3.1. Leaf Elongation Rates during Day and Night
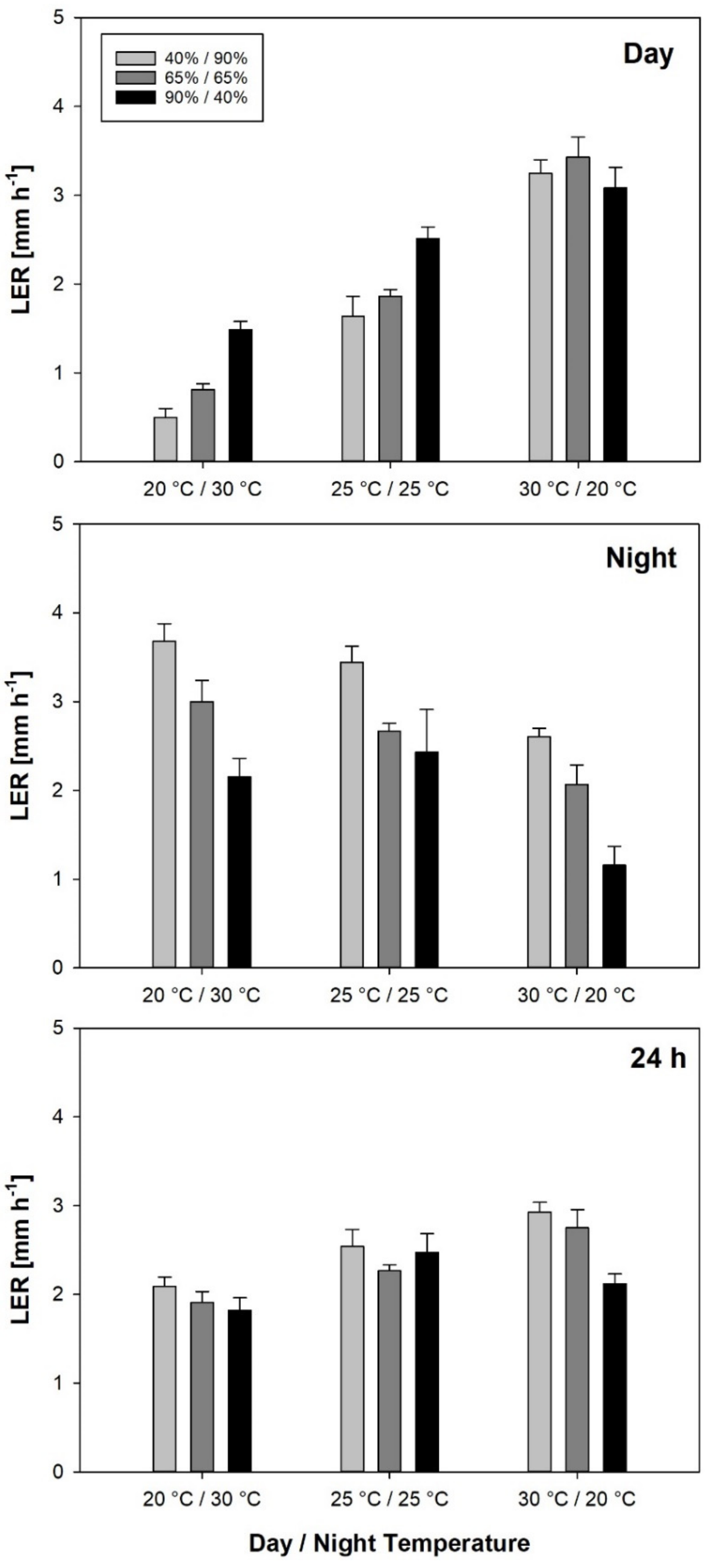
Daytime LER was closely correlated with daytime temperature, which had a much larger effect on LER than RH. The temperature dependence of LER has been demonstrated by several authors [
4,
21,
22]. In pressure chamber experiments using salt-treated barley, only daytime LER could be increased, as a result it has been argued that LER was controlled by the plant’s water status during the day, but not during the night [
12]. In our experiment, plants exposed to the highest evaporative demand during the light period (i.e., 2.5 kPa in Tnat/RHnat) had the second highest LER
Day and the highest LER
24h among all treatments. Thus, the positive effect of temperature on LER during the day seems to rule out the potentially negative effect of evaporative demand on plant water status. Rice has been described as an isohydric plant and shown to have a LER relatively insensitive to evaporative demand during the day [
23]. By contrast, in our experiment, nighttime LER was almost equally affected by temperature and RH. High sensitivity of nighttime LER to RH has not been described so far. Since g
s and, thus, also transpiration, are much lower during the night even at low RH, a large effect of nighttime RH on LER seems unlikely. However, despite low nighttime transpiration rates, evaporative demand could affect leaf water potential even during the night. Negative effects of high nighttime evaporative demand have been demonstrated for the leaf water potential of wine grape [
24] and the relative water content of wheat [
25]. Leaf elongation rates were found to be linearly correlated with leaf water potential in soybean [
26], while in ryegrass, the correlation was found only during the day, but not during the night [
11]. However, studies relating daytime and nighttime leaf growth to leaf water potentials are scarce and it has been claimed very recently that more investigations are required to address the question if nighttime VPD has a negative effect on LER, given that this is a well-established observation during daytime [
27].
Also, daytime and nighttime LER are not independent. Independent variation of day and night temperature showed that in rice, higher nighttime LER caused by higher night temperature was associated with a decrease in LER during the day, while LER
Night was not affected by higher day temperature and increased LER
Day [
28]. In barley, artificially increased leaf growth during the day was counterbalanced by growth reductions in the following night [
29]. Related to a larger variation of LER during the day, LER
24h was determined by LER
Day in our experiment. Here again, temperature had a larger effect than RH and high LER
24h was mainly associated with warm days and, to a lesser extent, with humid nights.
Even though the effect of RH on LER was rather weak, it should not be neglected. Leaf elongation rates have been used to calculate cardinal temperatures of crop genotypes [
4,
30,
31], and the causal relationship between leaf elongation and leaf appearance has been discussed [
32]. In our experiment, LER
Day linearly increased with temperature in the observed range, while LER
Night only slightly increased between 25 °C and 30 °C in the treatments around the same mean temperature and even decreased in the constant temperature treatments. In both cases, differences in LER
Night between 25 °C and 30 °C were not statistically significant. Therefore, we hypothesize that optimum temperature for LER
Night is below 30 °C, while for LER
Day, the linear relationship with temperature suggests an optimum temperature above the observed temperature range. The
x-axis intercept has been used to determine base temperatures (T
base) from the relationship between temperature and both development rates to the flowering [
33] and leaf appearance rates [
34]. Although our dataset is too small to provide robust information on T
base, taken as the
x-axis intercept from the regression of LER
Day vs. day temperature, we want to highlight that the
x-axis intercept of this regression varies with RH. While under low evaporative demand at 90% RH, tentative T
base is 10.6°C, which is relatively close to the T
base of 9.55°C described for IR64 [
35], tentative T
base increased with evaporative demand to 17.3 °C and 18.5 °C at 65% and 40% RH, respectively. In a previous estimation of cardinal temperatures based on LER for a large panel of rice accessions at 70% RH day and night, T
base was found to be systematically higher than values commonly used in crop models [
4]. It is clearly beyond the scope of this manuscript to provide cardinal temperatures for leaf elongation, but since precise cardinal temperatures are of such prominent importance for crop modeling, we would like to emphasize the possibility that high evaporative demand at the experimental site increases calculated T
base.
3.2. Leaf Elongation Rates and Leaf Gas Exchange
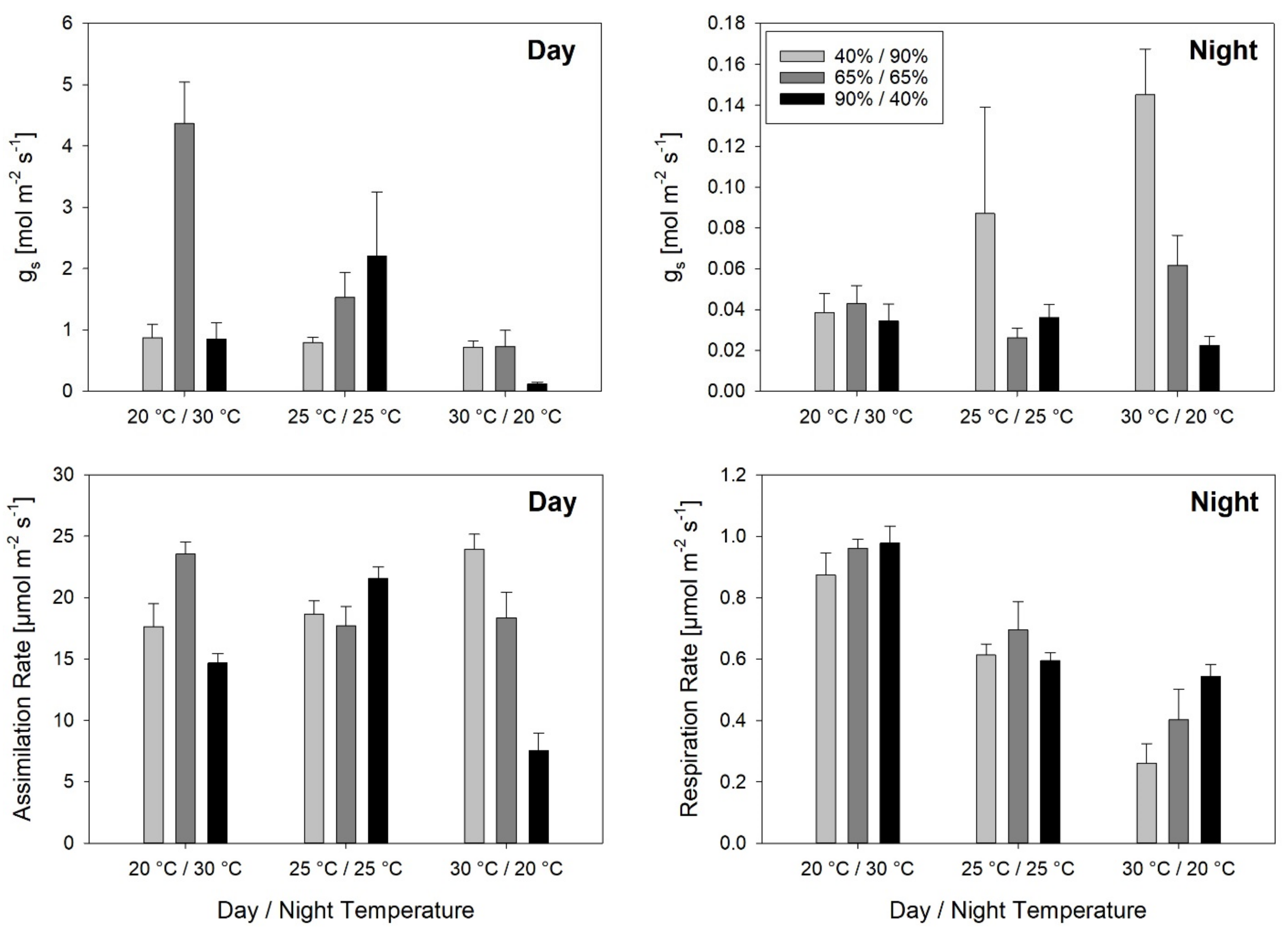
Stomatal conductance responded to both temperature and RH during the day, whereas during the night, it was significantly affected by RH alone. The response to inverted temperature and RH regimes was unexpected. Extremely high g
s values were measured with a maximum at Tinv/RHcon, which was in the range of the calculated maximum theoretical possible stomatal conductance [
36]. Measurement errors cannot be excluded here, but also in pre-experiments, where measurements resulted in elevated g
s at inverted day and night temperatures. High daytime g
s after warm nights has been observed before. In field experiments conducted in the semi-arid Sahel, noon-time g
s of different rice varieties was closely correlated with minimum meristem temperature, which was measured at the end of the night [
37]. The mechanism behind this remains unclear. Very low g
s values were found at Tnat/RHinv, indicating that low RH during the night might negatively affect daytime g
s. Effects of nighttime transpirational demand on daytime transpiration have been described for wheat [
38,
39]. In our experiment, unexpectedly no correlation between daytime VPD and g
s was found, which could be explained by the potential impact of nighttime transpiration on daytime g
s. In summary, the measured g
s values suggest that stomatal responses to environmental conditions remain difficult to understand. Similarly, but with less variation, A was affected by temperature and RH treatments. Tinv at constant RH led to a relatively high A, while Tnat at RHinv led to a very low A. However, nighttime respiration was closely correlated with temperature.
No significant correlation was found between A and LER, indicating that LER was not limited by carbon assimilation. Seneweera et al. [
10] found that LER at mid-tillering stage was depressed under CO
2 enrichment and concluded that tillers were stronger carbohydrate sinks than growing leaf blades. In contrast, a high negative correlation between nighttime respiration rates and LER
24h was found. However, as a result of the experimental set-up it is difficult to assess if the correlation of the two parameters indicates high respiration rates depleting the carbohydrate reserves, and as a result leading to reduced LER
24h. Alternatively, the correlation could be due to the inversion of temperature. High night temperatures lead to high respiration rates, which coincide with low LER
24h, which is induced by low day temperatures. Results of a previous experiment in the same experimental setup suggest the first possibility. There, carbohydrate depletion after periods of increased growth and respiration during the night has been demonstrated. Whereas day temperature did not affect sucrose levels of the leaves of IR64 at the end of the day, a higher night temperature led to significantly lower sucrose concentrations at the end of the night [
40].
3.3. Leaf Growth and Plant Growth
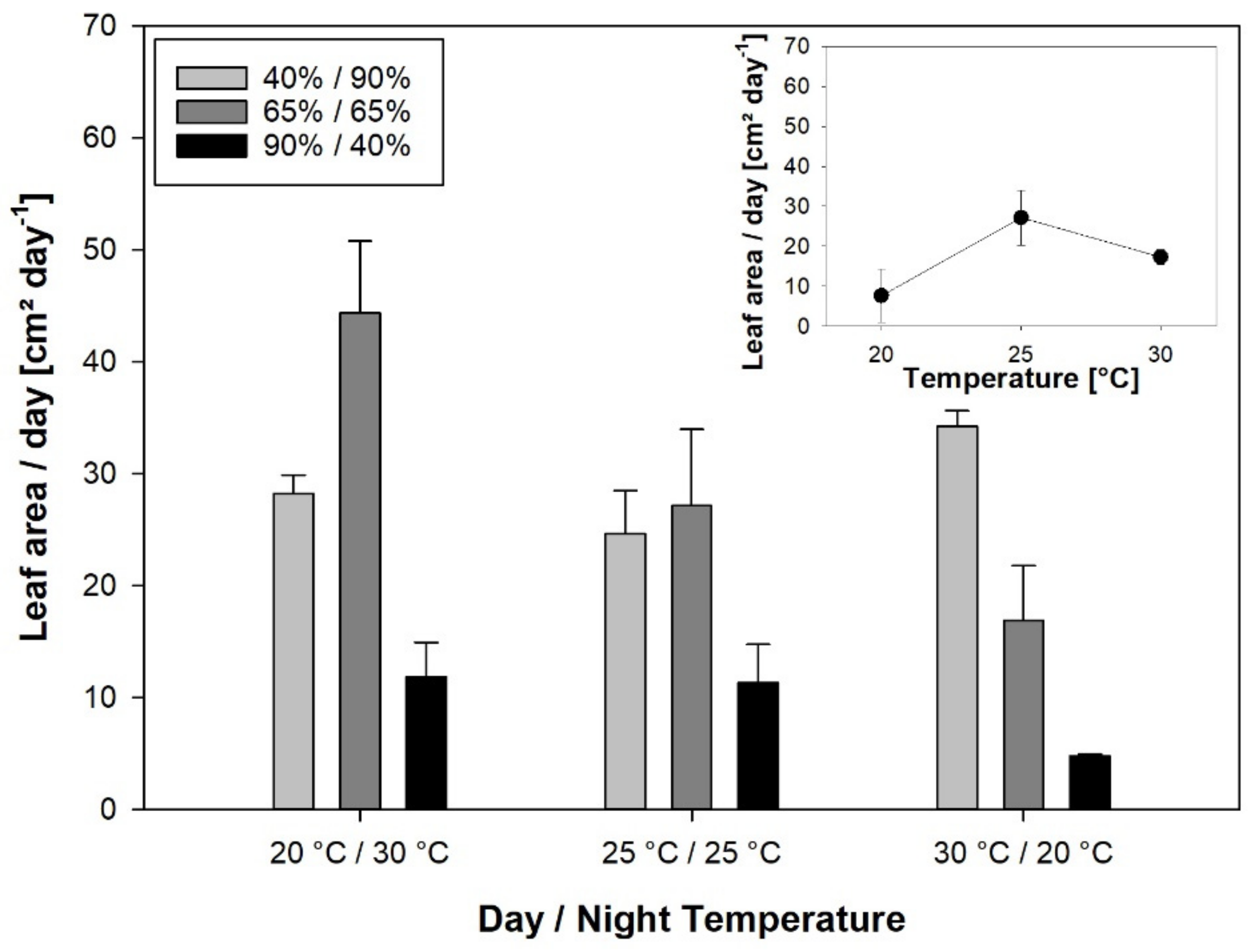
Unlike leaf elongation, the leaf area expansion rate was highly affected by RH, with a strong negative effect of inverted RH. In general, high air humidity has been found to have a positive effect on rice growth [
41,
42]. Consequently, the low leaf area expansion rate under inverted RH was a result of the low nighttime RH rather than of the high daytime RH. Although the reason for the strong negative effect of low nocturnal RH on leaf growth is still unclear, the diurnal regulation of aquaporin expression could be involved. Many aquaporins seem to be up-regulated in response to low air humidity [
42], but the expression of some aquaporins increases dramatically during the day and seems to be triggered by transpiration in the presence of light [
43]. As nighttime evaporative demand probably affected daytime g
s, effects on aquaporin expression during the day seem likely. In wheat plants, high evaporative demand during the night did not affect plant dry weight or leaf area, but negatively changed the root hydraulic properties [
25]. As rice has been described as sensitive to soil water deficit and evaporative demand, which are both related to its poor root system [
23], changes in root hydraulic properties could have a larger effect on rice than on wheat. Under natural conditions in the field, the vapor content of the atmosphere is usually almost saturated at night, and low RH is only observed in rare cases, which means the practical implications of the effects of low nighttime RH are limited. However, studying the underlying physiological mechanism could significantly improve our understanding of plant responses in artificial environments, which will most likely increase in importance in future plant production.
Unlike LER, leaf area expansion at the plant level was significantly correlated with the assimilation rate, demonstrating the carbon limitation of expansive growth. No correlation between LER
24h and leaf area expansion rate was found. Instead, leaf area expansion was correlated with tiller number (data not shown), showing that tillering is more important for leaf area development than the growth of individual leaves. However, a significant correlation was found between nighttime LER and the leaf area expansion rate. Leaf growth responses could be causally related to partitioning effects because day and night temperature, and RH treatments caused large shifts in dry matter partitioning [
20]. LER
Night was enhanced by warm and humid nights, whereas cool days and warm nights increased SLA, and high RH during the night was associated with a higher leaf mass fraction (LMF) [
20]. As leaf area expansion can be described as a function of carbon gain, LMF and SLA [
44], the same temperature and RH conditions that stimulate growth of individual leaves during the night also promote partitioning characteristics beneficial for leaf area expansion at the plant level.
In conclusion, the effects of nighttime RH on rice leaf growth were more pronounced than those of daytime RH. Low RH during the night has very negative effects on both the growth of individual leaves as well as the growth of overall leaf area. Since growth chamber experiments are often conducted under constant day and night RH, researchers should keep the potentially negative effect of low nighttime RH in mind. However, daytime RH also modified the growth responses to temperatures, and the results suggested an increase of Tbase with decreasing RH. Since crop growth models are usually based on fixed cardinal temperatures, the introduction of a correction factor for RH should be considered to improve model outputs.