Adherent but Not Suspension-Cultured Embryoid Bodies Develop into Laminated Retinal Organoids
Abstract
:1. Introduction
2. Materials and Methods
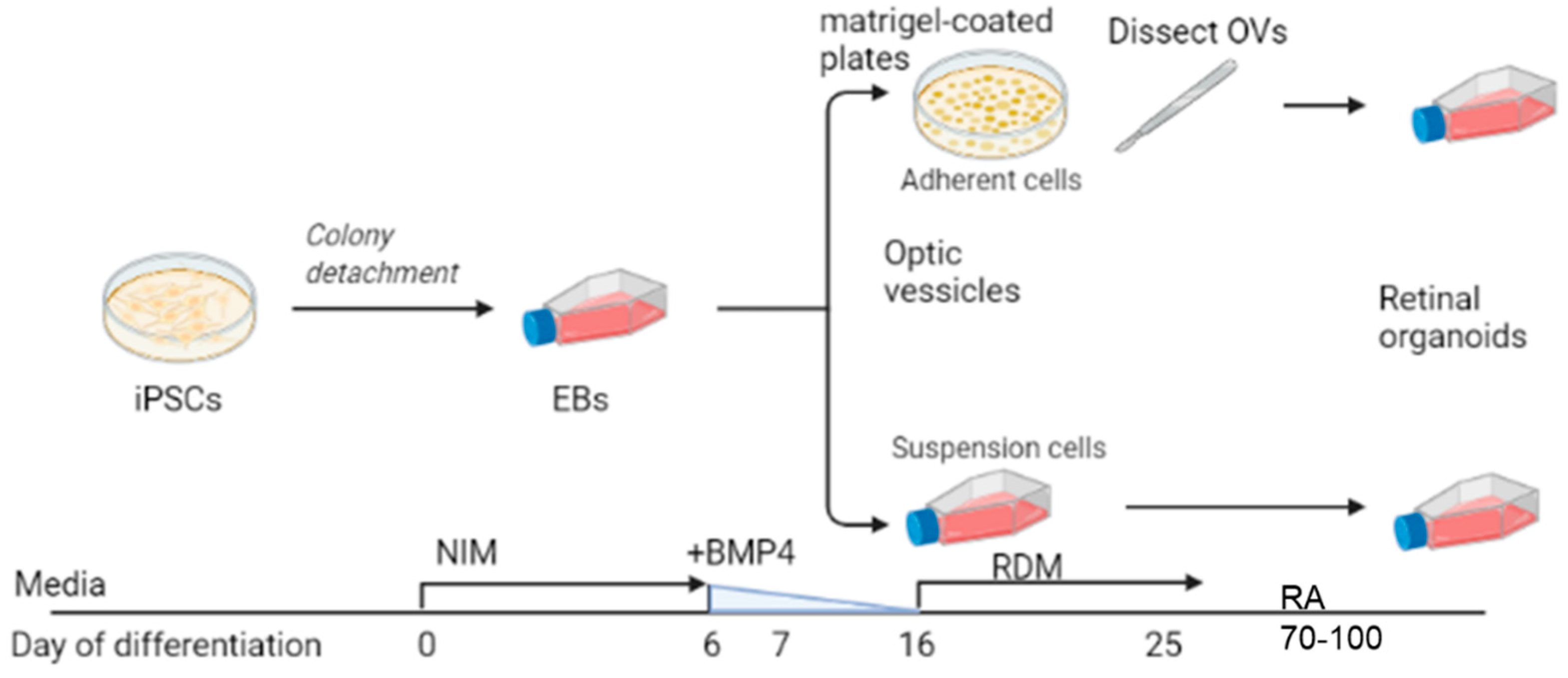
2.1. Differentiation Protocol
2.2. Immunohistochemistry (IHC)
2.3. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.4. Statistics
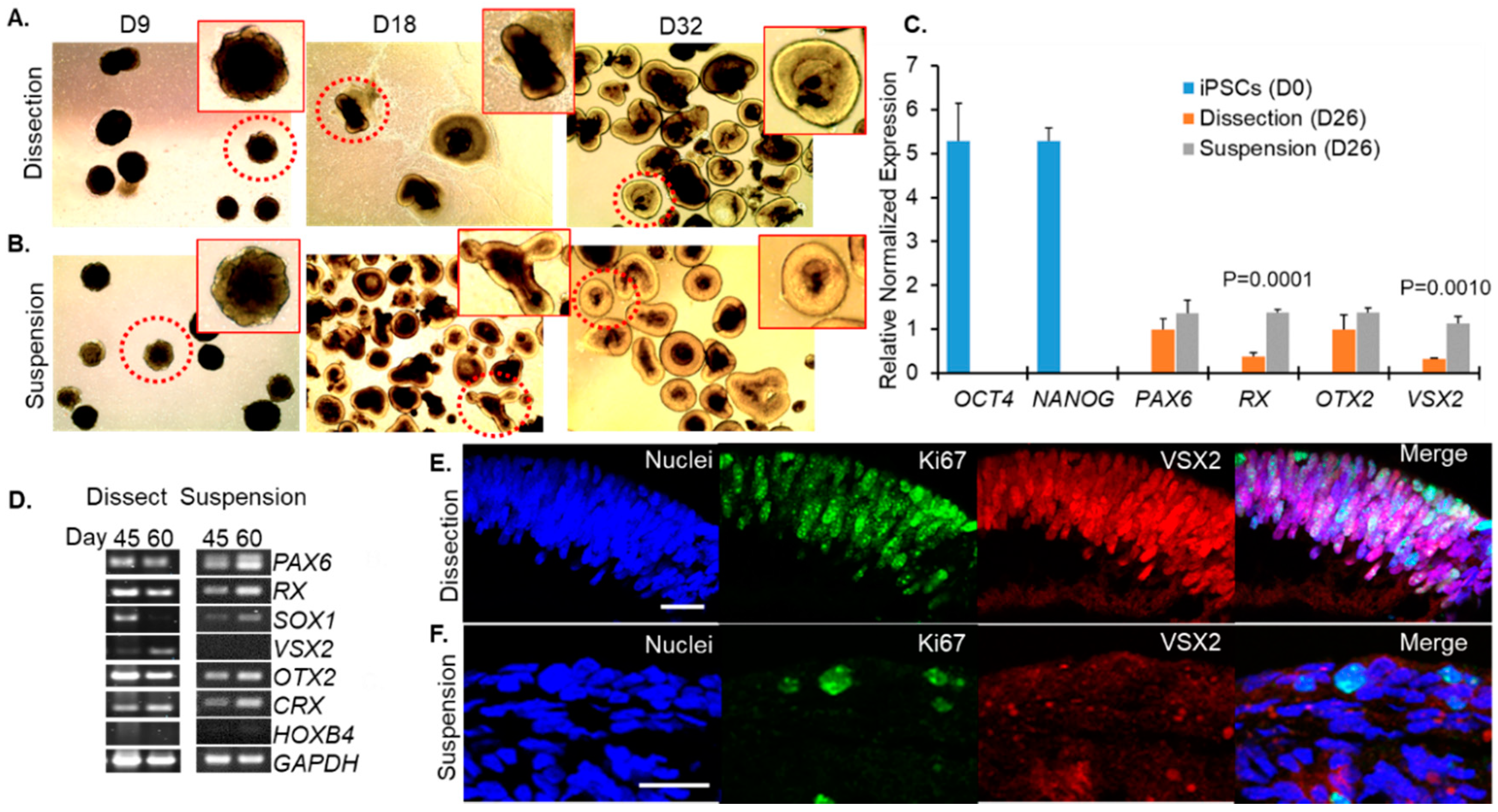
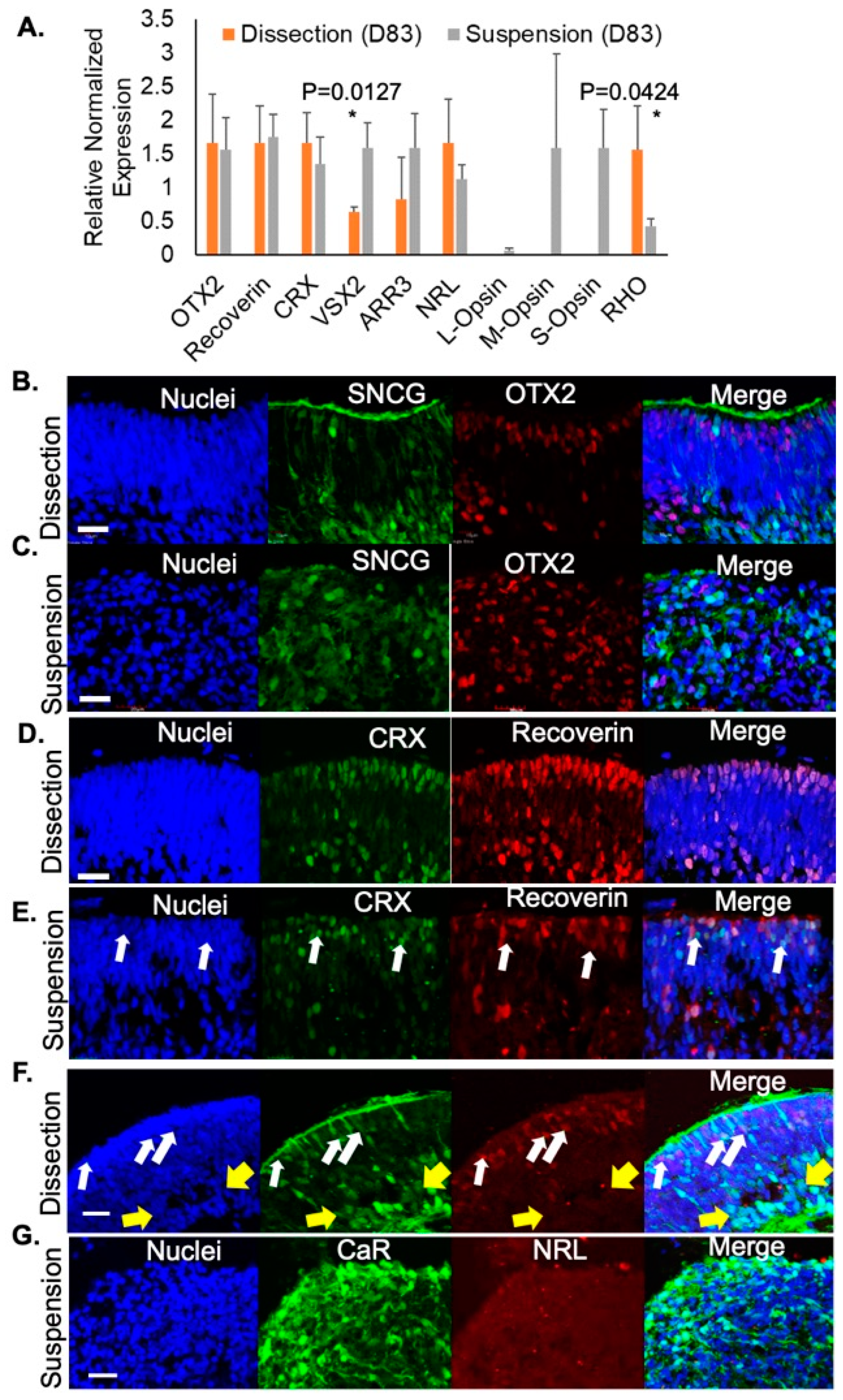
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Layer, P.G. Brains emerging: On modularity and self-organisation of neural development in vivo and in vitro. In Emergence and Modularity in Life Sciences; Wegner, L.H., Lüttge, U., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 145–169. [Google Scholar]
- Moscona, A. Development of heterotypic combinations of dissociated embryonic chick cells. Proc. Soc. Exp. Biol. Med. 1956, 92, 410–416. [Google Scholar] [CrossRef]
- Moscona, A. The development in vitro of chimeric aggregates of dissociated embryonic chick and mouse cells. Proc. Natl. Acad. Sci. USA 1957, 43, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Garber, B.; Kollar, E.J.; Moscona, A.A. Aggregation in vivo of dissociated cells. 3. Effect of state of differentiation of cells on feather development in hybrid aggregates of embryonic mouse and chick skin cells. J. Exp. Zool. 1968, 168, 455–472. [Google Scholar] [CrossRef]
- Layer, P.G.; Weikert, T.; Willbold, E. Chicken retinospheroids as developmental and pharmacological in vitro models: Acetylcholinesterase is regulated by its own and by butyrylcholinesterase activity. Cell Tissue Res. 1992, 268, 409–418. [Google Scholar] [CrossRef]
- Willbold, E.; Reinicke, M.; Lance-Jones, C.; Lagenaur, C.; Lemmon, V.; Layer, P.G. Muller glia stabilizes cell columns during retinal development: Lateral cell migration but not neuropil growth is inhibited in mixed chick-quail retinospheroids. Eur. J. Neurosci. 1995, 7, 2277–2284. [Google Scholar] [CrossRef]
- Willbold, E.; Mansky, P.; Layer, P.G. Lateral and radial growth uncoupled in reaggregated retinospheroids of embryonic avian retina. Int. J. Dev. Biol. 1996, 40, 1151–1159. [Google Scholar]
- Layer, P.G.; Robitzki, A.; Rothermel, A.; Willbold, E. Of layers and spheres: The reaggregate approach in tissue engineering. Trends Neurosci. 2002, 25, 131–134. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human ipscs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef] [Green Version]
- Wahlin, K.J.; Maruotti, J.A.; Sripathi, S.R.; Ball, J.; Angueyra, J.M.; Kim, C.; Grebe, R.; Li, W.; Jones, B.W.; Zack, D.J. Photoreceptor outer segment-like structures in long-term 3d retinas from human pluripotent stem cells. Sci. Rep. 2017, 7, 766. [Google Scholar] [CrossRef]
- Hallam, D.; Hilgen, G.; Dorgau, B.; Zhu, L.; Yu, M.; Bojic, S.; Hewitt, P.; Schmitt, M.; Uteng, M.; Kustermann, S.; et al. Human-induced pluripotent stem cells generate light responsive retinal organoids with variable and nutrient-dependent efficiency. Stem Cells 2018, 36, 1535–1551. [Google Scholar] [CrossRef] [Green Version]
- Kaya, K.D.; Chen, H.Y.; Brooks, M.J.; Kelley, R.A.; Shimada, H.; Nagashima, K.; de Val, N.; Drinnan, C.T.; Gieser, L.; Kruczek, K.; et al. Transcriptome-based molecular staging of human stem cell-derived retinal organoids uncovers accelerated photoreceptor differentiation by 9-cis retinal. Mol. Vis. 2019, 25, 663–678. [Google Scholar]
- Welby, E.; Lakowski, J.; di Foggia, V.; Budinger, D.; Gonzalez-Cordero, A.; Lun, A.T.L.; Epstein, M.; Patel, A.; Cuevas, E.; Kruczek, K.; et al. Isolation and comparative transcriptome analysis of human fetal and ipsc-derived cone photoreceptor cells. Stem Cell Rep. 2017, 9, 1898–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, J.L.; Pierce, E.A.; Laster, A.M.; Daiger, S.P.; Birch, D.G.; Ash, J.D.; Iannaccone, A.; Flannery, J.G.; Sahel, J.A.; Zack, D.J.; et al. Inherited retinal degenerations: Current landscape and knowledge gaps. Transl. Vis. Sci. Technol. 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, L.D.; Metz, G.; Klein, M.; Locke, K.G.; Khwaja, A.; Birch, D.G. Regional variations and intra-/intersession repeatability for scotopic sensitivity in normal controls and patients with inherited retinal degenerations. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1122–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, L.D.; Klein, M.; John, F.T.; Radojevic, B.; Jones, K.; Birch, D.G. Disease progression in patients with autosomal dominant retinitis pigmentosa due to a mutation in inosine monophosphate dehydrogenase 1 (impdh1). Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radojevic, B.; Jones, K.; Klein, M.; Mauro-Herrera, M.; Kingsley, R.; Birch, D.G.; Bennett, L.D. Variable expressivity in patients with autosomal recessive retinitis pigmentosa associated with the gene cngb1. Ophthalmic Genet. 2021, 42, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Noel, N.C.L.; MacDonald, I.M.; Allison, W.T. Zebrafish models of photoreceptor dysfunction and degeneration. Biomolecules 2021, 11, 78. [Google Scholar] [CrossRef]
- Collin, G.B.; Gogna, N.; Chang, B.; Damkham, N.; Pinkney, J.; Hyde, L.F.; Stone, L.; Naggert, J.K.; Nishina, P.M.; Krebs, M.P. Mouse models of inherited retinal degeneration with photoreceptor cell loss. Cells 2020, 9, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuck, M.W.; Conley, S.M.; Naash, M.I. The y141c knockin mutation in rds leads to complex phenotypes in the mouse. Hum. Mol. Genet. 2014, 23, 6260–6274. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, D.; Conley, S.M.; Zulliger, R.; Naash, M.I. The k153del prph2 mutation differentially impacts photoreceptor structure and function. Hum. Mol. Genet. 2016, 25, 3500–3514. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.-L.; Gao, M.-L.; Lei, X.-L.; Lv, J.-N.; Zhao, H.; He, K.-W.; Xia, X.-X.; Li, L.-Y.; Chen, Y.-C.; Li, Y.-P.; et al. Gene correction reverses ciliopathy and photoreceptor loss in ipsc-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Rep. 2018, 10, 1267–1281. [Google Scholar] [CrossRef] [Green Version]
- Parfitt, D.A.; Lane, A.; Ramsden, C.M.; Carr, A.-J.; Munro, P.M.; Jovanovic, K.; Schwarz, N.; Kanuga, N.; Muthiah, M.N.; Hull, S.; et al. Identification and correction of mechanisms underlying inherited blindness in human ipsc-derived optic cups. Cell Stem Cell 2016, 18, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Shen, W.; Kuai, D.; Martin, J.M.; Guo, X.; Smith, M.A.; Perez, E.T.; Phillips, M.J.; Simonett, J.M.; Wallace, K.A.; et al. Ips cell modeling of best disease: Insights into the pathophysiology of an inherited macular degeneration. Hum. Mol. Genet. 2013, 22, 593–607. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-formation of optic cups and storable stratified neural retina from human escs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Kallman, A.; Capowski, E.E.; Wang, J.; Kaushik, A.M.; Jansen, A.D.; Edwards, K.L.; Chen, L.; Berlinicke, C.A.; Phillips, M.J.; Pierce, E.A.; et al. Investigating cone photoreceptor development using patient-derived nrl null retinal organoids. Commun. Biol. 2020, 3, 82. [Google Scholar] [CrossRef] [Green Version]
- Capowski, E.E.; Samimi, K.; Mayerl, S.J.; Phillips, M.J.; Pinilla, I.; Howden, S.E.; Saha, J.; Jansen, A.D.; Edwards, K.L.; Jager, L.D.; et al. Reproducibility and staging of 3d human retinal organoids across multiple pluripotent stem cell lines. Development 2019, 146, dev171686. [Google Scholar] [CrossRef] [Green Version]
- Reichman, S.; Terray, A.; Slembrouck, A.; Nanteau, C.; Orieux, G.; Habeler, W.; Nandrot, E.F.; Sahel, J.A.; Monville, C.; Goureau, O. From confluent human ips cells to self-forming neural retina and retinal pigmented epithelium. Proc. Natl. Acad. Sci. USA 2014, 111, 8518–8523. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.S.; Shearer, R.L.; Capowski, E.E.; Wright, L.S.; Wallace, K.A.; McMillan, E.L.; Zhang, S.C.; Gamm, D.M. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 16698–16703. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.S.; Howden, S.E.; Wallace, K.A.; Verhoeven, A.D.; Wright, L.S.; Capowski, E.E.; Pinilla, I.; Martin, J.M.; Tian, S.; Stewart, R.; et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 2011, 29, 1206–1218. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chandra, A.J.; Lee, S.C.S.; Grunert, U. Melanopsin and calbindin immunoreactivity in the inner retina of humans and marmosets. Vis. Neurosci. 2019, 36, E009. [Google Scholar] [CrossRef]
- Hamano, K.; Kiyama, H.; Emson, P.C.; Manabe, R.; Nakauchi, M.; Tohyama, M. Localization of two calcium binding proteins, calbindin (28 kd) and parvalbumin (12 kd), in the vertebrate retina. J. Comp. Neurol. 1990, 302, 417–424. [Google Scholar] [CrossRef]
- Duparc, R.H.; Abdouh, M.; David, J.; Lepine, M.; Tetreault, N.; Bernier, G. Pax6 controls the proliferation rate of neuroepithelial progenitors from the mouse optic vesicle. Dev. Biol. 2007, 301, 374–387. [Google Scholar] [CrossRef] [Green Version]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef]
- Heavner, W.; Pevny, L. Eye development and retinogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008391. [Google Scholar] [CrossRef] [Green Version]
- Wikler, K.C.; Perez, G.; Finlay, B.L. Duration of retinogenesis: Its relationship to retinal organization in two cricetine rodents. J. Comp. Neurol. 1989, 285, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Morales, J.R.; del Bene, F.; Nica, G.; Hammerschmidt, M.; Bovolenta, P.; Wittbrodt, J. Differentiation of the vertebrate retina is coordinated by an fgf signaling center. Dev. Cell 2005, 8, 565–574. [Google Scholar] [CrossRef]
- Burmeister, M.; Novak, J.; Liang, M.-Y.; Basu, S.; Ploder, L.; Hawes, N.L.; Vidgen, D.; Hoover, F.; Goldman, D.; Kalnins, V.I.; et al. Ocular retardation mouse caused by chx10 homeobox null allele: Impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 1996, 12, 376–384. [Google Scholar] [CrossRef]
- Goodson, N.B.; Kaufman, M.A.; Park, K.U.; Brzezinski, J.A.T. Simultaneous deletion of prdm1 and vsx2 enhancers in the retina alters photoreceptor and bipolar cell fate specification, yet differs from deleting both genes. Development 2020, 147, dev190272. [Google Scholar] [CrossRef]
- Bachmann, G.; Frohns, F.; Thangaraj, G.; Bausch, A.; Layer, P.G. Ipl sublamination in chicken retinal spheroids is initiated via müller cells and cholinergic differentiation, and is disrupted by nmda signaling. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4759–4773. [Google Scholar] [CrossRef] [Green Version]
- Small, K.W.; DeLuca, A.P.; Whitmore, S.S.; Rosenberg, T.; Silva-Garcia, R.; Udar, N.; Puech, B.; Garcia, C.A.; Rice, T.A.; Fishman, G.A.; et al. North carolina macular dystrophy is caused by dysregulation of the retinal transcription factor prdm13. Ophthalmology 2016, 123, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Wiley, L.A.; Burnight, E.R.; DeLuca, A.P.; Anfinson, K.R.; Cranston, C.M.; Kaalberg, E.E.; Penticoff, J.A.; Affatigato, L.M.; Mullins, R.F.; Stone, E.M.; et al. Cgmp production of patient-specific ipscs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci. Rep. 2016, 6, 30742. [Google Scholar] [CrossRef]
- Finlay, B.L. The developing and evolving retina: Using time to organize form. Brain Res. 2008, 1192, 5–16. [Google Scholar] [CrossRef]
- Wang, L.; Hiler, D.; Xu, B.; Al Diri, I.; Chen, X.; Zhou, X.; Griffiths, L.; Valentine, M.; Shirinifard, A.; Sablauer, A.; et al. Retinal cell type DNA methylation and histone modifications predict reprogramming efficiency and retinogenesis in 3d organoid cultures. Cell Rep. 2018, 22, 2601–2614. [Google Scholar] [CrossRef] [Green Version]
- Hiler, D.; Chen, X.; Hazen, J.; Kupriyanov, S.; Carroll, P.A.; Qu, C.; Xu, B.; Johnson, D.; Griffiths, L.; Frase, S.; et al. Quantification of retinogenesis in 3d cultures reveals epigenetic memory and higher efficiency in ipscs derived from rod photoreceptors. Cell Stem Cell 2015, 17, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radojevic, B.; Conley, S.M.; Bennett, L.D. Adherent but Not Suspension-Cultured Embryoid Bodies Develop into Laminated Retinal Organoids. J. Dev. Biol. 2021, 9, 38. https://doi.org/10.3390/jdb9030038
Radojevic B, Conley SM, Bennett LD. Adherent but Not Suspension-Cultured Embryoid Bodies Develop into Laminated Retinal Organoids. Journal of Developmental Biology. 2021; 9(3):38. https://doi.org/10.3390/jdb9030038
Chicago/Turabian StyleRadojevic, Bojana, Shannon M. Conley, and Lea D. Bennett. 2021. "Adherent but Not Suspension-Cultured Embryoid Bodies Develop into Laminated Retinal Organoids" Journal of Developmental Biology 9, no. 3: 38. https://doi.org/10.3390/jdb9030038
APA StyleRadojevic, B., Conley, S. M., & Bennett, L. D. (2021). Adherent but Not Suspension-Cultured Embryoid Bodies Develop into Laminated Retinal Organoids. Journal of Developmental Biology, 9(3), 38. https://doi.org/10.3390/jdb9030038




