1. Introduction
Cauliflower (
Brassica oleracea L. var. Botrytis) is a cruciferous plant whose cultivation and consumption has increased dramatically in recent years after it was reported that this cruciferous was an important source of bioactive compounds with the ability to prevent and treat cardiovascular diseases and different types of cancer [
1]. The consumption of this cruciferous plant is reflected in a world production, together with broccoli, of 25.2 million tons, with Spain being the fourth largest producer of these crucifers [
2]. However, not everything is positive. In this sense, a large amount of this cruciferous plant becomes residue, and more than 50% of this residue is in the form of leaves, which has a high cost with negative effects for the environment. It is for these reasons that, in recent years, the possible reuse of this waste has been investigated [
3,
4].
Climate change is a significant threat not only from an ecological point of view. Thus, some studies have predicted that it is very possible that, before the end of this century, climate change will affect all levels of human activities [
5]. An increase in global temperature between 1.5 and 5.8 °C by the end of the century could lead to a reduction in crop production of approximately 16%. Experts in that area predict that this reduced production seems too low as compared to the population increase that is expected to occur by the end of this century [
6,
7]. Therefore, it is crucial to increase our knowledge about thermotolerance, in order to be able to create plants that are resistant to high temperatures.
It is known that as a result of heat stress (depending on the intensity and duration), cauliflower can show wounds that range from a decrease in protein stability, or an increase in membrane fluidity, to the inactivation of mitochondrial enzymes [
8]. Consequently, plants adjust their physiological and biochemical activities by altering their transcriptome, proteome, metabolome and lipidome to counteract these lesions and ensure cell survival [
9,
10].
Polyamines, including putrescine (Put), spermidine (Spd), and spermine (Spm), are a group of low molecular weight, polycationic, aliphatic, and nitrogenous compounds [
11]. These biogenic amines are present in all living organisms [
12]. Due to the cationic character of these aliphatic amines, they can bind to various negatively charged molecules such as RNA and DNA in cells, improving the synthesis of DNA, RNA, and proteins [
13]. Additionally, it has been observed that polyamines also function as gatekeepers to regulate ion flow [
13]. Thus, polyamines are involved in a wide range of biological processes in plants, including not only growth, plant development and senescence, but also providing protection to plant cells against various adverse conditions [
14]. In fact, a stimulation of polyamine synthesis in plants can confer tolerance to stress [
15]. Although the mechanism is not yet fully understood, it is known that the importance of polyamines lies above all in the fact that these biogenic amines are part of metabolic pathways that are interconnected with the formation of important metabolites involved in the plant’s response and signaling against stress [
16,
17]. Several previous studies have reported that the exogenous application of polyamines, especially putrescine, can increase the tolerance of crops to stress and promote the biosynthesis of bioactive compounds both in the floret and in the leaves of different plants [
17,
18,
19].
It is known that nitrogen (N) is one of the most important mineral nutrients for plants, as it affects not only their growth, but also several of their physiological aspects such as photosynthesis, stomatal conductance, the maximum potential quantum efficiency of photosystem II and the chlorophyll content. Therefore, nitrogen fertilizers were used very frequently in the past, causing significant negative impacts on the environment, such as water pollution [
20]. In recent years, attempts have been made to solve this problem with the efficient use of N fertilizers with a low amount of nitrates (NO
3−). However, the problem that arises now is that the lack of nutrients could lead to less healthy crops with a lower yield [
21]. It has been observed that nitrogen fertilization that provides ammonium (NH
4+), aside from promoting plant growth, can also reduce the negative effects of heat stress [
22,
23]. However, the most appropriate NO
3−/NH
4+ ratio depends on the species.
The goal of the present study was to try to understand how heat, different NO3−/NH4+ ratios, and putrescine can interact in cauliflower. For this, the problem of N leaching in the context of a heat wave was addressed. Furthermore, we gained insights into the possible potential synergistic effects of ammonium and putrescine. Thus, we will able to promote the use of cauliflower waste for nutraceutical purposes and increase the sustainability of this crop.
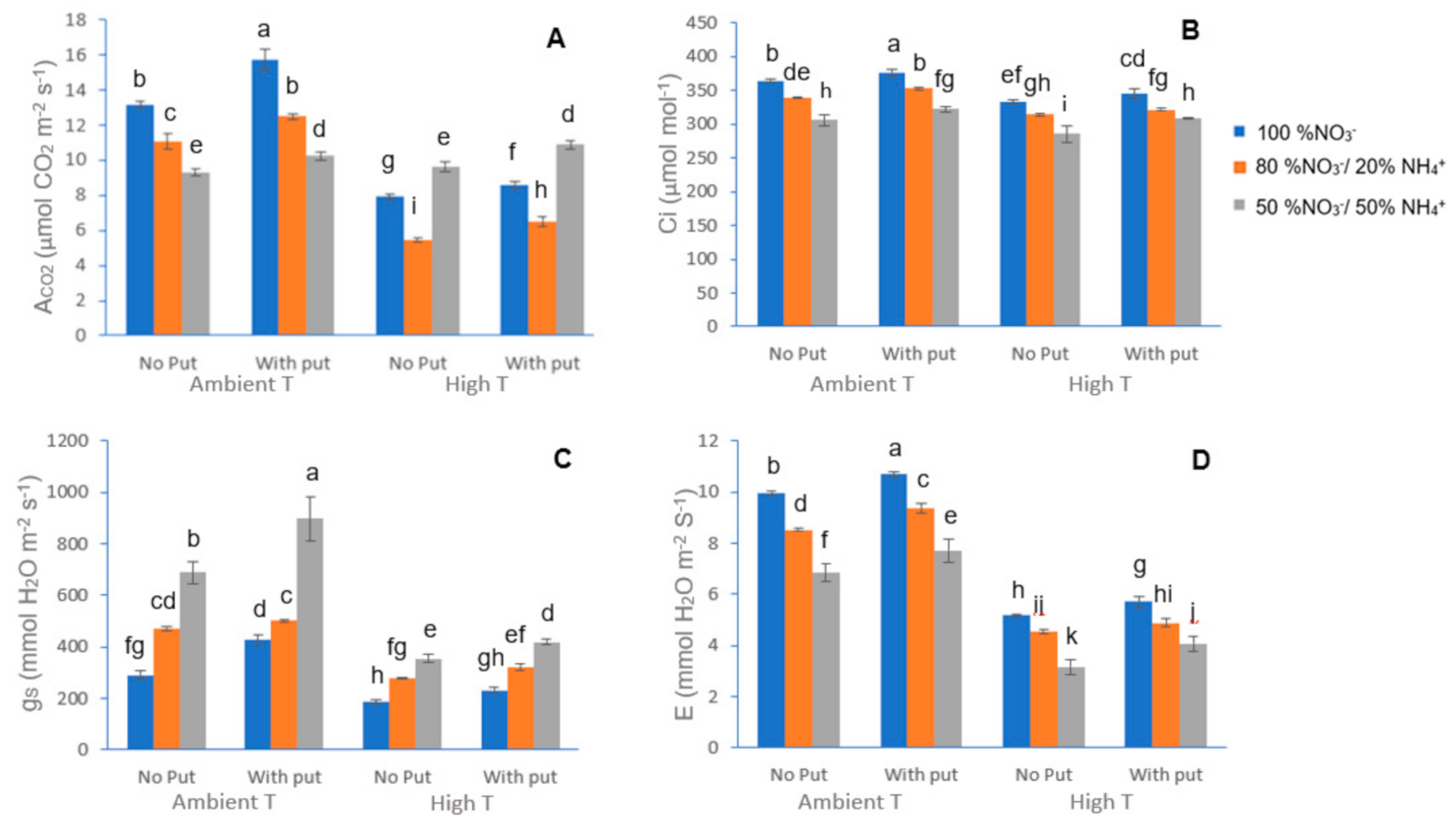
The level of stress obtained was analyzed according to the degree of change in the photosynthesis rate, stomatal conductance, water use efficiency, chlorophyll content, chlorophyll fluorescence, lipid peroxidation, protein content, and mineral content in cauliflower leaves.
3. Material and Methods
3.1. Experimental Conditions, Plant Material, and Treatments
Cauliflower (
Brassica oleracea var. botrytis L.) plants, cv. Moonshine, were obtained from a commercial seed supplier (El Jimenado S.A., Murcia, Spain) 30 days after their germination. Seedlings of similar size were transplanted into 5 L black pots filled with coconut fiber (Pelemix, Alhama de Murcia, Spain) and washed with 2 L of tap water. A total of sixty plants, one plant per pot, were used for carrying out this experiment and they were irrigated with different modified Hoagland solutions. The nutrition solution of the control treatment contained the following: Ca (NO
3)
2 4H
2O: 362.0 mg L
−1; KNO
3: 404.4 mg L
−1; K
2SO
4: 131.1 mg L
−1; MgSO
4 7H
2O: 123.2 mg L
−1; H
3PO
4: 0.101 mL. The nitrogen contribution in the other two treatments was in the form of NO
3−/NH
4+. Ammonium was introduced as (NH
4)
2SO
4 (105.6 mg L
−1 and 264 mg L
−1 for the 80/20 NO
3−/NH
4+ ratio treatment and the 50/50 NO
3−/NH
4+ ratio treatment, respectively). A total of twenty plants per nutrient treatment were used. The nutrients solutions used in the experiment were applied by self-compensating drippers (2 h L
−1) and drainage was evaluated daily to ensure drainage greater than 35% and for avoiding salt accumulation in the substrate. The experiment was conducted in a climate-controlled chamber designed by our department [
66] and located in Murcia, Spain (37°56′27.3″ N, 1°08′01.8″ W). In this chamber, all the environmental conditions were fully controlled: 60% RH, 400 µmol mol
−1 of CO
2, 16/8 h day/night, 28/16 °C during the first eighty-six days, and 43/30 °C on the last three days (heat stress). A photosynthetically active radiation (PAR) of 250 µmol m
−2 s
−1 was utilized, provided by a combination of fluorescent lamps (TL-D Master reflex 830 and 840, Koninklijke Philips Electronics N.V., from the Netherlands) and high-pressure lamps (Son-T Agro, Philips).
After eighty-six days, thirty plants (being five plants per treatment) were randomly selected and foliarly sprayed with 20 mL of a solution containing 2.5 mM putrescine plus 0.01% Tween-20 as a surfactant. After 4 days, half of the sprayed cauliflowers and half of the non-sprayed cauliflowers were harvested. The plants that were still in the chamber suffered the heat stress for three days. In total, the experiment lasted ninety-three days after transplantation.
3.2. Gas Exchange
Both in the ninetieth day and the final day of the total experiment, the net CO2 assimilation rate (ACO2), internal CO2 concentration (Ci), stomatal CO2 conductance (gs), and evapotranspiration (E) were measured on the mature fully-expanded leaf of each plant, using a CIRAS-2 portable photosynthesis system (PP system, Amesbury, MA, USA) with a PLC6 (U) Automatic Universal Leaf Cuvette, measuring both sides of the leaves. The cuvette provided light (LED) with a photon flux of 1300 µmol m−2 s−1, 400 µmol mol–1 CO2 and a leaf temperature of 25 °C.
3.3. Chlorophyll Content and Maximum Potential Quantum Efficiency of PSII
Chlorophylls a and b (Chl a and Chl b) were extracted from 1 g of frozen cauliflower leaves (−80 °C) with 25 mL of extraction buffer (acetone–hexane (2:3)). Leaf samples were homogenized using a Polytron and centrifuged at 3500 rpm for 6 min, at 4 °C. Afterwards, the absorbance of the supernatant was measured at 663, 645, 505, and 453 nm with a spectrophotometer (Shimadzu UV-1800 model with the CPS-240 cell holder, Shimadzu Europa GmbH, Duisburg, Germany). The contents of chlorophylls a and b, lycopene, and β-carotene were determined by using the Nagata and Yamashita [
67] equations:
On the same leaf used for gas exchange, the maximum potential quantum efficiency of PSII (Fv/Fm) was determined following the procedure previously described by Piñero et al. [
68]. These measurements were performed by using a portable modulated fluorometer OS-30P (Opti-Science, Hudson, NH, USA). Fv is the variable fluorescence from a dark-adapted leaf and Fm is the maximal fluorescence from a dark-adapted, mature fully expanded leaf. A special leaf clip holder was allocated to each leaf to maintain dark conditions for at least 30 min before reading. The analyses of chlorophyll, lycopene, β-Carotene, and Fv/Fm were run in five repetitions per treatment.
3.4. Determination of Total Protein
The total protein was measured in freeze-dried cauliflower leaves that had been dried at 65 °C, for at least 72 h. The analyses (of five replicates per treatment) were performed using a combustion nitrogen/protein analyzer (LECO FP-528, Leco Corporation, St. Joseph, MI, USA).
3.5. Lipid Peroxidation
Lipid peroxidation was measured through the thiobarbituric acid (TBA) reaction [
69]. Briefly, 0.1 g of lyophilized leaf samples were homogenized with 3 mL of extraction buffer (trichloroacetic acid (TCA), 20% (
w/v)) and centrifuged at 3500×
g for 20 min. After, 1 mL of supernatant was mixed with 1 mL of TCA (20%,
w/v) containing TBA (0.5%,
w/v) and 150 µL of BHT (4%,
w/v) in ethanol. This mixture, after being heated for 30 min at 95 °C, was cooled on ice and then centrifuged at 10,000×
g for 15 min. Thereafter, the absorbance was measured at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The concentration of thiobarbituric acid-reactive substances (TBARS) was calculated using an extinction coefficient of 155 mM
−1 cm
−1 [
70]. Results were expressed as TBARS µmol g
−1 FW. The analyses of lipid peroxidation were run in five replicates per treatment.
3.6. Leaf Mineral Content
The cauliflower leaf cations (Ca, K, Mg, B, Cu, Fe, Mn, and Zn) were extracted from ground lyophilized material (0.1 g) by acid digestion, using an ETHOS ONE microwave digestion system (Milestone Inc., Shelton, CT, USA), and were analyzed with an inductively-coupled plasma (ICP) spectrometer (Varian Vista MPX, Palo Alto, CA, USA).
The cauliflower leaf anions (Cl−, NO3−, PO43− and SO42−) were extracted from ground lyophilized material (0.4 g) with double-distilled water (20 mL) and analyzed with ion chromatography (METROHM 861 Advanced Compact IC; METROHM 838 Advanced Sampler). The analyses of cations and anions were run in five replicates per treatment.
3.7. Statistical Analysis
The experimental design was completely random, and five repetitions were carried out. Data were analyzed with SPSS v.21 (IBM, Chicago, IL, USA). First, data were tested for homogeneity of variance and normality of distribution and later, data were also subjected to a three-way (factor 1 = putrescine treatment; factor 2 = heat treatment; factor 3 = nutrition treatment) analysis of variance (ANOVA) and afterwards Tukey’s multiple-range test was utilized to compare the means. Differences were considered statistically significant at p ≤ 0.05.
4. Conclusions
From the results presented herein, it can be concluded that the cauliflower waste was richer in mineral nutrients than floret cauliflower. This is the first time that the strategy constituted of the application of putrescine and the appropriate ratio of nitrogen forms is proposed in order to improve not only the quality, but also the physiological changes in cauliflower. In this sense, in this work has been seen that putrescine and the appropriate ratio of nitrogen forms showed a synergistic effect on the enhancement of the tolerance capacity of the cauliflower cv Moonshine under heat stress. In fact, the results obtained in this study indicated a higher photosynthesis rate, a higher accumulation of both photosynthesis-related compounds and pigments, total proteins, and a change in the status of the different nutrients due to the application of 2.5 mM putrescine and a nutrition solution with a 50:50 NO3−/NH4+ ratio. For this, the application of both putrescine and fertilizing the plants with a 50:50 NO3−/NH4+ ratio could be proposed as an agricultural practice that is useful for increasing the thermotolerance of cauliflower cv Moonshine. Regarding the mineral content, it has been seen that under heat stress the content of cations, including Na, K, Ca, P, Fe, Zn, Cu, and Mn, and in anions, including chloride, sulphates and nitrate was decreased. Besides, some cations (Ca+, Mg2+, K+) and nitrate and some anions (Cl−, SO42− and PO43−) were increased in response to the ammonium effect. It could be as a consequence of changes in the one-way influx or efflux of ions and/or due to a more complex interaction with other cations, especially potassium.
Furthermore, our results suggest that this higher thermotolerance acquired may be correlated with a higher activity of antioxidant enzymes, which reduce lipid peroxidation. Lastly, due to decreased senescence, a lower lipid peroxidation was observed in the young leaves. Additionally, these beneficial effects are not only good for plants, but for humans as well. The occurrence of less lipid peroxidation in cauliflower leaves led to a higher accumulation of secondary metabolites with many health-promoting effects for humans. Therefore, the application of both treatments could be considered as a way to concentrate the content of these metabolites, especially in young cauliflower leaves. Thus, it could be easier to obtain ointments or nutraceutical products starting from young cauliflower leaves rich in these secondary metabolites. A very positive effect of using cauliflower leaves for producing nutraceutical products could be the increased sustainability of cauliflower cultivation.