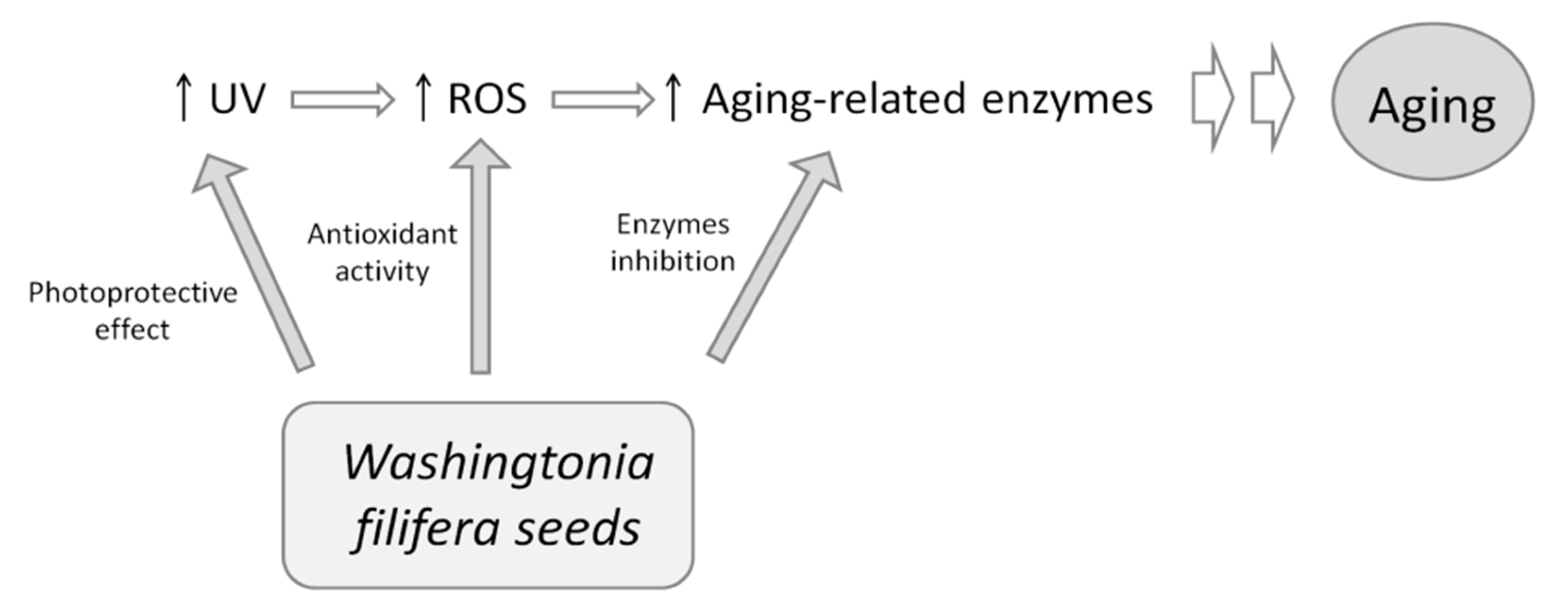
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumud, M.; Sanju, N. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodelling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, 005058. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Tsukahara, K.; Takema, Y.; Moriwaki, S.; Tsuji, N.; Suzuki, Y.; Fujimura, T.; Imokawa, G. Selective inhibition of skin fibroblast elastase elicits a concentration-dependent prevention of ultraviolet B-induced wrinkle formation. J. Investig. Derm. 2001, 117, 671–677. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.; Pawelek, J. L-tyrosine and L-DOPA as hormone-like regulators of melanocytes functions. Pigment Cell. Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- Thi Be Tu, P.; Tawata, S. Anti-oxidant, anti-aging, and anti melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar]
- Floris, S.; Fais, A.; Rosa, A.; Piras, A.; Marzouki, H.; Medda, R.; González-Paramás, A.M.; Kumar, A.; Santos-Buelga, C.; Era, B. Phytochemical composition and the cholinesterase and xanthine oxidase inhibitory properties of seed extracts from the Washingtonia filifera palm fruit. RSC Adv. 2019, 9, 21278–21287. [Google Scholar] [CrossRef]
- Gaagaiaa, D.E.; Bouakba, M.; Layachi, A. Thermo-physico-chemical and statistical mechanical properties of Washingtonian filifera new lignocellulosic fiber. Eng. Solid Mech. 2019, 7, 137–150. [Google Scholar] [CrossRef]
- Dewir, Y.H.; El-Mahrouk, M.E.; Seliem, M.K.; Murthy, H.N. Bioactive Compounds of California Fan Palm Washingtonia filifera (Linden ex André) H. Wendl. ex de Bary. In Bioactive Compounds in Underutilized Fruits and Nuts; Reference Series in Phytochemistry; Murthy, H., Bapat, V., Eds.; Springer: Cham, Switzerland, 2020; pp. 63–74. [Google Scholar]
- El-Sayed, N.H.; Ammar, N.M.; Al-Okbi, S.Y.; El-Kassem, L.T.A.; Mabry, T.J. Antioxidant activity and two new flavonoids from Washingtonia Filifera. Nat. Prod. Res. 2006, 20, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Brás, N.F.; Gonçalves, R.; Fernandes, P.A.; Mateus, N.; Ramos, M.J.; de Freitas, V. Understanding the binding of procyanidins to pancreatic elastase by experimental and computational methods. Biochemistry 2010, 49, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Brás, N.F.; Gonçalves, R.; Mateus, N.; Fernandes, P.A.; Ramos, M.J.; de Freitas, V. Inhibition of pancreatic elastase by polyphenolic compounds. J. Agric. Food Chem. 2010, 58, 10668–10676. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Yamaguchi, M.; Shigeyama, K.; Sakaguchi, I. The Anti-Aging Potential of Extracts from Chaenomeles sinensis. Cosmetics 2019, 6, 21. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Schurer, N.; Kohne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp. Derm. 1993, 2, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Wood, J.E.; Senthilmohan, S.T.; Peskin, A.V. Antioxidant activity of procyanidin-containing plant extracts at different pHs. Food Chem. 2002, 77, 155–161. [Google Scholar] [CrossRef]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharm. 1997, 95, 179–189. [Google Scholar]
- Di Petrillo, A.; González-Paramás, A.M.; Rosa, A.; Ruggiero, V.; Boylan, F.; Kumar, A.; Pintus, F.; Santos-Buelga, C.; Fais, A.; Era, B. Chemical composition and enzyme inhibition of Phytolacca dioica L.seeds extracts. J. Enzym. Inhib. Med. Chem. 2019, 34, 519–527. [Google Scholar] [CrossRef]
- Chompoo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S. Effect of Alpinia zerumbet components on antioxidant and skin diseases-related enzymes. BMC Complement. Altern. Med. 2012, 12, 106. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determinação Do Fator De Proteção Solar Por Espectrofotometria. Bras. Derm. Rio De Jan. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Pintus, F.; Spanò, D.; Corona, A.; Medda, R. Antityrosinase activity of Euphorbia characias extracts. PeerJ 2015, 3, e1305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fais, A.; Era, B.; Di Petrillo, A.; Floris, S.; Piano, D.; Montoro, P.; Tuberoso, C.I.G.; Medda, R.; Pintus, F. Selected enzyme inhibitory effects of Euphorbia characias extracts. Biomed. Res. Int. 2018, 2018, 1219367. [Google Scholar] [CrossRef] [PubMed]



| Plant Extracts | IC50 (µg/mL) | ||
|---|---|---|---|
| Tyrosinase * | Elastase * | Collagenase * | |
| EEG | 73.0 ± 5.09 a | 17.69 ± 2.81 a | 55.2 ± 19.09 a |
| EES | 89.0 ± 3.60 b | 19.75 ± 5.55 a | 50.04 ± 6.87 a |
| MEG | 89.5 ± 4.35 b | 10.76 ± 3.38 a | 50.03 ± 1.18 a |
| MES | 139.0 ± 3.34 c | 12.47 ± 3.11 a | 33.36 ± 13.06 a |
| AEG | 90.0 ± 2.11 b | 70.1 ± 4.56 b | ND |
| AES | 70.0 ± 3.17 a | 47.66 ± 2.88 c | ND |
| Kojic acid | 17.9 ± 0.98 d | - | - |
| Oleanolic acid | - | 11.75 ± 0.63 a | - |
| Epigallocatechin gallate | - | - | 120.8 ± 6.22 b |
| Elastase | |||
|---|---|---|---|
| Plant Extracts | Inhibition Type | KI (µg/mL) | KIS (µg/mL) |
| EEG | uncompetitive | - | 3.91 |
| EES | uncompetitive | - | 8.89 |
| MEG | noncompetitive | 9.66 | 9.55 |
| MES | noncompetitive | 9.48 | 9.57 |
| Collagenase | |||
| EEG | uncompetitive | - | 11.49 |
| EES | uncompetitive | - | 9.64 |
| MEG | uncompetitive | - | 13.04 |
| MES | uncompetitive | - | 7.58 |
| Wavelength (nm) | Absorbance | |||
|---|---|---|---|---|
| EEG | EES | MEG | MES | |
| 290 | 0.377 | 0.349 | 0.585 | 0.829 |
| 295 | 0.202 | 0.19 | 0.31 | 0.445 |
| 300 | 0.156 | 0.146 | 0.237 | 0.345 |
| 305 | 0.145 | 0.137 | 0.218 | 0.319 |
| 310 | 0.135 | 0.128 | 0.203 | 0.298 |
| 315 | 0.123 | 0.116 | 0.183 | 0.269 |
| 320 | 0.108 | 0.103 | 0.16 | 0.238 |
| SPF | 1.52 | 1.43 | 2.30 | 3.35 |
| Wavelength (nm) | EE × I |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0837 |
| 320 | 0.0180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Era, B.; Floris, S.; Sogos, V.; Porcedda, C.; Piras, A.; Medda, R.; Fais, A.; Pintus, F. Anti-Aging Potential of Extracts from Washingtonia filifera Seeds. Plants 2021, 10, 151. https://doi.org/10.3390/plants10010151
Era B, Floris S, Sogos V, Porcedda C, Piras A, Medda R, Fais A, Pintus F. Anti-Aging Potential of Extracts from Washingtonia filifera Seeds. Plants. 2021; 10(1):151. https://doi.org/10.3390/plants10010151
Chicago/Turabian StyleEra, Benedetta, Sonia Floris, Valeria Sogos, Clara Porcedda, Alessandra Piras, Rosaria Medda, Antonella Fais, and Francesca Pintus. 2021. "Anti-Aging Potential of Extracts from Washingtonia filifera Seeds" Plants 10, no. 1: 151. https://doi.org/10.3390/plants10010151
APA StyleEra, B., Floris, S., Sogos, V., Porcedda, C., Piras, A., Medda, R., Fais, A., & Pintus, F. (2021). Anti-Aging Potential of Extracts from Washingtonia filifera Seeds. Plants, 10(1), 151. https://doi.org/10.3390/plants10010151





