Maternal Transcripts of Hox Genes Are Found in Oocytes of Platynereis dumerilii (Annelida, Nereididae)
Abstract
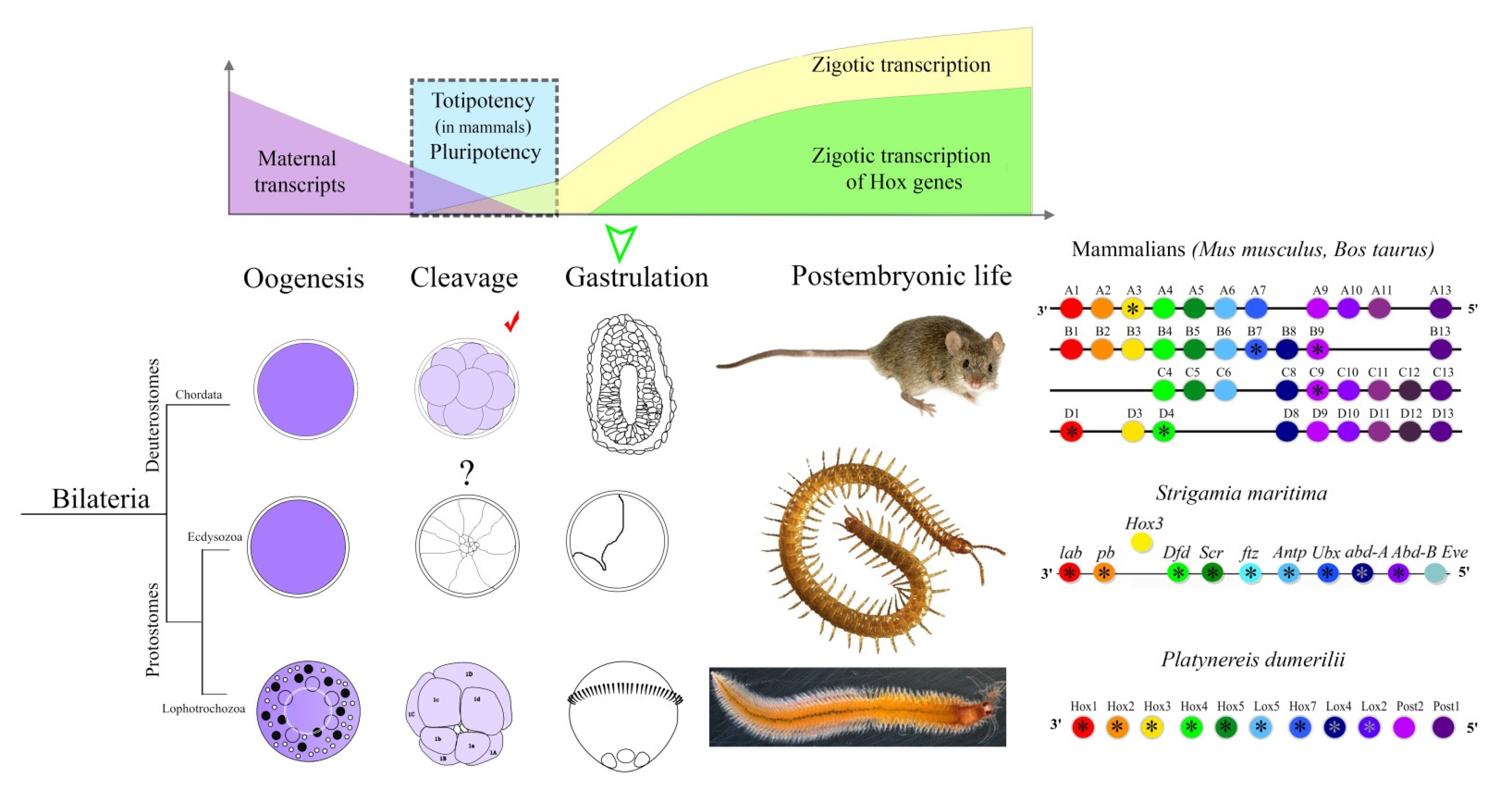
:1. Introduction
2. Materials and Methods
2.1. Collection of Material and Fixation of Oocytes for In Situ Hybridization
2.2. Isolation of RNA and Synthesis of cDNA
2.3. RT-PCR
2.4. In Situ Hybridization
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hpf | hours post fertilization |
| FPKM | fragments per kilobase per million mapped reads |
References
- Lee, J.J.; Calzone, F.J.; Britten, R.J.; Angerer, R.C.; Davidson, E.H. Activation of sea urchin actin genes during embryogenesis. Measurement of transcript accumulation from five different genes in Strongylocentrotus purpuratus. J. Mol. Biol. 1986, 188, 173–183. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Kinloch, R.A. Gene expression during oogenesis in mice. Mutat. Res. 1992, 296, 3–15. [Google Scholar] [CrossRef]
- Dalbies-Tran, R.; Cadoret, V.; Desmarchais, A.; Elis, S.; Maillard, V.; Monget, P.; Monniaux, D.; Reynaud, K.; Saint-Dizier, M.; Uzbekova, S. A Comparative Analysis of Oocyte Development in Mammals. Cells 2020, 9, 1002. [Google Scholar] [CrossRef] [Green Version]
- Akam, M. The molecular basis for metameric pattern in the Drosophila embryo. Development 1987, 101, 1–22. [Google Scholar] [CrossRef]
- Kimelman, D.; Martin, B.L. Anterior-posterior patterning in early development: Three strategies. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 253–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachs, M.; Onodera, C.; Blaschke, K.; Ebata, K.T.; Song, J.S.; Ramalho-Santos, M. Bivalent chromatin marks developmental regulatory genes in the mouse embryonic germline in vivo. Cell Rep. 2013, 3, 1777–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, D.; Bridoux, L.; Rezsöhazy, R.; Donnay, I. HOX genes are expressed in bovine and mouse oocytes and early embryos. Mol. Reprod. Dev. 2011, 78, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, K.; Dorresteijn, A.W.; Fröbius, A.C. Activation of Hox genes during caudal regeneration of the polychaete annelid Platynereis dumerilii. Dev. Genes Evol. 2012, 222, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, M.; Bakalenko, N.; Novikova, E.; Cook, C.E.; Eliseeva, E.; Steinmetz, P.R.; Kostyuchenko, R.P.; Dondua, A.; Arendt, D.; Akam, M.; et al. Hox gene expression in larval development of the polychaetes Nereis virens and Platynereis dumerilii (Annelida, Lophotrochozoa). Dev. Genes Evol. 2007, 217, 39–54. [Google Scholar] [CrossRef]
- Irvine, S.Q.; Chaga, O.; Martindale, M.Q. Larval ontogenetic stages of Chaetopterus: Developmental heterochrony in the evolution of chaetopterid polychaetes. Biol. Bull. 1999, 197, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Dorresteijn, A.W. Quantitative analysis of cellular differentiation during early embryogenesis of Platynereis dumerilii. Roux’s Arch. Dev. Biol. 1990, 199, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, S.; Shu, W.; Guo, Y.; Guan, Y.; Zeng, J.; Wang, H.; Han, L.; Zhang, J.; Liu, X.; et al. Characterization of Metabolic Patterns in Mouse Oocytes during Meiotic Maturation. Mol. Cell. 2020, 80, 525–540.e9. [Google Scholar] [CrossRef]
- Dobrzycki, T.; Krecsmarik, M.; Monteiro, R. Genotyping and Quantification of In Situ Hybridization Staining in Zebrafish. J. Vis. Exp. 2020, 155, e59956. [Google Scholar] [CrossRef] [PubMed]
- Barucca, M.; Canapa, A.; Biscotti, M.A. An Overview of Hox Genes in Lophotrochozoa: Evolution and Functionality. J. Dev. Biol. 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- de Rosa, R.; Grenier, J.K.; Andreeva, T.; Cook, C.E.; Adoutte, A.; Akam, M.; Carroll, S.B.; Balavoine, G. Hox genes in brachiopods and priapulids and protostome evolution. Nature 1999, 399, 772–776. [Google Scholar] [CrossRef]
- Raible, F.; Tessmar-Raible, K.; Osoegawa, K.; Wincker, P.; Jubin, C.; Balavoine, G.; Ferrier, D.; Benes, V.; de Jong, P.; Weissenbach, J.; et al. Vertebrate-type intron-rich genes in the marine annelid Platynereis dumerilii. Science 2005, 310, 1325–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessmar-Raible, K.; Raible, F.; Christodoulou, F.; Guy, K.; Rembold, M.; Hausen, H.; Arendt, D. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: Insights into hypothalamus evolution. Cell 2007, 129, 1389–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denes, A.S.; Jékely, G.; Steinmetz, P.R.; Raible, F.; Snyman, H.; Prud’homme, B.; Ferrier, D.E.; Balavoine, G.; Arendt, D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 2007, 129, 277–288. [Google Scholar] [CrossRef]
- Arendt, D.; Tessmar, K.; de Campos-Baptista, M.I.; Dorresteijn, A.; Wittbrodt, J. Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in Bilateria. Development 2002, 129, 1143–1154. [Google Scholar] [CrossRef]
- Chou, H.C.; Pruitt, M.M.; Bastin, B.R.; Schneider, S.Q. A transcriptional blueprint for a spiral-cleaving embryo. BMC Genom. 2016, 17, 552. [Google Scholar] [CrossRef] [Green Version]
- Sauvegarde, C.; Paul, D.; Bridoux, L.; Jouneau, A.; Degrelle, S.; Hue, I.; Rezsohazy, R.; Donnay, I. Dynamic Pattern of HOXB9 Protein Localization during Oocyte Maturation and Early Embryonic Development in Mammals. PLoS ONE 2016, 11, e0165898. [Google Scholar] [CrossRef]
- Chipman, A.D.; Ferrier, D.E.; Brena, C.; Qu, J.; Hughes, D.S.; Schröder, R.; Torres-Oliva, M.; Znassi, N.; Jiang, H.; Almeida, F.C.; et al. The first myriapod genome sequence reveals conservative arthropod gene content and genome organisation in the centipede Strigamia maritima. PLoS Biol. 2014, 12, e1002005. [Google Scholar] [CrossRef] [Green Version]
- Tadros, W.; Lipshitz, H.D. The maternal-to-zygotic transition: A play in two acts. Development 2009, 136, 3033–3042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, K.N.; Harrison, M.M. Mechanisms regulating zygotic genome activation. Nat. Rev. Genet. 2019, 20, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Banreti, A.; Hudry, B.; Sass, M.; Saurin, A.J.; Graba, Y. Hox proteins mediate developmental and environmental control of autophagy. Dev. Cell. 2014, 28, 56–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnello, M.; Chiarelli, R.; Martino, C.; Bosco, L.; Roccheri, M.C. Autophagy is required for sea urchin oogenesis and early development. Zygote 2016, 24, 918–926. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslakov, G.P.; Kulishkin, N.S.; Surkova, A.A.; Kulakova, M.A. Maternal Transcripts of Hox Genes Are Found in Oocytes of Platynereis dumerilii (Annelida, Nereididae). J. Dev. Biol. 2021, 9, 37. https://doi.org/10.3390/jdb9030037
Maslakov GP, Kulishkin NS, Surkova AA, Kulakova MA. Maternal Transcripts of Hox Genes Are Found in Oocytes of Platynereis dumerilii (Annelida, Nereididae). Journal of Developmental Biology. 2021; 9(3):37. https://doi.org/10.3390/jdb9030037
Chicago/Turabian StyleMaslakov, Georgy P., Nikita S. Kulishkin, Alina A. Surkova, and Milana A. Kulakova. 2021. "Maternal Transcripts of Hox Genes Are Found in Oocytes of Platynereis dumerilii (Annelida, Nereididae)" Journal of Developmental Biology 9, no. 3: 37. https://doi.org/10.3390/jdb9030037
APA StyleMaslakov, G. P., Kulishkin, N. S., Surkova, A. A., & Kulakova, M. A. (2021). Maternal Transcripts of Hox Genes Are Found in Oocytes of Platynereis dumerilii (Annelida, Nereididae). Journal of Developmental Biology, 9(3), 37. https://doi.org/10.3390/jdb9030037





