Dynein Heavy Chain 64C Differentially Regulates Cell Survival and Proliferation of Wingless-Producing Cells in Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Strains
2.2. Immunohistochemistry
2.3. Adult Wing Mounting
3. Results
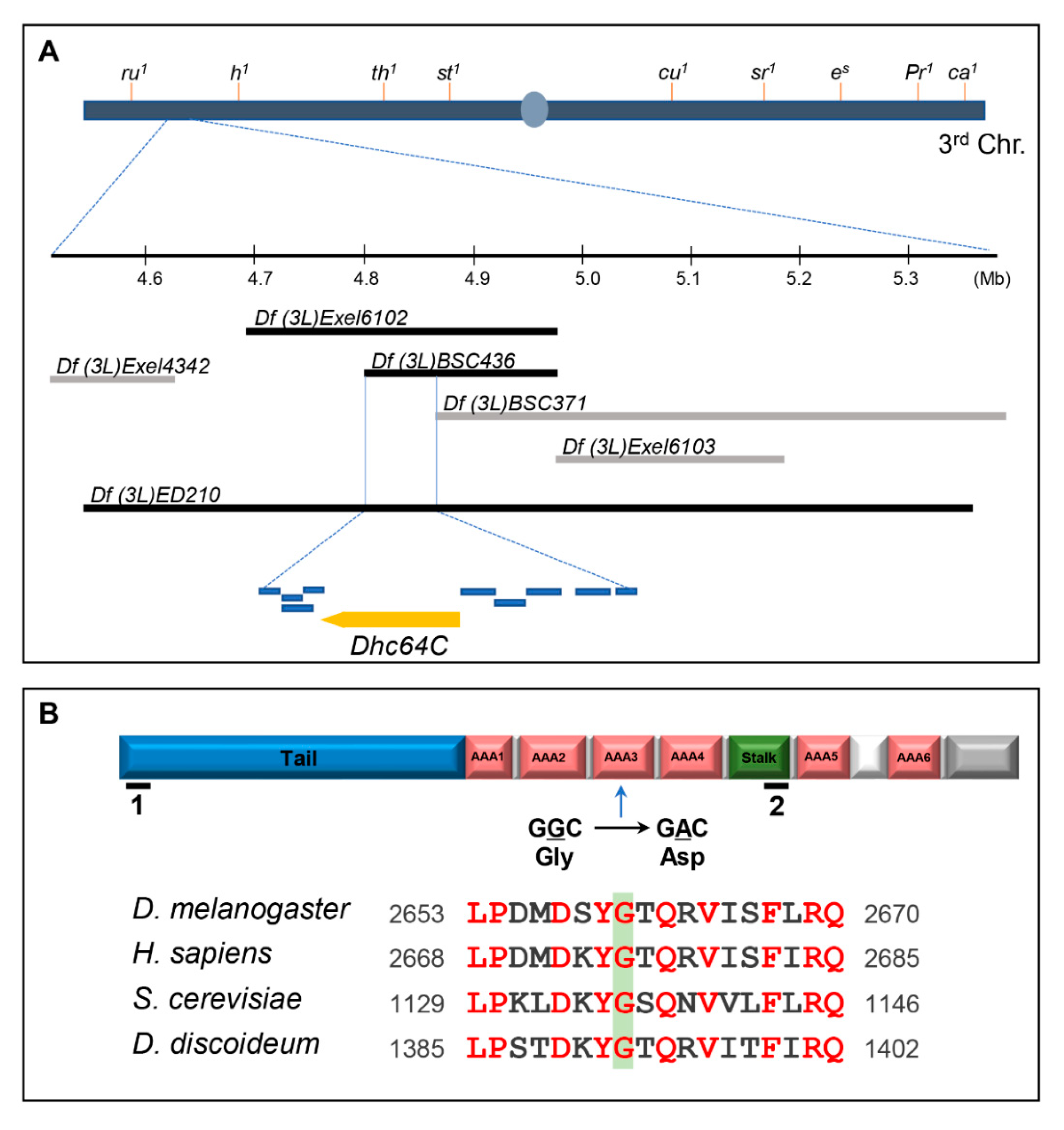
3.1. A Sona Suppressor m115 has a Mutation in the ATPases Associated with Various Cellular Activities 3 (AAA3) Domain of Dhc64C Protein
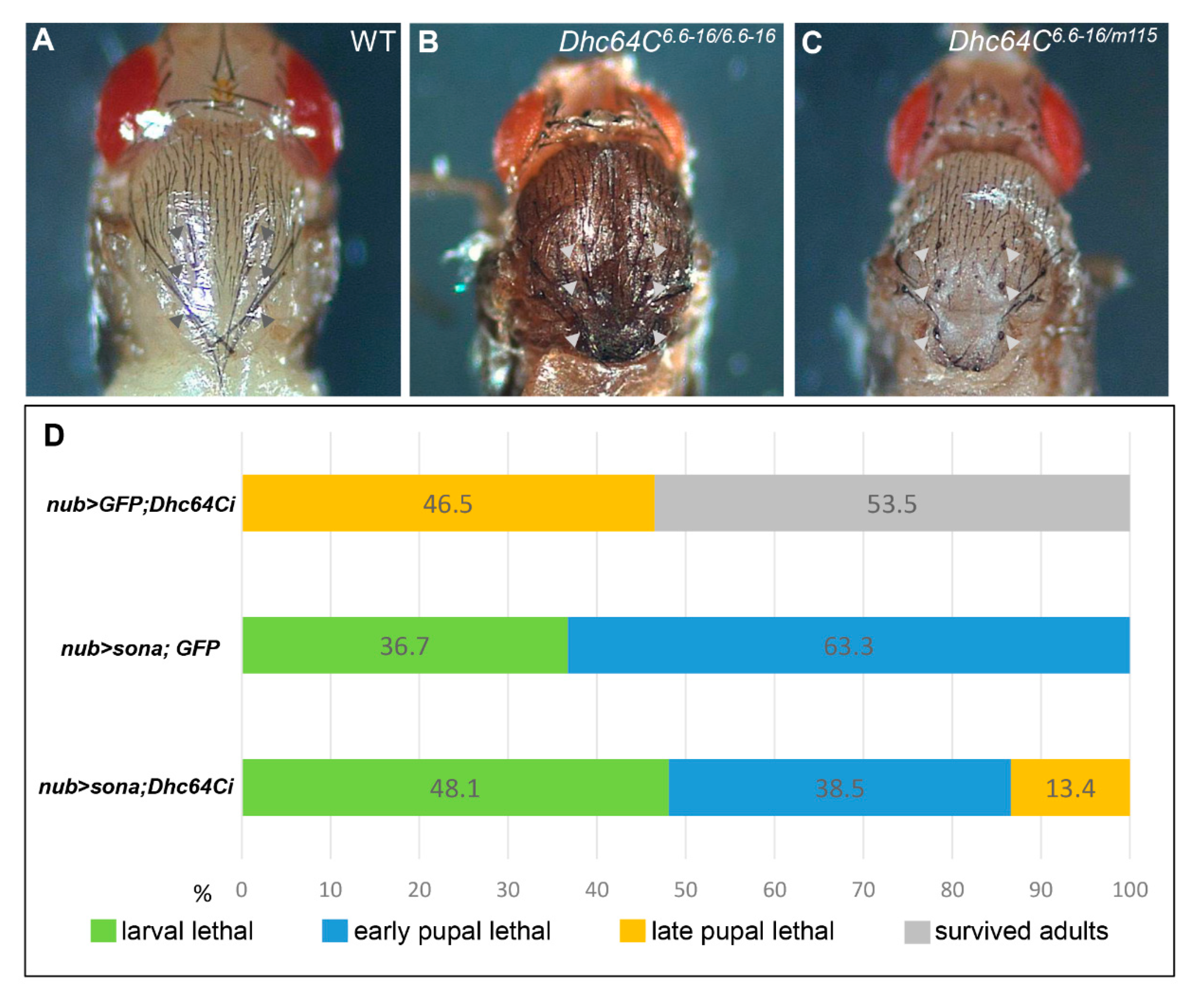
3.2. Knockdown of Dhc64C Reduces the Severity of Sona-Induced Lethality
3.3. Knockdown of Dhc64C Induces Apoptosis
3.4. Knockdown of Dhc64C Induces Overproliferation in a Non-Cell Autonomous Manner
3.5. The Knockdown of Dhc64C Increases the Level of Wg in the Entire Wing Pouch
3.6. Knockdown of Dhc64C Induces Cell Death of Wg-Expressing Cells in the Apical Region
3.7. Knockdown of Dhc64C Does Not Induce Cell Death but Induces Overproliferation of Wg-Expressing Cells in the Inner Hinge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, H.D.; Gorenc, T.; Steller, H. Apoptotic Cells Can Induce Compensatory Cell Proliferation through the JNK and the Wingless Signaling Pathways. Dev. Cell 2004, 7, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Baehrecke, E.H.; Thummel, C.S. Steroid regulated programmed cell death during Drosophila metamorphosis. Development 1997, 124, 4673–4683. [Google Scholar] [CrossRef] [PubMed]
- Pazdera, T.M.; Janardhan, P.; Minden, J.S. Patterned epidermal cell death in wild-type and segment polarity mutant Drosophila embryos. Development 1998, 125, 3427–3436. [Google Scholar] [CrossRef] [PubMed]
- Wolff, T.; Ready, D.F. Cell death in normal and rough eye mutants of Drosophila. Development 1991, 113, 825–839. [Google Scholar] [CrossRef]
- Karpen, G.H.; Schubiger, G. Extensive regulatory capabilities of a Drosophila imaginal disk blastema. Nature 1981, 294, 744–747. [Google Scholar] [CrossRef]
- El Hour, M.; Moncada-Pazos, A.; Blacher, S.; Masset, A.; Cal, S.; Berndt, S.; Detilleux, J.; Host, L.; Obaya, A.J.; Maillard, C.; et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene 2010, 29, 3025–3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, P.H.Y.; Lung, H.L.; Cheung, A.K.L.; Apte, S.S.; Chan, K.W.; Kwong, F.M.; Ko, J.M.Y.; Cjeng, Y.; Law, S.; Srivastava, G.; et al. Extracellular protease ADAMTS9 suppresses esophageal and nasopharyngeal carcinoma tumor formation by inhibiting angiogenesis. Cancer Res. 2010, 70, 5567–5576. [Google Scholar] [CrossRef] [Green Version]
- Moncada-Pazos, A.; Obaya, A.J.; Fraga, M.F.; Viloria, C.G.; Capellá, G.; Gausachs, M.; Esteller, M.; López-Otín, C.; Cal, S. The ADAMTS12 metalloprotease gene is epigenetically silenced in tumor cells and transcriptionally activated in the stroma during progression of colon cancer. J. Cell Sci. 2009, 122, 2906–2913. [Google Scholar] [CrossRef] [Green Version]
- Rocks, N.; Paulissen, G.; El Hour, M.; Quesada, F.; Crahay, C.; Gueders, M.; Foidart, J.; Noel, A.; Cataldo, D. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie 2008, 90, 369–379. [Google Scholar] [CrossRef]
- Kim, G.-W.; Won, J.-H.; Lee, O.-K.; Lee, S.-S.; Han, J.-H.; Tsogtbaatar, O.; Nam, S.; Kim, Y.; Cho, K.-O. Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling. Sci. Rep. 2016, 6, 31863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsogtbaatar, O.; Won, J.H.; Kim, G.W.; Han, J.H.; Bae, Y.K.; Cho, K.O. An ADAMTS Sol narae is required for cell survival in Drosophila. Sci. Rep. 2019, 9, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.-H.; Kim, Y.; Cho, K.-O. Exosomal arrow (Arr)/lipoprotein receptor protein 6 (LRP6) in Drosophila melanogaster increases the extracellular level of Sol narae (Sona) in a Wnt-independent manner. Cell Death Dis. 2020, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, K.-O. POU domain motif3 (Pdm3) induces wingless (wg) transcription and is essential for development of larval neuromuscular junctions in Drosophila. Sci. Rep. 2020, 10, 517. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.; Cho, K.-O. Wingless and Archipelago, a fly E3 ubiquitin ligase and a homolog of human tumor suppressor FBW7, show an antagonistic relationship in wing development. BMC Dev. Biol. 2020, 20, 14. [Google Scholar] [CrossRef]
- Cho, D.-G.; Lee, S.-S.; Cho, A.K.-O. Anastral Spindle 3/Rotatin Stabilizes Sol narae and Promotes Cell Survival in Drosophila melanogaster. Mol. Cells 2021, 44, 13–25. [Google Scholar] [CrossRef]
- Asai, D.J.; Koonce, M.P. The dynein heavy chain: Structure, mechanics and evolution. Trends Cell Biol. 2001, 11, 196–202. [Google Scholar] [CrossRef]
- Asai, D.J.; Wilkes, D.E. The dynein heavy chain family. J. Eukaryot. Microbiol. 2004, 51, 23–29. [Google Scholar] [CrossRef]
- McLean, I.W.; Nakane, P.K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 1974, 22, 1077–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.-O.; Chern, J.; Izaddoost, S.; Choi, K.-W. Novel Signaling from the Peripodial Membrane Is Essential for Eye Disc Patterning in Drosophila. Cell 2000, 103, 331–342. [Google Scholar] [CrossRef] [Green Version]
- DeWitt, M.A.; Cypranowska, C.A.; Cleary, F.B.; Belyy, V.; Yildiz, A. The AAA3 domain of cytoplasmic dynein acts as a switch to facilitate microtubule release. Nat. Struct. Mol. Biol. 2015, 22, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Bitan, A.; Guild, G.M.; Bar-Dubin, D.; Abdu, U. Asymmetric Microtubule Function Is an Essential Requirement for Polarized Organization of the Drosophila Bristle. Mol. Cell. Biol. 2010, 30, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Jack, J.; Dorsett, D.; DeLotto, Y.; Liu, S. Expression of the cut locus in the Drosophila wing margin is required for cell type specification and is regulated by a distant enhancer. Development 1991, 113, 735–747. [Google Scholar] [CrossRef]
- Seto, E.S.; Bellen, H.J. Internalization is required for proper Wingless signaling in Drosophila melanogaster. J. Cell Biol. 2006, 173, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Won, J.-H.; Kim, G.-W.; Kim, J.-Y.; Cho, D.-G.; Kwon, B.; Bae, Y.-K.; Cho, K.-O. ADAMTS Sol narae cleaves extracellular Wingless to generate a novel active form that regulates cell proliferation in Drosophila. Cell Death Dis. 2019, 10, 564. [Google Scholar] [CrossRef] [Green Version]
- Garijo, A.P.; Shlevkov, E.; Morata, G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development 2009, 136, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Batlevi, Y.; Martin, D.N.; Pandey, U.B.; Simon, C.R.; Powers, C.M.; Taylor, J.P.; Baehrecke, E.H. Dynein light chain 1 is required for autophagy, protein clearance, and cell death in Drosophila. Proc. Natl. Acad. Sci. USA 2010, 107, 742–747. [Google Scholar] [CrossRef] [Green Version]
- Dick, T.; Ray, K.; Salz, H.K.; Chia, W. Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. Mol. Cell. Biol. 1996, 16, 1966–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, A.L.; Kim, H.; Srinivasan, D.; Lakonishok, M.; Larson, A.G.; Gelfand, V.I. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc. Natl. Acad. Sci. USA 2010, 107, 12151–12156. [Google Scholar] [CrossRef] [Green Version]
- Saxton, W.M.; Hicks, J.; Goldstein, L.S.; Raff, E.C. Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell 1991, 64, 1093–1102. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Nam, S.-C.; Choi, K.-W. Kinesin II is required for cell survival and adherens junction positioning in Drosophila photoreceptors. Genesis 2010, 48, 522–530. [Google Scholar] [CrossRef]
- Ray, K.; Perez, S.E.; Yang, Z.; Xu, J.; Ritchings, B.W.; Steller, H.; Goldstein, L.S. Kinesin-II Is Required for Axonal Transport of Choline Acetyltransferase in Drosophila. J. Cell Biol. 1999, 147, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, S.C.; Martín, R.; Morata, G. Tissue Homeostasis in the Wing Disc of Drosophila melanogaster: Immediate Response to Massive Damage during Development. PLoS Genet. 2013, 9, e1003446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verghese, S.; Su, T.T. Drosophila Wnt and STAT Define Apoptosis-Resistant Epithelial Cells for Tissue Regeneration after Irradiation. PLoS Biol. 2016, 14, e1002536. [Google Scholar] [CrossRef]
- Ballesteros-Arias, L.; Saavedra, V.; Morata, G. Cell competition may function either as tumour-suppressing or as tumour-stimulating factor in Drosophila. Oncogene 2014, 33, 4377–4384. [Google Scholar] [CrossRef] [Green Version]
- Marois, E.; Mahmoud, A.; Eaton, S. The endocytic pathway and formation of the Wingless morphogen gradient. Development 2006, 133, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Satoh, D.; Sato, D.; Tsuyama, T.; Saito, M.; Ohkura, H.; Rolls, M.; Ishikawa, F.; Uemura, T. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nature 2008, 10, 1164–1171. [Google Scholar] [CrossRef]
- Lin, S.X.; A Collins, C. Immunolocalization of cytoplasmic dynein to lysosomes in cultured cells. J. Cell Sci. 1992, 101, 125–137. [Google Scholar] [CrossRef]
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nature 2012, 14, 1036–1045. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Tsogtbaatar, O.; Cho, K.-O. Dynein Heavy Chain 64C Differentially Regulates Cell Survival and Proliferation of Wingless-Producing Cells in Drosophila melanogaster. J. Dev. Biol. 2021, 9, 43. https://doi.org/10.3390/jdb9040043
Kim J-Y, Tsogtbaatar O, Cho K-O. Dynein Heavy Chain 64C Differentially Regulates Cell Survival and Proliferation of Wingless-Producing Cells in Drosophila melanogaster. Journal of Developmental Biology. 2021; 9(4):43. https://doi.org/10.3390/jdb9040043
Chicago/Turabian StyleKim, Ja-Young, Orkhon Tsogtbaatar, and Kyung-Ok Cho. 2021. "Dynein Heavy Chain 64C Differentially Regulates Cell Survival and Proliferation of Wingless-Producing Cells in Drosophila melanogaster" Journal of Developmental Biology 9, no. 4: 43. https://doi.org/10.3390/jdb9040043
APA StyleKim, J.-Y., Tsogtbaatar, O., & Cho, K.-O. (2021). Dynein Heavy Chain 64C Differentially Regulates Cell Survival and Proliferation of Wingless-Producing Cells in Drosophila melanogaster. Journal of Developmental Biology, 9(4), 43. https://doi.org/10.3390/jdb9040043





