Metabolism in the Zebrafish Retina
Abstract
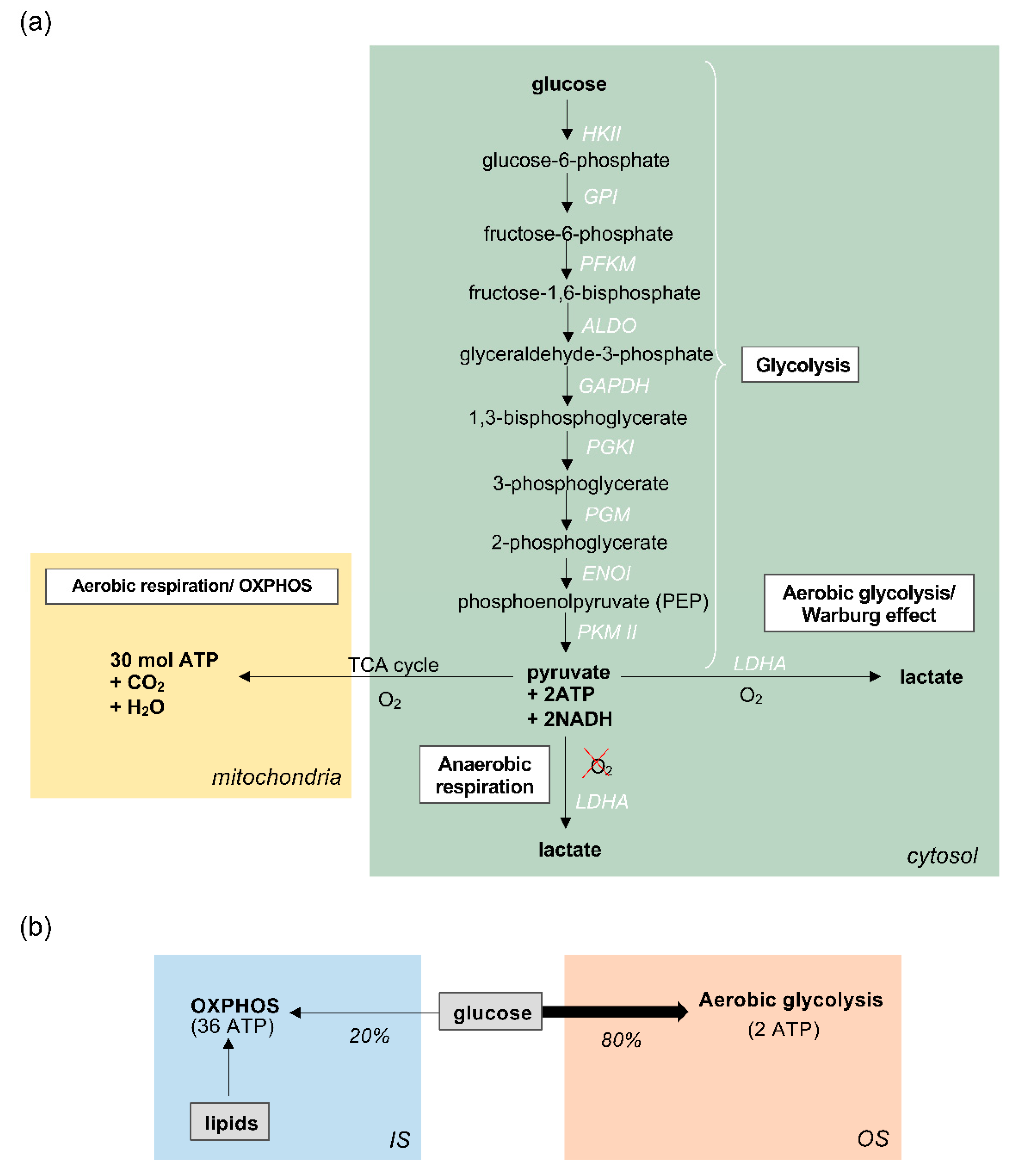
1. Introduction
2. The Zebrafish Retina
2.1. Advantages of the Zebrafish as a Model Organism to Study Retinal Metabolism and Disease
2.2. Conserved Structure of the Zebrafish Retina
2.3. Zebrafish Photoreceptors
2.4. Mitochondria in Zebrafish Photoreceptors
3. Metabolism in Photoreceptors
3.1. Energy Production in Zebrafish Photoreceptors
3.2. The role of Aerobic Glycolysis in Photoreceptors
3.3. Metabolic Changes in Light and Darkness
4. Metabolism in the Retinal Pigmented Epithelium (RPE) and Müller Glia
5. Metabolic Imbalance and Retinal Degeneration
5.1. Retinitis Pigmentosa (RP)
5.2. Leber Congenital Amaurosis (LCA)
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ames, A.; Li, Y.Y.; Heher, E.C.; Kimble, C.R. Energy metabolism of rabbit retina as related to function: High cost of Na+ transport. J. Neurosci. 1992. [Google Scholar] [CrossRef]
- Narayan, D.S.; Chidlow, G.; Wood, J.P.M.; Casson, R.J. Glucose metabolism in mammalian photoreceptor inner and outer segments. Clin. Exp. Ophthalmol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.J.; Dowling, J.E. The Retina: An Approachable Part of the Brain. Am. J. Psychol. 1988. [Google Scholar] [CrossRef]
- Country, M.W. Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kanow, M.A.; Giarmarco, M.M.; Jankowski, C.S.R.; Tsantilas, K.; Engel, A.L.; Du, J.; Linton, J.D.; Farnsworth, C.C.; Sloat, S.R.; Rountree, A.; et al. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. eLife 2017. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci. Res. 2015. [Google Scholar] [CrossRef]
- Winkler, B.S. Glycolytic and oxidative metabolism in relation to retinal function. J. Gen. Physiol. 1981. [Google Scholar] [CrossRef] [PubMed]
- Chinchore, Y.; Begaj, T.; Wu, D.; Drokhlyansky, E.; Cepko, C.L. Glycolytic reliance promotes anabolism in photoreceptors. eLife 2017. [Google Scholar] [CrossRef]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye 2017. [Google Scholar] [CrossRef] [PubMed]
- Avanesov, A.; Malicki, J. Analysis of the Retina in the Zebrafish Model. Methods Cell Biol. 2010, 100, 153–204. [Google Scholar] [CrossRef]
- Malicki, J.; Pooranachandran, N.; Nikolaev, A.; Fang, X.; Avanesov, A. Analysis of the retina in the zebrafish model. Methods Cell Biol. 2016. [Google Scholar] [CrossRef]
- Angueyra, J.M.; Kindt, K.S. Leveraging zebrafish to study retinal degenerations. Front. Cell Dev. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a better understanding of human eye disease: Insights from the zebrafish, Danio rerio. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Stenkamp, D.L. Development of the Vertebrate Eye and Retina. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Goldsmith, P.; Harris, W.A. The zebrafish as a tool for understanding the biology of visual disorders. Semin. Cell Dev. Biol. 2003. [Google Scholar] [CrossRef]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A model system for the study of eye genetics. Prog. Retin. Eye Res. 2008. [Google Scholar] [CrossRef]
- Raymond, P.A.; Colvin, S.M.; Jabeen, Z.; Nagashima, M.; Barthel, L.K.; Hadidjojo, J.; Popova, L.; Pejaver, V.R.; Lubensky, D.K. Patterning the cone mosaic array in zebrafish retina requires specification of ultraviolet-sensitive cones. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Easter, S.S.; Nicola, G.N. The development of vision in the zebrafish (Danio rerio). Dev. Biol. 1996. [Google Scholar] [CrossRef]
- Gestri, G.; Link, B.A.; Neuhauss, S.C.F. The visual system of zebrafish and its use to model human ocular Diseases. Dev. Neurobiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.C. The genetics of ocular disorders: Insights from the zebrafish. Birth Defects Res. Part C Embryo Today Rev. 2011. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, P.; Mills-Henry, I.; Padmanabhan, K.R.; Pascuzzi, P.; Hassan, M.; Zhang, J.; Zhang, X.; Ma, P.; Pang, C.P.; Dowling, J.E.; et al. Rods contribute to visual behavior in larval zebrafish. Investig. Ophthalmol. Vis. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Moosajee, M.; Gregory-Evans, K.; Ellis, C.D.; Seabra, M.C.; Gregory-Evans, C.Y. Translational bypass of nonsense mutations in zebrafish rep1, pax2.1 and lamb1 highlights a viable therapeutic option for untreatable genetic eye disease. Hum. Mol. Genet. 2008. [Google Scholar] [CrossRef]
- Ganzen, L.; Venkatraman, P.; Pang, C.P.; Leung, Y.F.; Zhang, M. Utilizing zebrafish visual behaviors in drug screening for retinal degeneration. Int. J. Mol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gollisch, T.; Meister, M. Eye Smarter than Scientists Believed: Neural Computations in Circuits of the Retina. Neuron 2010. [Google Scholar] [CrossRef]
- Tarboush, R.; Chapman, G.B.; Connaughton, V.P. Ultrastructure of the distal retina of the adult zebrafish, Danio rerio. Tissue Cell 2012. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, E.; Chang, B.S.; Oh, C.S.; Mun, G.H.; Chung, Y.H.; Shin, D.H. The presence of megamitochondria in the ellipsoid of photoreceptor inner segment of the zebrafish retina. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2005. [Google Scholar] [CrossRef]
- Robinson, J.; Schmitt, E.A.; Hárosi, F.I.; Reece, R.J.; Dowling, J.E. Zebrafish ultraviolet visual pigment: Absorption spectrum, sequence, and localization. Proc. Natl. Acad. Sci. USA 1993. [Google Scholar] [CrossRef]
- Nawrocki, L.; Bremiller, R.; Streisinger, G.; Kaplan, M. Larval and adult visual pigments of the zebrafish, Brachydanio rerio. Vision Res. 1985. [Google Scholar] [CrossRef]
- Zimmermann, M.J.Y.; Nevala, N.E.; Yoshimatsu, T.; Osorio, D.; Nilsson, D.E.; Berens, P.; Baden, T. Zebrafish Differentially Process Color across Visual Space to Match Natural Scenes. Curr. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Salbreux, G.; Barthel, L.K.; Raymond, P.A.; Lubensky, D.K. Coupling Mechanical Deformations and Planar Cell Polarity to Create Regular Patterns in the Zebrafish Retina. PLoS Comput. Biol. 2012. [Google Scholar] [CrossRef]
- Patterson, B.W.; Abraham, A.O.; MacIver, M.A.; McLean, D.L. Visually guided gradation of prey capture movements in larval zebrafish. J. Exp. Biol. 2013. [Google Scholar] [CrossRef]
- Yoshimatsu, T.; Schröder, C.; Nevala, N.E.; Berens, P.; Baden, T. Fovea-like Photoreceptor Specializations Underlie Single UV Cone Driven Prey-Capture Behavior in Zebrafish. Neuron 2020. [Google Scholar] [CrossRef]
- Hagerman, G.F.; Noel, N.C.L.; Cao, S.Y.; DuVal, M.G.; Oel, A.P.; Allison, W.T. Rapid recovery of visual function associated with blue cone ablation in Zebrafish. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Endeman, D.; Klaassen, L.J.; Kamermans, M. Action Spectra of Zebrafish Cone Photoreceptors. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Allison, W.T.; Haimberger, T.J.; Hawryshyn, C.W.; Temple, S.E. Visual pigment composition in zebrafish: Evidence for a rhodopsin-porphyropsin interchange system. Vis. Neurosci. 2004. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Nelson, R.; Connaughton, V.P. Color processing in zebrafish retina. Front. Cell. Neurosci. 2018. [Google Scholar] [CrossRef]
- Branchek, T.; Bremiller, R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J. Comp. Neurol. 1984. [Google Scholar] [CrossRef]
- Cameron, D.A. Mapping absorbance spectra, cone fractions, and neuronal mechanisms to photopic spectral sensitivity in the zebrafish. Vis. Neurosci. 2002. [Google Scholar] [CrossRef] [PubMed]
- Carter-Dawson, L.D.; Lavail, M.M. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 1979. [Google Scholar] [CrossRef]
- Kostic, C.; Arsenijevic, Y. Animal modelling for inherited central vision loss. J. Pathol. 2016. [Google Scholar] [CrossRef]
- Cameron, D.A.; Carney, L.H. Cell mosaic patterns in the native and regenerated inner retina of zebrafish: Implications for retinal assembly. J. Comp. Neurol. 2000. [Google Scholar] [CrossRef]
- Fadool, J.M. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev. Biol. 2003. [Google Scholar] [CrossRef]
- Stenkamp, D.L. Neurogenesis in the Fish Retina. Int. Rev. Cytol. 2007. [Google Scholar] [CrossRef]
- Huckenpahler, A.L.; Wilk, M.A.; Cooper, R.F.; Moehring, F.; Link, B.A.; Carroll, J.; Collery, R.F. Imaging the adult zebrafish cone mosaic using optical coherence tomography. Vis. Neurosci. 2016. [Google Scholar] [CrossRef]
- Perkins, B.D.; Fadool, J.M.; Dowling, J.E. Photoreceptor structure and development: Analyses using GFP transgenes. Methods Cell Biol. 2004. [Google Scholar] [CrossRef]
- Raymond, P.A.; Barthel, L.K. A moving wave patterns the cone photoreceptor mosaic array in the zebrafish retina. Int. J. Dev. Biol. 2004. [Google Scholar] [CrossRef]
- Allison, W.T.; Barthel, L.K.; Skebo, K.M.; Takechi, M.; Kawamura, S.; Raymond, P.A. Ontogeny of cone photoreceptor mosaics in zebrafish. J. Comp. Neurol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Tarboush, R.; Novales Flamarique, I.; Chapman, G.B.; Connaughton, V.P. Variability in mitochondria of zebrafish photoreceptor ellipsoids. Vis. Neurosci. 2014. [Google Scholar] [CrossRef]
- Masuda, T.; Wada, Y.; Kawamura, S. ES1 is a mitochondrial enlarging factor contributing to form mega-mitochondria in zebrafish cones. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M. Energy metabolism of the visual system. Eye Brain 2010. [Google Scholar] [CrossRef]
- Cohen, L.H.; Noell, W.K. Glucose Catabolism of Rabbit Retina Before and After Development of Visual Function. J. Neurochem. 1960, 5, 253–276. [Google Scholar] [CrossRef]
- Zou, C.; Wang, Y.; Shen, Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J. Biochem. Biophys. Methods 2005. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, D.W.; Kim, W.H.; Um, J.I.; Yim, S.H.; Oh, W.K.; Williams, D.R. Development of a highly visual, simple, and rapid test for the discovery of novel insulin mimetics in living vertebrates. ACS Chem. Biol. 2013. [Google Scholar] [CrossRef]
- Tabassum, N.; Tai, H.; Jung, D.W.; Williams, D.R. Fishing for Nature’s Hits: Establishment of the Zebrafish as a Model for Screening Antidiabetic Natural Products. Evidence-based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Giarmarco, M.; Kanow, M.A.; Lindsay, K.J.; Du, J.; Hurley, J. Confocal imaging reveals glucose uptake by photoreceptors in vivo. Invest. Ophthalmol. Vis. Sci. 2016, 57, 1760. [Google Scholar]
- Hantzidiamantis, P.J.; Lappin, S.L. Physiology, Glucose; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lindsay, K.J.; Du, J.; Sloat, S.R.; Contreras, L.; Linton, J.D.; Turner, S.J.; Sadilek, M.; Satrústegui, J.; Hurley, J.B. Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc. Natl. Acad. Sci. USA 2014. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Rountree, A.; Cleghorn, W.M.; Contreras, L.; Lindsay, K.J.; Sadilek, M.; Gu, H.; Djukovic, D.; Raftery, D.; Satrústegui, J.; et al. Phototransduction influences metabolic flux and nucleotide metabolism in mouse retina. J. Biol. Chem. 2016. [Google Scholar] [CrossRef]
- Medrano, C.J.; Fox, D.A. Oxygen consumption in the rat outer and inner retina: Light- and pharmacologically-induced inhibition. Exp. Eye Res. 1995. [Google Scholar] [CrossRef]
- Otto Warburg, B.; Wind, F.; Negelein, N. The metabolism of tumours in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016. [Google Scholar] [CrossRef]
- Léveillard, T. Cancer metabolism of cone photoreceptors. Oncotarget 2015. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Törnquist, P.; Bill, A. Glucose metabolism of the inner retina in pigs in darkness and light. Acta Physiol. Scand. 1997. [Google Scholar] [CrossRef]
- Wang, L.; Törnquist, P.; Bill, A. Glucose metabolism in pig outer retina in light and darkness. Acta Physiol. Scand. 1997. [Google Scholar] [CrossRef]
- Wang, L.; Kondo, M.; Bill, A. Glucose metabolism in cat outer retina: Effects of light and hyperoxia. Investig. Ophthalmol. Vis. Sci. 1997, 38, 48–55. [Google Scholar]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013. [Google Scholar] [CrossRef]
- Joyal, J.S.; Sun, Y.; Gantner, M.L.; Shao, Z.; Evans, L.P.; Saba, N.; Fredrick, T.; Burnim, S.; Kim, J.S.; Patel, G.; et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 2016. [Google Scholar] [CrossRef]
- Lowry, O.H.; Roberts, N.R.; Lewis, C. The quantitative histochemistry of the retina. J. Biol. Chem. 1956. [Google Scholar] [CrossRef]
- Calzia, D.; Garbarino, G.; Caicci, F.; Pestarino, M.; Manni, L.; Traverso, C.E.; Panfoli, I.; Candiani, S. Evidence of Oxidative Phosphorylation in Zebrafish Photoreceptor Outer Segments at Different Larval Stages. J. Histochem. Cytochem. 2018. [Google Scholar] [CrossRef]
- Gospe, S.M.; Baker, S.A.; Arshavsky, V.Y. Facilitative glucose transporter Glut1 is actively excluded from rod outer segments. J. Cell Sci. 2010. [Google Scholar] [CrossRef] [PubMed]
- Chertov, A.O.; Holzhausen, L.; Kuok, I.T.; Couron, D.; Parker, E.; Linton, J.D.; Sadilek, M.; Sweet, I.R.; Hurley, J.B. Roles of glucose in photoreceptor survival. J. Biol. Chem. 2011. [Google Scholar] [CrossRef]
- Ng, S.K.; Wood, J.P.M.; Chidlow, G.; Han, G.; Kittipassorn, T.; Peet, D.J.; Casson, R.J. Cancer-like metabolism of the mammalian retina. Clin. Exp. Ophthalmol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, F.; Safa, H.; Finnemann, S.C. Understanding photoreceptor outer segment phagocytosis: Use and utility of RPE cells in culture. Exp. Eye Res. 2014. [Google Scholar] [CrossRef]
- Kevany, B.M.; Palczewski, K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 2010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Justus, S.; Hsu, C.W.; Bonet-Ponce, L.; Wu, W.H.; Tsai, Y.T.; Wu, W.P.; Jia, Y.; Duong, J.K.; et al. Reprogramming metabolism by targeting sirtuin 6 attenuates retinal degeneration. J. Clin. Investig. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rajala, R.V.S.; Rajala, A.; Kooker, C.; Wang, Y.; Anderson, R.E. The warburg effect mediator pyruvate kinase M2 expression and regulation in the retina. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rueda, E.M.; Johnson, J.E.; Giddabasappa, A.; Swaroop, A.; Brooks, M.J.; Sigel, I.; Chaney, S.Y.; Fox, D.A. The cellular and compartmental profile of mouse retinal glycolysis, tricarboxylic acid cycle, oxidative phosphorylation, and ~p transferring kinases. Mol. Vis. 2016, 22, 847. [Google Scholar]
- Rajala, R.V.S. Aerobic Glycolysis in the Retina: Functional Roles of Pyruvate Kinase Isoforms. Front. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fiske, B.P.; Vander Heiden, M.G. Seeing the Warburg effect in the developing retina. Nat. Cell Biol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Agathocleous, M.; Love, N.K.; Randlett, O.; Harris, J.J.; Liu, J.; Murray, A.J.; Harris, W.A. Metabolic differentiation in the embryonic retina. Nat. Cell Biol. 2012. [Google Scholar] [CrossRef]
- Linsenmeier, R.A. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J. Gen. Physiol. 1986. [Google Scholar] [CrossRef]
- Okawa, H.; Sampath, A.P.; Laughlin, S.B.; Fain, G.L. ATP Consumption by Mammalian Rod Photoreceptors in Darkness and in Light. Curr. Biol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Hagins, W.A.; Yoshikami, S. A role for Ca2+ in excitation of retinal rods and cones. Exp. Eye Res. 1974. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001. [Google Scholar] [CrossRef]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal oxygen: Fundamental and clinical aspects. Arch. Ophthalmol. 2003. [Google Scholar] [CrossRef]
- Taylor, M.R.; Van Epps, H.A.; Kennedy, M.J.; Saari, J.C.; Hurley, J.B.; Brockerhoff, S.E. Biochemical analysis of phototransduction and visual cycle in zebrafish larvae. Methods Enzymol. 2000. [Google Scholar] [CrossRef]
- Aquila, M.; Benedusi, M.; Fasoli, A.; Rispoli, G. Characterization of Zebrafish green cone photoresponse recorded with pressure-polished patch pipettes, yielding efficient intracellular dialysis. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.J.; Dunn, F.A.; Hurley, J.B. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron 2004. [Google Scholar] [CrossRef]
- Ward, R.; Sundaramurthi, H.; Di Giacomo, V.; Kennedy, B.N. Enhancing understanding of the visual cycle by applying CRISPR/Cas9 gene editing in zebrafish. Front. Cell Dev. Biol. 2018. [Google Scholar] [CrossRef]
- Goldberg, A.F.X.; Moritz, O.L.; Williams, D.S. Molecular basis for photoreceptor outer segment architecture. Prog. Retin. Eye Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gibert, Y.; McGee, S.L.; Ward, A.C. Metabolic profile analysis of zebrafish embryos. J. Vis. Exp. 2013. [Google Scholar] [CrossRef]
- Bond, S.T.; McEwen, K.A.; Yoganantharajah, P.; Gibert, Y. Live metabolic profile analysis of zebrafish embryos using a seahorse XF 24 extracellular flux analyzer. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Masland, R.H. The Neuronal Organization of the Retina. Neuron 2012. [Google Scholar] [CrossRef] [PubMed]
- Hutto, R.A.; Bisbach, C.M.; Abbas, F.; Brock, D.C.; Cleghorn, W.M.; Parker, E.D.; Bauer, B.H.; Ge, W.; Vinberg, F.; Hurley, J.B.; et al. Increasing Ca2+ in photoreceptor mitochondria alters metabolites, accelerates photoresponse recovery, and reveals adaptations to mitochondrial stress. Cell Death Differ. 2020. [Google Scholar] [CrossRef] [PubMed]
- Giarmarco, M.M.; Cleghorn, W.M.; Sloat, S.R.; Hurley, J.B.; Brockerhoff, S.E. Mitochondria maintain distinct Ca2+ pools in cone photoreceptors. J. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta Bioenerg. 2009. [Google Scholar] [CrossRef]
- Bisbach, C.M.; Hutto, R.A.; Poria, D.; Cleghorn, W.M.; Abbas, F.; Vinberg, F.; Kefalov, V.J.; Hurley, J.B.; Brockerhoff, S.E. Mitochondrial Calcium Uniporter (MCU) deficiency reveals an alternate path for Ca2+ uptake in photoreceptor mitochondria. Sci. Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.Y.; Lewis, A.; Barabas, P.; Stearns, G.; Suzuki, S.; Krizaj, D.; Brockerhoff, S.E. Loss of Pde6 reduces cell body Ca2+ transients within photoreceptors. Cell Death Dis. 2013. [Google Scholar] [CrossRef] [PubMed]
- Fain, G.L. Why photoreceptors die (and why they don’t). BioEssays 2006. [Google Scholar] [CrossRef]
- Vinberg, F.; Chen, J.; Kefalov, V.J. Regulation of calcium homeostasis in the outer segments of rod and cone photoreceptors. Prog. Retin. Eye Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.R.; Knight, K.; Engel, A.L.; Jankowski, C.; Wang, Y.; Manson, M.A.; Gu, H.; Djukovic, D.; Raftery, D.; Hurley, J.B.; et al. Human retinal pigment epithelial cells prefer proline as a nutrient and transport metabolic intermediates to the retinal side. J. Biol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. The blood-retinal barriers. Doc. Ophthalmol. 1976. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yanagida, A.; Knight, K.; Engel, A.L.; Vo, A.H.; Jankowski, C.; Sadilek, M.; Tran, V.T.B.; Manson, M.A.; Ramakrishnan, A.; et al. Reductive carboxylation is a major metabolic pathway in the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2016. [Google Scholar] [CrossRef]
- Rohrer, B.; Bandyopadhyay, M.; Beeson, C. Reduced metabolic capacity in aged primary retinal pigment epithelium (RPE) is correlated with increased susceptibility to oxidative stress. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Zhao, C.; Yasumura, D.; Li, X.; Matthes, M.; Lloyd, M.; Nielsen, G.; Ahern, K.; Snyder, M.; Bok, D.; Dunaief, J.L.; et al. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J. Clin. Investig. 2011. [Google Scholar] [CrossRef]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019. [Google Scholar] [CrossRef]
- Harik, S.I.; Kalaria, R.N.; Whitney, P.M.; Andersson, L.; Lundahl, P.; Ledbetter, S.R.; Perry, G. Glucose transporters are abundant in cells with “occluding” junctions at the blood-eye barriers. Proc. Natl. Acad. Sci. USA 1990. [Google Scholar] [CrossRef]
- Mantych, G.J.; Devaskar, S.U.; Devaskar, S.U. Characterization of glucose transporter isoforms in the adult and developing human eye. Endocrinology 1993. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.L.; Benedicto, I.; Philp, N.J.; Rodriguez-Boulan, E. Plasma membrane protein polarity and trafficking in RPE cells: Past, present and future. Exp. Eye Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Kasahara, T.; Kasahara, M.; Ezaki, O.; Hirano, H. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in cells of the blood-retinal barrier in the rat. Investig. Ophthalmol. Vis. Sci. 1992, 33, 377–383. [Google Scholar]
- Takata, K.; Kasahara, T.; Kasahara, M.; Ezaki, O.; Hirano, H. Erythrocyte/HEPG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochem. Biophys. Res. Commun. 1990. [Google Scholar] [CrossRef]
- Swarup, A.; Samuels, I.S.; Bell, B.A.; Han, J.Y.S.; Du, J.; Massenzio, E.; Abel, E.D.; Boesze-Battaglia, K.; Peachey, N.S.; Philp, N.J. Modulating GLUT1 expression in retinal pigment epithelium decreases glucose levels in the retina: Impact on photoreceptors and müller glial cells. Am. J. Physiol. Cell Physiol. 2019. [Google Scholar] [CrossRef]
- Zheng, P.P.; Romme, E.; Van Der Spek, P.J.; Dirven, C.M.F.; Willemsen, R.; Kros, J.M. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann. Neurol. 2010. [Google Scholar] [CrossRef]
- Jensen, P.J.; Gitlin, J.D.; Carayannopoulos, M.O. GLUT1 deficiency links nutrient availability and apoptosis during embryonic development. J. Biol. Chem. 2006. [Google Scholar] [CrossRef]
- Xu, R.; Ritz, B.K.; Wang, Y.; Huang, J.; Zhao, C.; Gong, K.; Liu, X.; Du, J. The retina and retinal pigment epithelium differ in nitrogen metabolism and are metabolically connected. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Zeevalk, G.D.; Nicklas, W.J. Contribution of glial metabolism to neuronal damage caused by partial inhibition of energy metabolism in retina. Exp. Eye Res. 1997. [Google Scholar] [CrossRef] [PubMed]
- Charlton-Perkins, M.; Almeida, A.D.; MacDonald, R.B.; Harris, W.A. Genetic control of cellular morphogenesis in Müller glia. Glia 2019. [Google Scholar] [CrossRef]
- MacDonald, R.B.; Randlett, O.; Oswald, J.; Yoshimatsu, T.; Franze, K.; Harris, W.A. Müller glia provide essential tensile strength to the developing retina. J. Cell Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Thummel, R.; Kassen, S.C.; Enright, J.M.; Nelson, C.M.; Montgomery, J.E.; Hyde, D.R. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 2008. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.B.; Charlton-Perkins, M.; Harris, W.A. Mechanisms of Müller glial cell morphogenesis. Curr. Opin. Neurobiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Arnold, M.J.; Brassell, M.A.; Puro, D.G. Energy metabolism in human retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3183–3190. [Google Scholar]
- Ola, M.S.; LaNoue, K.F. Molecular basis for increased lactate formation in the Müller glial cells of retina. Brain Res. Bull. 2019. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Starnes, C.A.; Sauer, M.W.; Firouzgan, Z.; Chen, S.C. Cultured retinal neuronal cells and Müller cells both show net production of lactate. Neurochem. Int. 2004. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Biedermann, B.; Francke, M.; Iandiev, I.; Grosche, J.; Wiedemann, P.; Albrecht, J.; Reichenbach, A. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem. Int. 2009. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006. [Google Scholar] [CrossRef] [PubMed]
- Punzo, C.; Kornacker, K.; Cepko, C.L. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat. Neurosci. 2009. [Google Scholar] [CrossRef] [PubMed]
- Punzo, C.; Xiong, W.; Cepko, C.L. Loss of daylight vision in retinal degeneration: Are oxidative stress and metabolic dysregulation to blame? J. Biol. Chem. 2012, 287, 1642–1648. [Google Scholar] [CrossRef]
- Umino, Y.; Everhart, D.; Solessio, E.; Cusato, K.; Pan, J.C.; Nguyen, T.H.; Brown, E.T.; Hafler, R.; Frio, B.A.; Knox, B.E.; et al. Hypoglycemia leads to age-related loss of vision. Proc. Natl. Acad. Sci. USA 2006. [Google Scholar] [CrossRef] [PubMed]
- Zieger, M.; Punzo, C. Improved cell metabolism prolongs photoreceptor survival upon retinal-pigmented epithelium loss in the sodium iodate induced model of geographic atrophy. Oncotarget 2016. [Google Scholar] [CrossRef]
- Astuti, G.D.N.; van den Born, L.I.; Khan, M.I.; Hamel, C.P.; Bocquet, B.; Manes, G.; Quinodoz, M.; Ali, M.; Toomes, C.; McKibbin, M.; et al. Identification of inherited retinal disease-associated genetic variants in 11 candidate genes. Genes 2018. [Google Scholar] [CrossRef]
- Pacione, L.R.; Szego, M.J.; Ikeda, S.; Nishina, P.M.; McInnes, R.R. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu. Rev. Neurosci. 2003. [Google Scholar] [CrossRef]
- Rattner, A.; Sun, H.; Nathans, J. Molecular genetics of human retinal disease. Annu. Rev. Genet. 1999. [Google Scholar] [CrossRef]
- Nishiwaki, Y.; Komori, A.; Sagara, H.; Suzuki, E.; Manabe, T.; Hosoya, T.; Nojima, Y.; Wada, H.; Tanaka, H.; Okamoto, H.; et al. Mutation of cGMP phosphodiesterase 6α′-subunit gene causes progressive degeneration of cone photoreceptors in zebrafish. Mech. Dev. 2008. [Google Scholar] [CrossRef]
- Rachel, R.A.; Li, T.; Swaroop, A. Photoreceptor sensory cilia and ciliopathies: Focus on CEP290, RPGR and their interacting proteins. Cilia 2012. [Google Scholar] [CrossRef] [PubMed]
- Eley, L.; Yates, L.M.; Goodship, J.A. Cilia and disease. Curr. Opin. Genet. Dev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Saari, J.C. Vitamin a metabolism in rod and cone visual cycles. Annu. Rev. Nutr. 2012. [Google Scholar] [CrossRef]
- Besch, D.; Jägle, H.; Scholl, H.P.N.; Seeliger, M.W.; Zrenner, E. Inherited multifocal RPE-diseases: Mechanisms for local dysfunction in global retinoid cycle gene defects. Vision Res. 2003. [Google Scholar] [CrossRef]
- Kiser, P.D.; Palczewski, K. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, E. Retinitis pigmentosa. In Retinitis Pigmentosa: Causes, Diagnosis and Treatment; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2010. [Google Scholar] [CrossRef]
- Daiger, S.P.; Sullivan, L.S.; Bowne, S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013. [Google Scholar] [CrossRef] [PubMed]
- Saade, C.J.; Alvarez-Delfin, K.; Fadool, J.M. Rod photoreceptors protect from cone degeneration-induced retinal remodeling and restore visual responses in zebrafish. J. Neurosci. 2013. [Google Scholar] [CrossRef]
- Mohand-Said, S.; Hicks, D.; Léveillard, T.; Picaud, S.; Porto, F.; Sahel, J.A. Rod-cone interactions: Developmental and clinical significance. Prog. Retin. Eye Res. 2001. [Google Scholar] [CrossRef]
- Sahel, J.A.; Mohand-Said, S.; Léveillard, T.; Hicks, D.; Picaud, S.; Dreyfus, H. Rod-cone interdependence: Implications for therapy of photoreceptor cell diseases. Prog. Brain Res. 2001. [Google Scholar] [CrossRef]
- Mohand-Said, S.; Hicks, D.; Dreyfus, H.; Sahel, J.A. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch. Ophthalmol. 2000. [Google Scholar] [CrossRef]
- Léveillard, T.; Mohand-Saïd, S.; Lorentz, O.; Hicks, D.; Fintz, A.C.; Clérin, E.; Simonutti, M.; Forster, V.; Cavusoglu, N.; Chalmel, F.; et al. Identification and characterization of rod-derived cone viability factor. Nat. Genet. 2004. [Google Scholar] [CrossRef] [PubMed]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; Blond, F.; Perrocheau, L.; Reichman, S.; Byrne, L.C.; et al. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 2015. [Google Scholar] [CrossRef]
- Yang, Y.; Mohand-Said, S.; Danan, A.; Simonutti, M.; Fontaine, V.; Clerin, E.; Picaud, S.; Léveillard, T.; Sahel, J.A. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol. Ther. 2009. [Google Scholar] [CrossRef] [PubMed]
- Cepko, C.; Punzo, C. Cell metabolism: Sugar for sight. Nature 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kini, A.; Wang, Y.; Liu, T.; Chen, Y.; Vukmanic, E.; Emery, D.; Liu, Y.; Lu, X.; Jin, L.; et al. Metabolic Deregulation of the Blood-Outer Retinal Barrier in Retinitis Pigmentosa. Cell Rep. 2019. [Google Scholar] [CrossRef]
- Sahel, J.A.; Léveillard, T. Maintaining cone function in rod-cone dystrophies. Adv. Exp. Med. Biol. 2018. [Google Scholar] [CrossRef]
- Clérin, E.; Marussig, M.; Sahel, J.A.; Léveillard, T. Metabolic and redox signaling of the nucleoredoxin-like-1 gene for the treatment of genetic retinal diseases. Int. J. Mol. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, P.; Baier, H.; Harris, W.A. Two Zebrafish Mutants, ebony and ivory, Uncover Benefits of Neighborhood on Photoreceptor Survival. J. Neurobiol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.C.; Schroeter, E.H.; Bilotta, J.; Wong, R.O.L.; Fadool, J.M. Cone survival despite rod degeneration in XOPS-mCFP transgenic zebrafish. Investig. Ophthalmol. Vis. Sci. 2005. [Google Scholar] [CrossRef]
- Kondkar, A.A.; Abu-Amero, K.K. Leber congenital amaurosis: Current genetic basis, scope for genetic testing and personalized medicine. Exp. Eye Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sergouniotis, P.I.; Davidson, A.E.; Mackay, D.S.; Li, Z.; Yang, X.; Plagnol, V.; Moore, A.T.; Webster, A.R. Recessive mutations in KCNJ13, encoding an inwardly rectifying potassium channel subunit, cause leber congenital amaurosis. Am. J. Hum. Genet. 2011. [Google Scholar] [CrossRef] [PubMed]
- Toms, M.; Burgoyne, T.; Tracey-White, D.; Richardson, R.; Dubis, A.M.; Webster, A.R.; Futter, C.; Moosajee, M. Phagosomal and mitochondrial alterations in RPE may contribute to KCNJ13 retinopathy. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mirra, S.; Marfany, G. Mitochondrial Gymnastics in Retinal Cells: A Resilience Mechanism Against Oxidative Stress and Neurodegeneration. Adv. Exp. Med. Biol. 2019. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaroszynska, N.; Harding, P.; Moosajee, M. Metabolism in the Zebrafish Retina. J. Dev. Biol. 2021, 9, 10. https://doi.org/10.3390/jdb9010010
Jaroszynska N, Harding P, Moosajee M. Metabolism in the Zebrafish Retina. Journal of Developmental Biology. 2021; 9(1):10. https://doi.org/10.3390/jdb9010010
Chicago/Turabian StyleJaroszynska, Natalia, Philippa Harding, and Mariya Moosajee. 2021. "Metabolism in the Zebrafish Retina" Journal of Developmental Biology 9, no. 1: 10. https://doi.org/10.3390/jdb9010010
APA StyleJaroszynska, N., Harding, P., & Moosajee, M. (2021). Metabolism in the Zebrafish Retina. Journal of Developmental Biology, 9(1), 10. https://doi.org/10.3390/jdb9010010





