The Roles of Aquaporins in Plant Stress Responses
Abstract
:1. Background and Discovery of Aquaporins
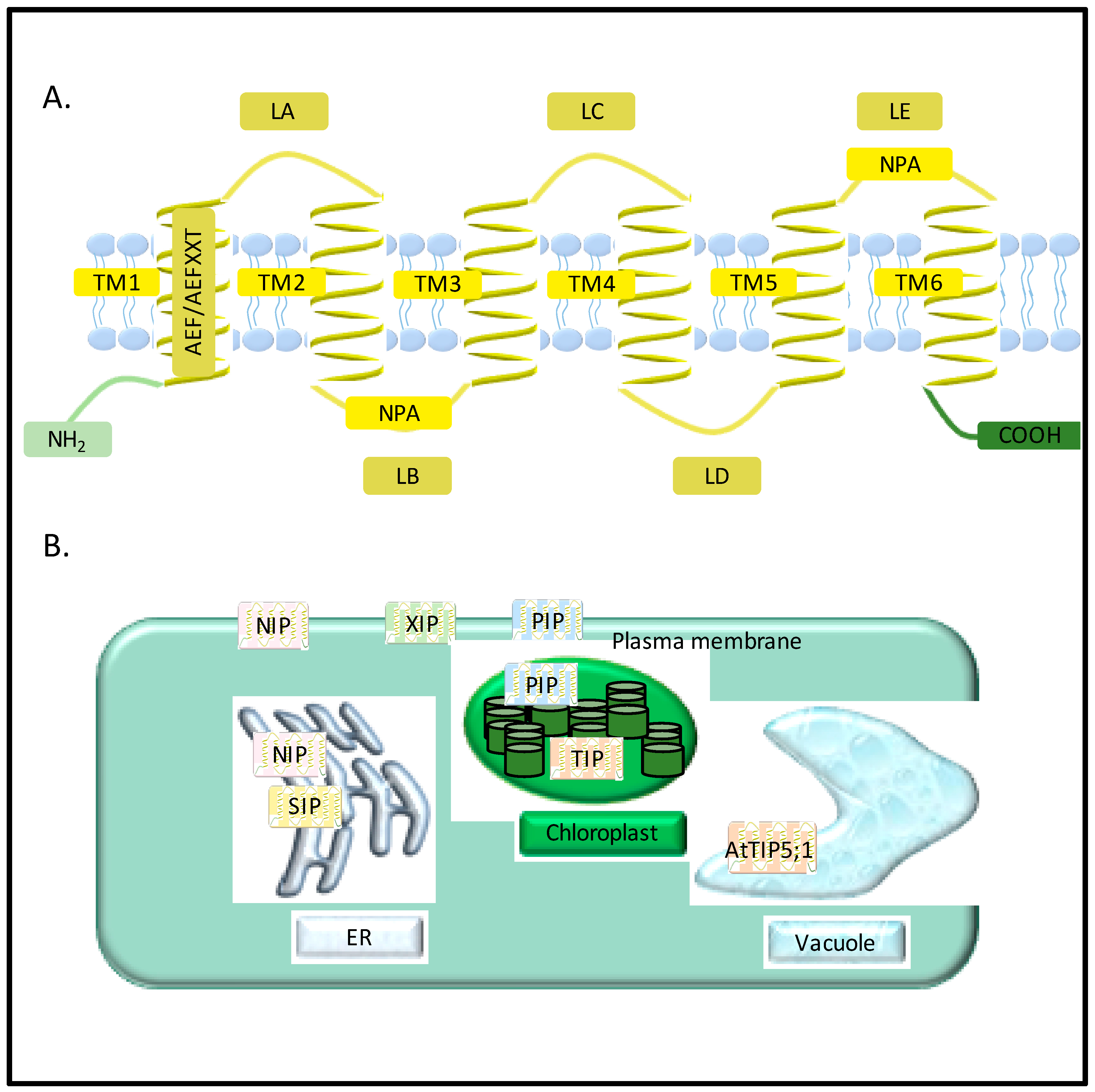
2. Diversity of Aquaporins in Plants
3. Roles of Aquaporins in Plant–Water Relations
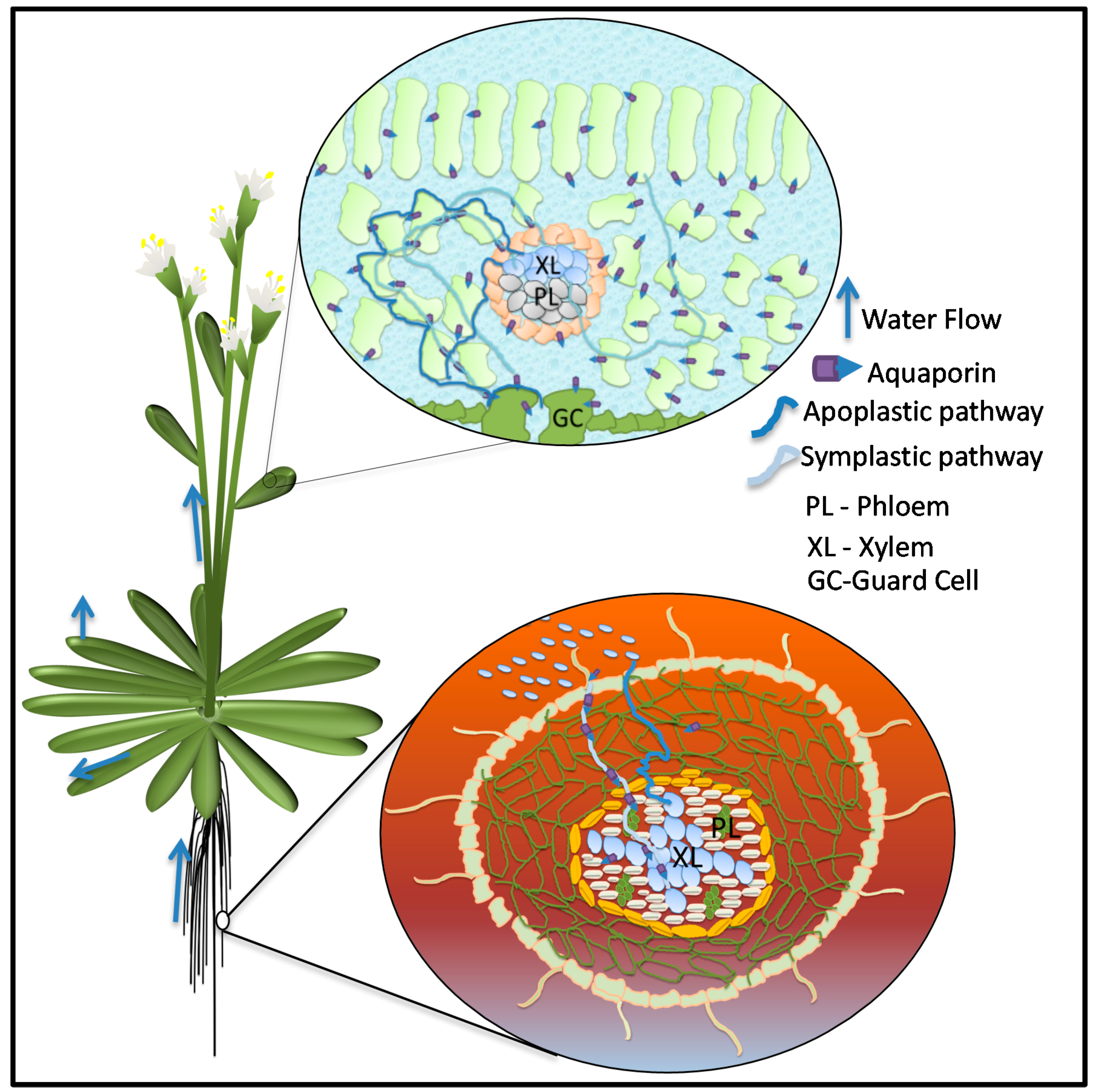
4. Plant Aquaporins in Water Stress
5. Aquaporins in CO2 Homeostasis in Water Deficit Conditions
6. Roles of Aquaporins in Salt Stress
7. Cold Stress and Aquaporins
8. Aquaporins in Micronutrient Homeostasis and Heavy Metal Stress
9. Aquaporins in Plant Symbiotic and Pathogenic Relations
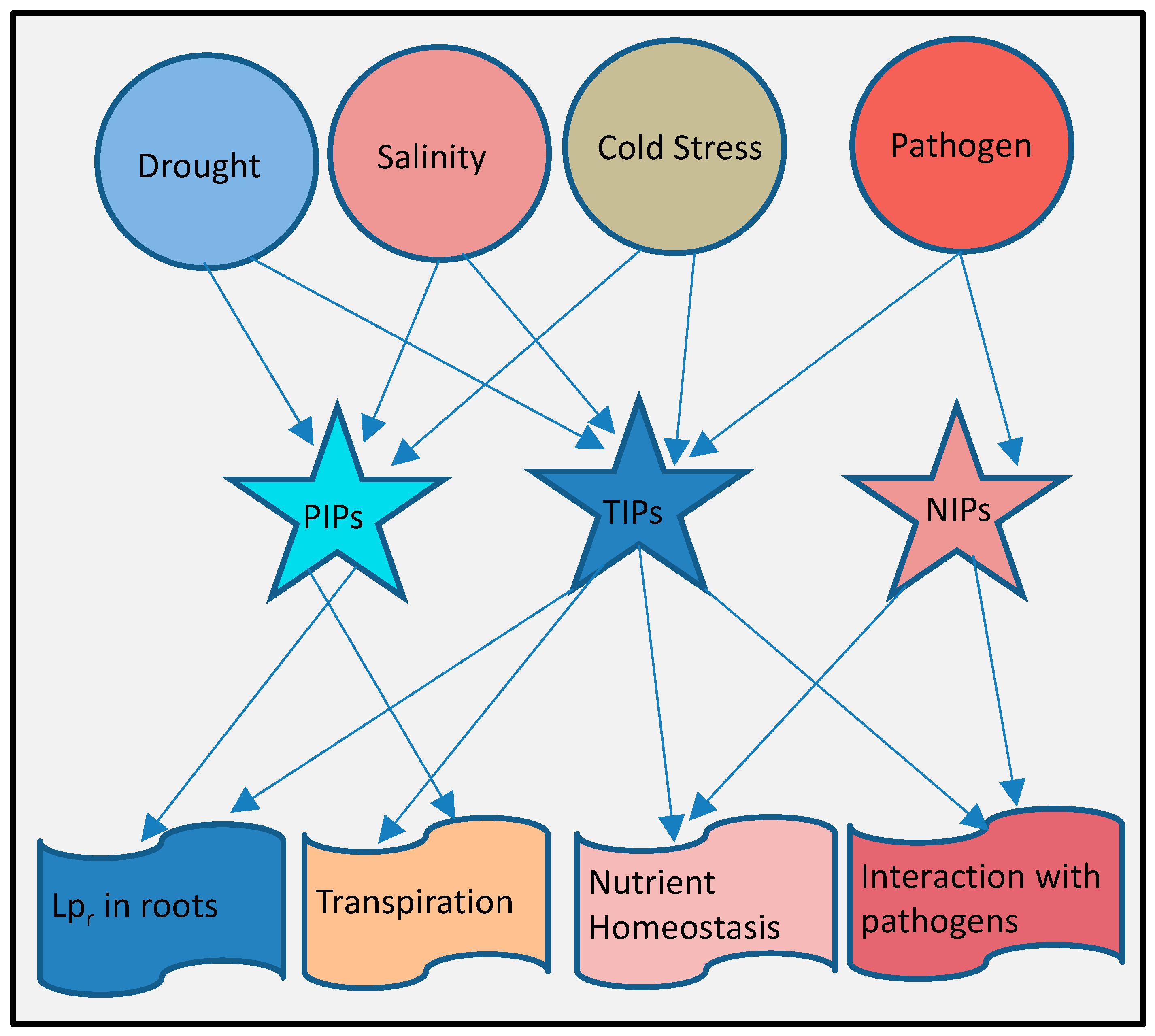
10. Complex Integrated Roles of Aquaporins in Plant Stresses
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Lpr | Root hydraulic Conductivity |
| MIPs | Major intrinsic proteins |
| MIPs | Major intrinsic protein genes |
| PIPs | Plasma membrane intrinsic proteins |
| PIPs | Plasma membrane intrinsic protein genes |
| TIPs | Tonoplast intrinsic proteins |
| TIPs | Tonoplast intrinsic protein genes |
| NIPs | Nodulin-26 like intrinsic proteins |
| NIPs | Nodulin-26 like intrinsic protein genes |
| SIPs | Small basic intrinsic proteins |
| SIPs | Small basic intrinsic protein genes |
| GIPs | GlpF-like intrinsic proteins |
| HIPs | Hybrid intrinsic proteins |
| AQPs | Aquaporins |
| XIPs | Uncategorized X intrinsic proteins |
| XIPs | Uncategorized X intrinsic protein genes |
| ROS | Reactive oxygen species |
| GLPs | Glycerol-facilitators |
| ER | Endoplasmic reticulum |
| ΔΨ | Water potential |
| CMV | Cucumber mosaic virus |
References
- Maurel, C.; Tacnet, F.; Guclu, J.; Guern, J.; Ripoche, P. Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity. Proc. Natl. Acad. Sci. USA 1997, 94, 7103–7108. [Google Scholar] [CrossRef] [PubMed]
- Koefoed-Johnsen, V. The contributions of diffusion and flow to the passage of D2O through living membranes: Effect of neurohypophysenl hormone 011 isolated anuran skin. Acta Physiol. Scand. 1953, 28, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Macey, R.L.; Farmer, R.E. Inhibition of water and solute permeability in human red cells. Biochim. Biophys. Acta Biomembr. 1970, 211, 104–106. [Google Scholar] [CrossRef]
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel MR 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in xenopus oocytes expressing red cell chip28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.G.; Morrison, N.A.; Verma, D.P.S. Nodulin-26, a peribacteroid membrane nodulin is expressed independently of the development of the peribacteroid compartment. Nucleic Acids Res. 1987, 15, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Reizer, J.; Schroeder, J.I.; Chrispeels, M.J. The vacuolar membrane protein gamma-tip creates water specific channels in xenopus oocytes. EMBO J. 1993, 12, 2241–2247. [Google Scholar] [PubMed]
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and evolution of membrane intrinsic proteins. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.R.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjovall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.O.; Barrieu, F.O.; Wojcik, E.; Chrispeels, M.J.; Jung, R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.D.P.S.; Pedrosa, A.M.; Du, D.; Goncalves, L.P.; Yu, Q.; Gmitter, F.G., Jr.; Costa, M.G.C. Genome-wide characterization and expression analysis of major intrinsic proteins during abiotic and biotic stresses in sweet orange (Citrus sinensis L. Osb.). PLoS ONE 2015, 10, e0138786. [Google Scholar]
- Reuscher, S.; Akiyama, M.; Mori, C.; Aoki, K.; Shibata, D.; Shiratake, K. Genome-wide identification and expression analysis of aquaporins in tomato. PLoS ONE 2013, 8, e79052. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.B.; Sankararamakrishnan, R. Genome-wide analysis of major intrinsic proteins in the tree plant populus trichocarpa: Characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Wang, C.B.; Xu, L.; Yi, J.X.; Xu, Z.L.; Liu, X.Q.; He, X.L.; Huang, Y.H.; Khan, I.A.; Trethowan, R. Genome-wide sequence characterization and expression analysis of major intrinsic proteins in soybean (Glycine max L.). PLoS ONE 2013, 8, e56312. [Google Scholar]
- Shekhawat, U.K.; Ganapathi, T.R. Overexpression of a native plasma membrane aquaporin for development of abiotic stress tolerance in banana. Plant Biotechnol. J. 2013, 11, 942–952. [Google Scholar]
- Park, W.; Scheffler, B.E.; Bauer, P.J.; Campbell, B.T. Genome-wide identification of differentially expressed genes under water deficit stress in upland cotton (Gossypium hirsutum L.). BMC Plant Biol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Rao, T.S.R.B.; Sharma, K.K.; Vadez, V. Genome-wide identification and characterization of the aquaporin gene family in Sorghum bicolor (L.). Plant Gene 2015, 1, 18–28. [Google Scholar] [CrossRef]
- Karkouri, K.; Gueune, H.; Delamarche, C. MIPDB: A relational database dedicated to MIP family proteins. Biol. Cell 2005, 97, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R. Phylogeny and evolution of the major intrinsic protein family. Biol. Cell 2005, 97, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Bishai, W.R.; Preston, G.M.; Guggino, W.B.; Agre, P. Molecular cloning and characterization of AQPZ, a water channel from escherichia coli. J. Biol. Chem. 1995, 270, 29063–29066. [Google Scholar] [PubMed]
- Carbrey, J.M.; Bonhivers, M.l.; Boeke, J.D.; Agre, P. Aquaporins in saccharomyces: Characterization of a second functional water channel protein. Proc. Natl. Acad. Sci. USA 2001, 98, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.N.; Yoshino, R.; Morio, T.; Yokoyama, M.; Maeda, M.; Urushihara, H.; Tanaka, Y. Loss of a member of the aquaporin gene family, AQPA affects spore dormancy in dictyostelium. Gene 2000, 251, 131–139. [Google Scholar] [CrossRef]
- Kozono, D.; Ding, X.; Iwasaki, I.; Meng, X.; Kamagata, Y.; Agre, P.; Kitagawa, Y. Functional expression and characterization of an archaeal aquaporin aqpm from methanothermobacter marburgensis. J. Biol. Chem. 2003, 278, 10649–10656. [Google Scholar] [CrossRef] [PubMed]
- Beuron, F.; le Caherec, F.; Guillam, M.T.; Cavalier, A.; Garret, A.; Tassan, J.P.; Delamarche, C.; Schultz, P.; Mallouh, V.; Rolland, J.P. Structural analysis of a mip family protein from the digestive tract of cicadella viridis. J. Biol. Chem. 1995, 270, 17414–17422. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin chip: The archetypal molecular water channel. Am. J. Physiol. Renal Physiol. 1993, 265, F463–F476. [Google Scholar]
- Zardoya, R.; Ding, X.; Kitagawa, Y.; Chrispeels, M.J. Origin of plant glycerol transporters by horizontal gene transfer and functional recruitment. Proc. Natl. Acad. Sci. USA 2002, 99, 14893–14896. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Karlsson, M.; Johanson, U.; Larsson, C.; Kjellbom, P. The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta Biomembr. 2000, 1465, 324–342. [Google Scholar] [CrossRef]
- Maurel, C.; Javot, H.; Lauvergeat, V.; Gerbeau, P.; Tournaire, C.; Santoni, V.; Heyes, J. Molecular physiology of aquaporins in plants. Int. Rev. Cytol. 2002, 215, 105–148. [Google Scholar] [PubMed]
- Nielsen, S.R.; King, L.S.; Christensen, B.M.N.; Agre, P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am. J. Physiol. Cell Physiol. 1997, 273, C1549–C1561. [Google Scholar]
- Forrest, K.L.; Bhave, M. Major intrinsic proteins (MIPs) in plants: A complex gene family with major impacts on plant phenotype. Funct. Integr. Genomics 2007, 7, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Hove, R.M.; Bhave, M. Plant aquaporins with non-aqua functions: Deciphering the signature sequences. Plant Mol. Biol. 2011, 75, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.; Suga, S.; Uemura, T.; Sato, M.H.; Maeshima, M. Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in arabidopsis thaliana. FEBS Lett. 2005, 579, 5814–5820. [Google Scholar] [CrossRef] [PubMed]
- Wudick, M.M.; Luu, D.T.; Tournaire-Roux, C.; Sakamoto, W.; Maurel, C. Vegetative and sperm cell-specific aquaporins of arabidopsis highlight the vacuolar equipment of pollen and contribute to plant reproduction. Plant Physiol. 2014, 164, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. Aquaporin subfamily with unusual NPA boxes. Biochim. Biophys. Acta Biomembr. 2006, 1758, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Kuwahara, M.; Kageyama, Y.; Sasaki, S.; Suzuki, M.; Imai, M. Molecular cloning of a new aquaporin superfamily in mammals. In Molecular Biology and Physiology of Water and Solute Transport; Springer: NewYork, NY, USA, 2000; pp. 123–126. [Google Scholar]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed]
- Zangi, R.; Filella, M. Transport routes of metalloids into and out of the cell: A review of the current knowledge. Chemico Biol. Interact. 2012, 197, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Bronner, G.L.; Brunel, N.; Auguin, D.; Bourgerie, S.; Brignolas, F.; Carpin, S.; Tournaire-Roux, C.; Maurel, C.; Fumanal, B. Insights into populus XIP aquaporins: Evolutionary expansion, protein functionality, and environmental regulation. J. Exp. Bot. 2012, 63, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E. The cohesion-tension mechanism and the acquisition of water by plant roots. Annu. Rev. Plant Biol. 2001, 52, 847–875. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S. Biological Science, 3rd ed.; Benjamin Cummins: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Tyerman, S.; Niemietz, C.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002, 25, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E.; Peterson, C.A. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Ribas-Carbo, M.; Sans, J.F.; Lovisolo, C.; Heckwolf, M.; Uehlein, N. Aquaporins and plant water balance. Plant Cell Environ. 2008, 31, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Venisse, J.S.P.; Fumanal, B.; Chaumont, F.O.; Guillot, E.; Daniels, M.J.; Cochard, H.; Julien, J.L.; Gousset-Dupont, A.L. Aquaporins and leaf hydraulics, poplar sheds new light. Plant Cell Physiol. 2013, 54, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.S.; Choi, W.G.; Roberts, D.M. The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Siefritz, F.; Tyree, M.T.; Lovisolo, C.; Schubert, A.; Kaldenhoff, R. PIP1 plasma membrane aquaporins in tobacco from cellular effects to function in plants. Plant Cell 2002, 14, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Hukin, D.; Doering-Saad, C.; Thomas, C.; Pritchard, J. Sensitivity of cell hydraulic conductivity to mercury is coincident with symplasmic isolation and expression of plasmalemma aquaporin genes in growing maize roots. Planta 2002, 215, 1047–1056. [Google Scholar] [PubMed]
- Park, W.J.; Campbell, B.T. Aquaporins as targets for stress tolerance in plants: Genomic complexity and perspectives. Turk. J. Bot. 2015, 39, 879–886. [Google Scholar] [CrossRef]
- Boursiac, Y.; Boudet, J.; Postaire, O.; Doan, T.L.; Colette, T.R.; Maurel, C. Stimulus induced downregulation of root water transport involves reactive oxygen species activated cell signalling and plasma membrane intrinsic protein internalization. Plant J. 2008, 56, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Koizumi, M.; Urao, S.; Shinozaki, K. Molecular cloning and characterization of 9 cDNAs for genes that are responsive to desiccation in Arabidopsis thaliana: Sequenceanalysis of one cDNA clone that encodes a putative transmembrane channel protein. Plant Cell Physiol. 1992, 33, 217–224. [Google Scholar]
- Alexandersson, E.; Fraysse, L.; Sjovall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005, 59, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Danielson, J.A.; Rade, J.; Moparthi, V.K.; Fontes, M.; Kjellbom, P.; Johanson, U. Transcriptional regulation of aquaporins in accessions of arabidopsis in response to drought stress. Plant J. 2010, 61, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, D.G.; Kim, Y.O.; Kim, J.S.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Secchi, F.; Lovisolo, C.; Schubert, A. Expression of OePIP2;1 aquaporin gene and water relations of OLEA europaea twigs during drought stress and recovery. Ann. Appl. Biol. 2007, 150, 163–167. [Google Scholar] [CrossRef]
- Mahdieh, M.; Mostajeran, A.; Horie, T.; Katsuhara, M. Drought stress alters water relations and expression of PIP-type aquaporin genes in Nicotiana tabacum plants. Plant Cell Physiol. 2008, 49, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, S.; Ohshima, I.; Gemma, H.; Iwahori, S. Expression analysis of genes encoding aquaporins during the development of peach fruit. In Proceedings of the XXVI International Horticultural Congress: Environmental Stress and Horticulture Crops 618, Toronto, AB, Canada, 11–17 August 2002; pp. 363–370.
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Ergul, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C. Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles. Funct. Integr. Genomics 2007, 7, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Z.Y.; Lin, H.; Cui, W.E.; Chen, J.; Liu, M.; Chen, Z.L.; Qu, L.J.; Gu, H. Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 2006, 16, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Li, L.G.; Li, S.F.; Tao, Y.; Kitagawa, Y. Molecular cloning of a novel water channel from rice: Its products expression in xenopus oocytes and involvement in chilling tolerance. Plant Sci. 2000, 154, 43–51. [Google Scholar] [CrossRef]
- Malz, S.; Sauter, M. Expression of two pip genes in rapidly growing internodes of rice is not primarily controlled by meristem activity or cell expansion. Plant Mol. Biol. 1999, 40, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Suga, S.; Komatsu, S.; Maeshima, M. Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiol. 2002, 43, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ban, L.; Wen, H.; Wang, Z.; Dzyubenko, N.; Chapurin, V.; Gao, H.; Wang, X. An aquaporin protein is associated with drought stress tolerance. Biochem. Biophys. Res. Commun. 2015, 459, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Galmes, J.; Pou, A.; Alsina, M.M.; Tomas, M.; Medrano, H.l.; Flexas, J. Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): Relationship with ecophysiological status. Planta 2007, 226, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Surbanovski, N.; Sargent, D.J.; Else, M.A.; Simpson, D.W.; Zhang, H.; Grant, O.M. Expression of fragaria vesca PIP aquaporins in response to drought stress: PIP down-regulation correlates with the decline in substrate moisture content. PLoS ONE 2013, 8, e74945. [Google Scholar]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Barzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.O.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant Microb. Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Umeda, M.; Uchimiya, H. Isolation and expression analysis of two rice genes encoding the major intrinsic protein. Plant Mol. Biol. 1994, 26, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.; Rotter, B.R.; Horres, R.; Udupa, S.M.; Besser, B.; Bellarmino, L.; Baum, M.; Matsumura, H.; Terauchi, R.; Kahl, G.N. Supersage: The drought stress-responsive transcriptome of chickpea roots. BMC Genomics 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Martre, P.; Morillon, R.L.; Barrieu, F.O.; North, G.B.; Nobel, P.S.; Chrispeels, M.J. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol. 2002, 130, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Kaldenhoff, R.; Grote, K.; Zhu, J.J.; Zimmermann, U. Significance of plasmalemma aquaporins for water transport in arabidopsis thaliana. Plant J. 1998, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.L.N.; Lauvergeat, V.; Santoni, V.R.; Martin-Laurent, F.; Guclu, J.; Vinh, J.L.; Heyes, J.; Franck, K.I.; Schaffner, A.R.; Bouchez, D. Role of a single aquaporin isoform in root water uptake. Plant Cell 2003, 15, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Lienard, D.; Durambur, G.; Kiefer-Meyer, M.C.; Nogue, F.; Menu-Bouaouiche, L.; Charlot, F.; Gomord, V.R.; Lassalles, J.P. Water transport by aquaporins in the extant plant physcomitrella patens. Plant Physiol. 2008, 146, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Hu, Y.; Li, J.; Wu, Q.; Lin, Z. Sense and antisense expression of plasma membrane aquaporin bnpip1 from brassica napus in tobacco and its effects on plant drought resistance. Plant Sci. 2005, 169, 647–656. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, W.; Deng, X.; Ma, Z.; Chen, L.; Huang, C.; Wang, C.; Wang, J.; He, Y.; Yang, G. Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS ONE 2012, 7, e52439. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.H.; Hao, F.S.; Chen, H.; Chen, J.; Wang, X.C. Expression of the vicia faba VFPIP1 gene in arabidopsis thaliana plants improves their drought resistance. J. Plant Res. 2008, 121, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, W.; Liu, J.; Zhang, J.; Jia, C.; Miao, H.; Xu, B.; Jin, Z. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Vinocur, B.J.; Diber, A.; Shatil, A.; Ronen, G.; Nissan, H.; Wallach, R.; Karchi, H.; Moshelion, M. Improving plant stress tolerance and yield production: Is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol. 2009, 181, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Aharon, R.; Shahak, Y.; Wininger, S.; Bendov, R.; Kapulnik, Y.; Galili, G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 2003, 15, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Vander Willigen, C.; Pammenter, N.; Mundree, S.G.; Farrant, J.M. Mechanical stabilization of desiccated vegetative tissues of the resurrection grass eragrostis nindensis: Does a TIP3;1 and/or compartmentalization of subcellular components and metabolites play a role? J. Exp. Bot. 2004, 55, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Rodriguez, A.M.; Cooke, J.E.; Yeh, F.; Zwiazek, J.J. Functional characterization of drought responsive aquaporins in populus balsamifera and populus simonii X balsamifera clones with different drought resistance strategies. Physiol. Plant. 2010, 140, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.L.; Yu, X.; Ye, Q.; Ding, X.S.; Kitagawa, Y.; Kwak, S.S.; Su, W.A.; Tang, Z.C. The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol. 2004, 45, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Smith-Espinoza, C.; Richter, A.; Salamini, F.; Bartels, D. Dissecting the response to dehydration and salt (NACL) in the resurrection plant craterostigma plantagineum. Plant Cell Environ. 2003, 26, 1307–1315. [Google Scholar] [CrossRef]
- Zhuang, L.; Liu, M.; Yuan, X.; Yang, Z.; Huang, B. Physiological effects of aquaporin in regulating drought tolerance through overexpressing of festuca arundinacea aquaporin gene FaPIP2;1. J. Am. Soc. Hortic. Sci. 2015, 140, 404–412. [Google Scholar]
- Flexas, J.; Miquel, R.C.; Hanson, D.T.; Bota, J.; Otto, B.; Cifre, J.; McDowell, N.; Medrano, H.; Kaldenhoff, R. Tobacco aquaporin NTAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006, 48, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Boudichevskaia, A.; Heckwolf, M.; Kaldenhoff, R. T-DNA insertion in aquaporin gene AtPIP1;2 generates transcription profiles reminiscent of a low CO2 response. Plant Cell Environ. 2015, 38, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Sperling, H.; Heckwolf, M.; Kaldenhoff, R. The arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant Cell Environ. 2012, 35, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.N.M.; CarriquÃ, M.; DÃaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihira, A.; Hanba, Y.T.; Kato, N.; Doi, T.; Kawazu, T.; Maeshima, M. Effect of overexpression of radish plasma membrane aquaporins on water-use efficiency, photosynthesis and growth of eucalyptus trees. Tree Physiol. 2010, 30, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Hanba, Y.T.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K.; Terashima, I.; Katsuhara, M. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 2004, 45, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, M.; Akiyama, Y.; Koshio, K.; Shibasaka, M.; Kasamo, K. Functional analysis of water channels in barley roots. Plant Cell Physiol. 2002, 43, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, M.; Koshio, K.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K. Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant Cell Physiol. 2003, 44, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Katsuhara, M.; Kelly, W.B.; Michalowski, C.B.; Bohnert, H.J. A family of transcripts encoding water channel proteins: Tissue-specific expression in the common ice plant. Plant Cell 1995, 7, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Kirch, H.H.; Vera-Estrella, R.; Golldack, D.; Quigley, F.; Michalowski, C.B.; Barkla, B.J.; Bohnert, H.J. Expression of water channel proteins in mesembryanthemum crystallinum. Plant Physiol. 2000, 123, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Schraut, D.; Hartung, W.; SchÃffner, A.R. Differential responses of maize MIP genes to salt stress and ABA. J. Exp. Bot. 2005, 56, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Li, L.; Ren, F.; Lu, P.; Wei, P.; Cai, J.; Xin, L.; Zhang, J.; Chen, J.; Wang, X. Overexpression of the tonoplast aquaporin AtTIP5;1 conferred tolerance to boron toxicity in arabidopsis. J. Genet. Genomics 2010, 37, 389–397. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, W.; Cai, W.; Arora, R. Overexpression of a panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic arabidopsis plants. Planta 2007, 226, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Dhonukshe, P.; Aniento, F.; Hwang, I.; Robinson, D.G.; Mravec, J.; Stierhof, Y.D.; Friml, J.Ã. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in arabidopsis. Curr. Biol. 2007, 17, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Yang, Y.; Li, R.; He, Q.; Fang, X.; Luu, D.T.; Maurel, C.; Lin, J. Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of arabidopsis plasma membrane aquaporin regulation. Plant Cell 2011, 23, 3780–3797. [Google Scholar] [CrossRef] [PubMed]
- Luu, D.T.; Martiniere, A.; Sorieul, M.; Runions, J.; Maurel, C. Fluorescence recovery after photobleaching reveals high cycling dynamics of plasma membrane aquaporins in arabidopsis roots under salt stress. Plant J. 2012, 69, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; He, X.; Zhao, B.; Zhou, C.; Liang, Y.; Ge, R.; Shen, Y.; Huang, Z. Overexpressing a putative aquaporin gene from wheat, TaNIP, enhances salt tolerance in transgenic arabidopsis. Plant Cell Physiol. 2010, 51, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, S.; Hu, W.; Deng, X.; Wei, S.; Yang, G.; He, G. The wheat aquaporin gene TAAQP7 confers tolerance to cold stress in transgenic tobacco. Z. Naturforschung. C 2014, 69, 142–148. [Google Scholar] [CrossRef]
- Ahamed, A.; Murai-Hatano, M.; Ishikawa-Sakurai, J.; Hayashi, H.; Kawamura, Y.; Uemura, M. Cold stress-induced acclimation in rice is mediated by root-specific aquaporins. Plant Cell Physiol. 2012, 53, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Amodeo, G.; Fernandez-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.B.T.; de Rosa, V.E.; Menossi, M.; Ulian, E.N.C.; Arruda, P. RNA expression profiles and data mining of sugarcane response to low temperature. Plant Physiol. 2003, 132, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Wada, M.; Ludewig, U.; Schaaf, G.; von Wiren, N.; Fujiwara, T. The arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Choi, W.G.; Wallace, I.S.; Baudry, J.; Roberts, D.M. Arabidopsis thaliana NIP7;1: An anther-specific boric acid transporter of the aquaporin superfamily regulated by an unusual tyrosine in HELIX 2 of the transport pore. Biochemistry 2011, 50, 6633–6641. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Wallace, I.S.; Takano, J.; Roberts, D.M.; Fujiwara, T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in arabidopsis. Plant Cell 2008, 20, 2860–2875. [Google Scholar] [CrossRef] [PubMed]
- Schnurbusch, T.; Hayes, J.; Hrmova, M.; Baumann, U.; Ramesh, S.A.; Tyerman, S.D.; Langridge, P.; Sutton, T. Boron toxicity tolerance in barley through reduced expression of the multifunctional aquaporin HvNIP2;1. Plant Physiol. 2010, 153, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Mitatni, N.; Ma, J.F. A transporter regulating silicon distribution in rice shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Tanaka, M.; Mitani, N.; Ma, J.F.; Maeshima, M.; Fujiwara, T. NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of arabidopsis thaliana. J. Biol. Chem. 2009, 284, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Thorsen, M.; Schussler, M.D.; Nilsson, H.R.; Wagner, A.; Tamas, M.J.; Jahn, T.P. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008, 6. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Fujiwara, T. Arabidopsis NIP1;1 transports antimonite and determines antimonite sensitivity. Plant Cell Physiol. 2009, 50, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Roberts, D.M. Arabidopsis NIP2;1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. J. Biol. Chem. 2007, 282, 24209–24218. [Google Scholar] [CrossRef] [PubMed]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hooijmaijers, C.; Rhee, J.Y.; Kwak, K.J.; Chung, G.C.; Horie, T.; Katsuhara, M.; Kang, H. Hydrogen peroxide permeability of plasma membrane aquaporins of arabidopsis thaliana. J. Plant Res. 2012, 125, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Rodriguez-Zas, S.; Aldea, M.; Li, M.; Zhu, J.; Gonzalez, D.O.; Vodkin, L.O.; DeLucia, E.; Clough, S.J. Expression profiling soybean response to pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol. Plant Microb. Interact. 2005, 18, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Aritua, V.; Achor, D.; Gmitter, F.G.; Albrigo, G.; Wang, N. Transcriptional and microscopic analyses of citrus stem and root responses to Candidatus Liberibacter asiaticus infection. PLoS ONE 2013, 8, e73742. [Google Scholar]
- Lawrence, S.; Novak, N.; Xu, H.; Cooke, J. Herbivory of maize by southern corn rootworm induces expression of the major intrinsic protein ZmNIP1;1 and leads to the discovery of a novel aquaporin ZmPIP2;8. Plant Signal. Behav. 2013, 8, e24937. [Google Scholar] [CrossRef] [PubMed]
- Opperman, C.H.; Taylor, C.G.; Conkling, M.A. Root-knot nematode-directed expression of a plant root-specific gene. Science 1994, 263, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.L.; Wade, R.N. Defending the leaf surface: Intra-and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.S.; Carvunis, A.R.; Dreze, M.; Epple, P.; Steinbrenner, J.; Moore, J.; Tasan, M.; Galli, M.; Hao, T.; Nishimura, M.T. Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science 2011, 333, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.R.; Paek, K.H. Arabidopsis tonoplast proteins Tip1 and Tip2 interact with the cucumber mosaic virus 1A replication protein. J. Gen. Virol. 2006, 87, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Cavez, D.; Miled, N.; Chaumont, F.O.; Masmoudi, K. Identification and characterization of two plasma membrane aquaporins in durum wheat (Triticum turgidum L. Subsp. Durum) and their role in abiotic stress tolerance. Plant Physiol. Biochem. 2011, 49, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Lee, S.H.; Rhee, J.Y.; Chung, G.C.; Ahn, S.J.; Kang, H. Transgenic arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. Plant Mol. Biol. 2007, 64, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, E.; Borst, J.W.; Muylaert, M.L.; Batoko, H.; Hemminga, M.A.; Chaumont, F.O. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc. Natl. Acad. Sci. USA 2007, 104, 12359–12364. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yin, X.; Wang, L.; Tian, J.; Yang, R.; Liu, D.; Yu, Z.; Ma, N.; Gao, J. Involvement of rose aquaporin RhPIP1;1 in ethylene-regulated petal expansion through interaction with RhPIP2;1. Plant Mol. Biol. 2013, 83, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Ruan, X.M.; Zhang, J.; Wu, Y.J.; Wang, X.L.; Li, X.B. Cotton plasma membrane intrinsic protein 2s (PIP2s) selectively interact to regulate their water channel activities and are required for fibre development. New Phytol. 2013, 199, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Burnotte, E.; Bienert, G.P.; Chevalier, A.S.; Errachid, A.; Grefen, C.; Blatt, M.R.; Chaumont, F.O. Selective regulation of maize plasma membrane aquaporin trafficking and activity by the snare SYP121. Plant Cell 2012, 24, 3463–3481. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Laloux, T.E.; Reinhardt, H.; Cavez, D.; Degand, H.; Grefen, C.; de Rycke, R.; InzÃ, D.; Blatt, M.R.; Russinova, E. Arabidopsis snares syp61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability. Plant Cell 2014, 26, 3132–3147. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Veljanovski, V.; Reinhardt, H.; Guillaumot, D.; Vanhee, C.; Chaumont, F.O.; Batoko, H. The arabidopsis abiotic stress-induced tspo-related protein reduces cell-surface expression of the aquaporin PIP2;7 through protein-protein interactions and autophagic degradation. Plant Cell 2014, 26, 4974–4990. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Cho, S.K.; Son, O.; Xu, Z.; Hwang, I.; Kim, W.T. Drought stress-induced RMA1H1, a ring membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic arabidopsis plants. Plant Cell 2009, 21, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Masalkar, P.; Wallace, I.S.; Hwang, J.H.; Roberts, D.M. Interaction of cytosolic glutamine synthetase of soybean root nodules with the C-terminal domain of the symbiosome membrane nodulin 26 aquaglyceroporin. J. Biol. Chem. 2010, 285, 23880–23888. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.N.; Rodriguez, C.S.; Pertl-Obermeyer, H.; Obermeyer, G.; Schulze, W.X. Sucrose-induced receptor kinase SIRK1 regulates a plasma membrane aquaporin in arabidopsis. Mol. Cell. Proteomics 2013, 12, 2856–2873. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, A.S.; Chaumont, F.O. Trafficking of plant plasma membrane aquaporins: Multiple regulation levels and complex sorting signals. Plant Cell Physiol. 2015, 56, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Vera-Estrella, R.; Barkla, B.J.; Bohnert, H.J.; Pantoja, O. Novel regulation of aquaporins during osmotic stress. Plant Physiol. 2004, 135, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The Roles of Aquaporins in Plant Stress Responses. J. Dev. Biol. 2016, 4, 9. https://doi.org/10.3390/jdb4010009
Afzal Z, Howton TC, Sun Y, Mukhtar MS. The Roles of Aquaporins in Plant Stress Responses. Journal of Developmental Biology. 2016; 4(1):9. https://doi.org/10.3390/jdb4010009
Chicago/Turabian StyleAfzal, Zunaira, T. C. Howton, Yali Sun, and M. Shahid Mukhtar. 2016. "The Roles of Aquaporins in Plant Stress Responses" Journal of Developmental Biology 4, no. 1: 9. https://doi.org/10.3390/jdb4010009
APA StyleAfzal, Z., Howton, T. C., Sun, Y., & Mukhtar, M. S. (2016). The Roles of Aquaporins in Plant Stress Responses. Journal of Developmental Biology, 4(1), 9. https://doi.org/10.3390/jdb4010009





