Retinoic Acid and the Development of the Endoderm
Abstract
:1. Introduction
2. What is Endoderm?
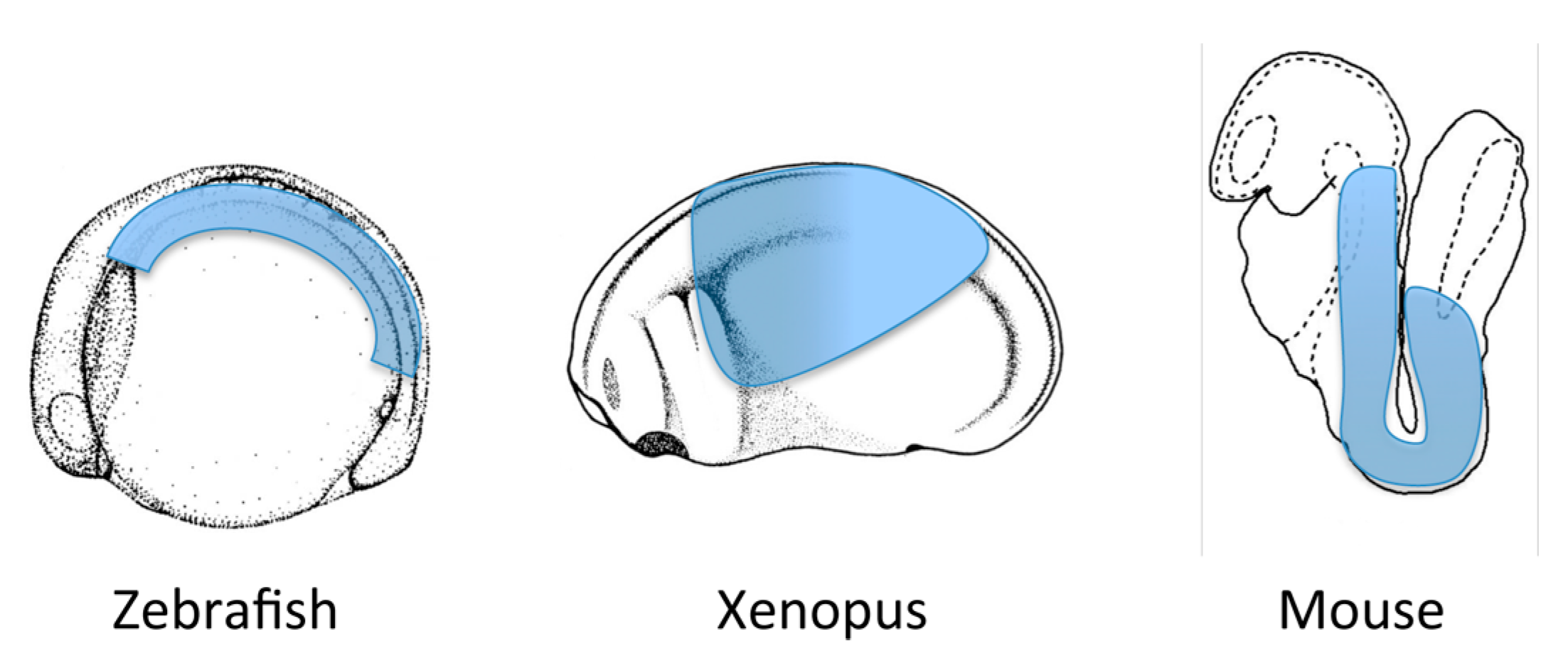
3. Retinoic Acid Signaling
3.1. Generating Retinoic Acid
3.2. Movement of Retinoic Acid
3.3. Interaction with DNA
4. The Role of Retinoic Acid in Anterior-Posterior Patterning of the Endoderm
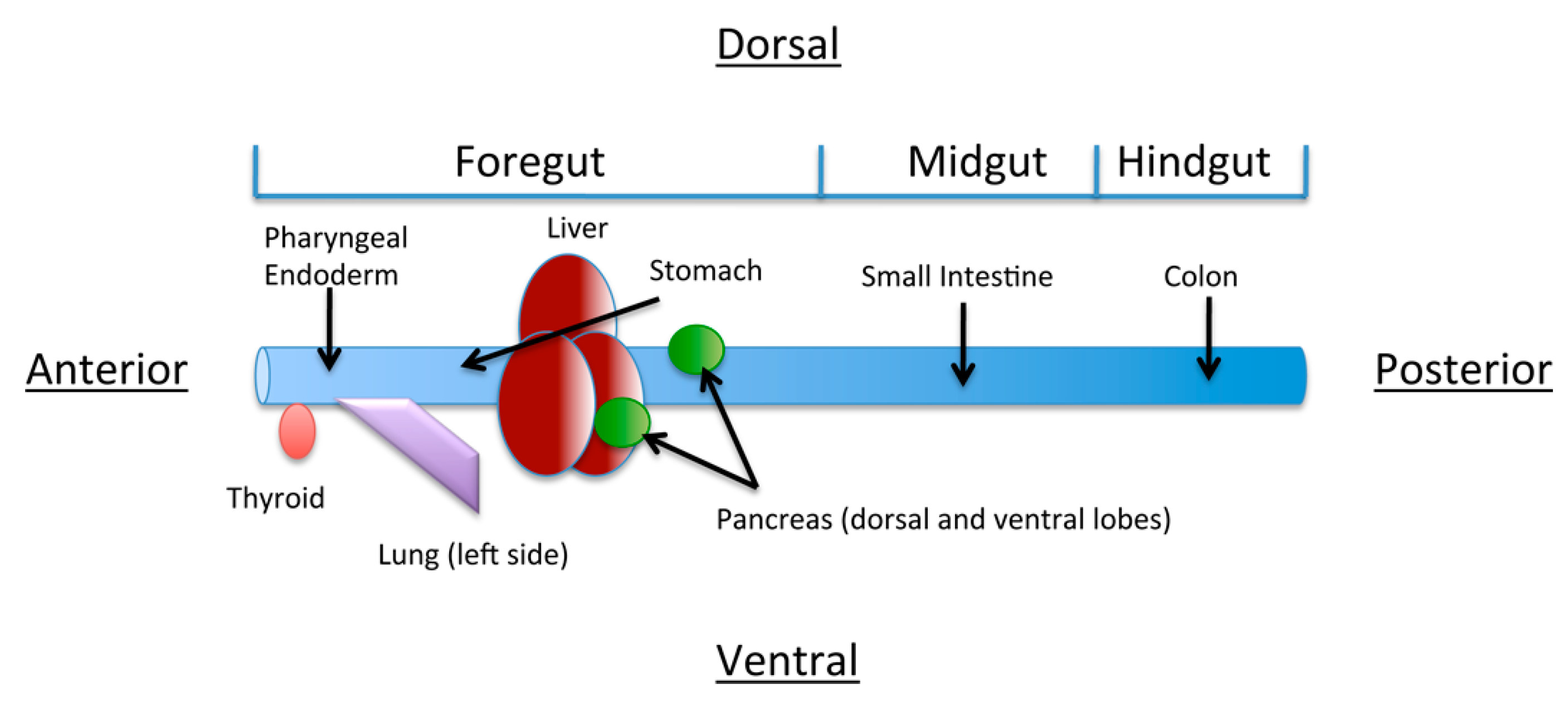
5. Role of Retinoic acid in Individual Organ Systems
5.1. Retinoic Acid and the Thyroid
5.2. Retinoic Acid and the Anterior Foregut
5.3. Retinoic Acid and the Pharynx
5.4. Retinoic Acid and the Lung
5.5. Retinoic Acid and the Pancreas
5.6. Retinoic Acid and the Liver
5.7. Retinoic Acid and the Stomach
5.8. Retinoic acid and Posterior Endoderm
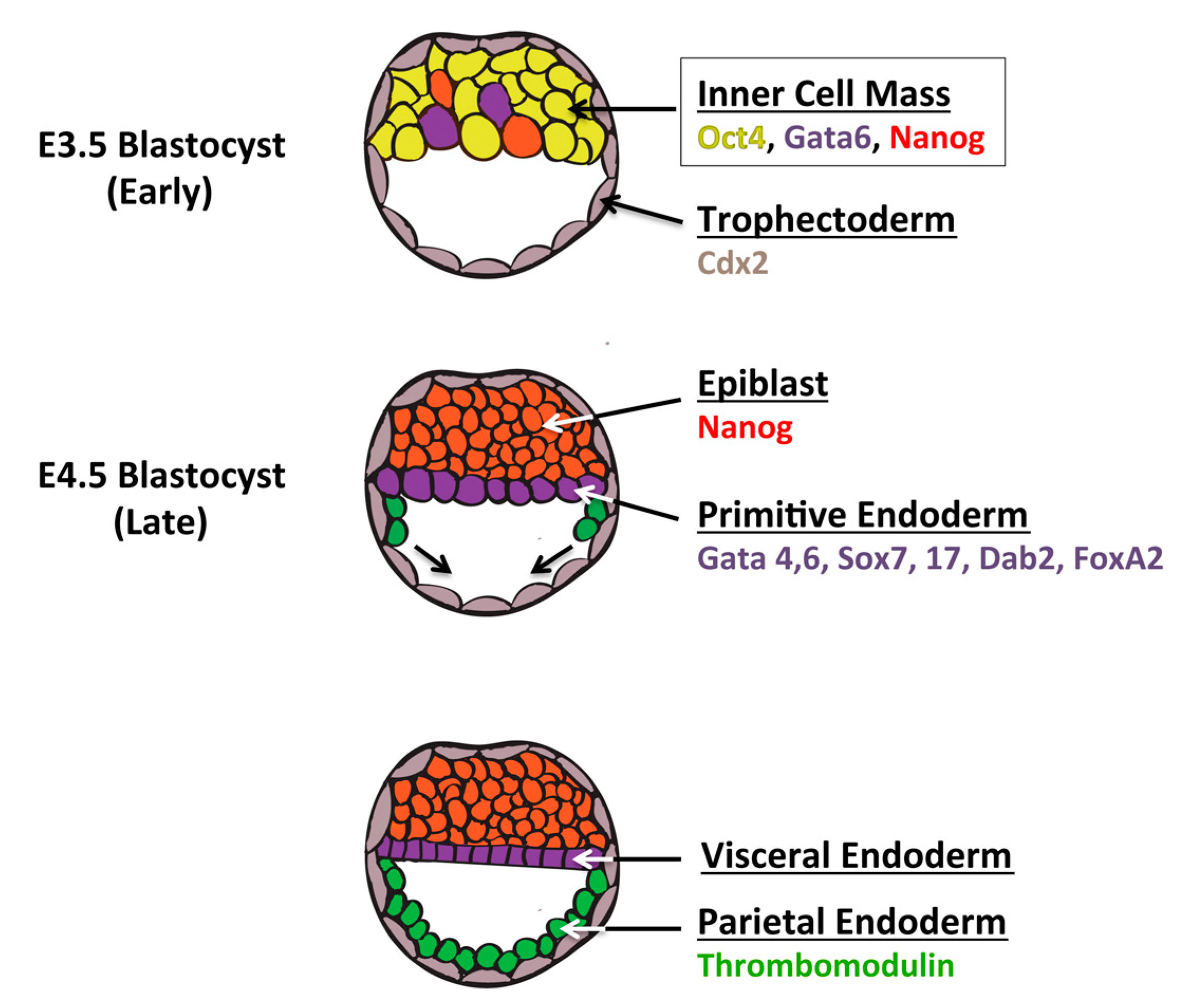
6. Definitive Endoderm vs. the Extraembryonic Endoderm Lineage
7. Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [PubMed]
- Rhinn, M.; Dolle, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Kawamura, K. Acquisition of retinoic acid signaling pathway and innovation of the chordate body plan. Zoolog. Sci. 2003, 20, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Marletaz, F.; Holland, L.Z.; Laudet, V.; Schubert, M. Retinoic acid signaling and the evolution of chordates. Int. J. Biol. Sci. 2006, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Canestro, C.; Postlethwait, J.H.; Gonzalez-Duarte, R.; Albalat, R. Is retinoic acid genetic machinery a chordate innovation? Evol. Dev. 2006, 8, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Simoes-Costa, M.S.; Azambuja, A.P.; Xavier-Neto, J. The search for non-chordate retinoic acid signaling: Lessons from chordates. J. Exp. Zool. B Mol. Dev. Evol. 2008, 310, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Albalat, R. The retinoic acid machinery in invertebrates: Ancestral elements and vertebrate innovations. Mol. Cell. Endocrinol 2009, 313, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Canestro, C.; Postlethwait, J.H. Development of a chordate anterior-posterior axis without classical retinoic acid signaling. Dev. Biol. 2007, 305, 522–538. [Google Scholar] [CrossRef]
- Stafford, D.; Prince, V.E. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr. Biol. 2002, 12, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, F.C.; Brandes, N.; Afelik, S.; Solter, M.; Pieler, T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in xenopus. Dev. Biol. 2004, 271, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Bayha, E.; Jorgensen, M.C.; Serup, P.; Grapin-Botton, A. Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One 2009, 4, e5845. [Google Scholar] [CrossRef] [PubMed]
- Hashimshony, T.; Feder, M.; Levin, M.; Hall, B.K.; Yanai, I. Spatiotemporal transcriptomics reveals the evolutionary history of the endoderm germ layer. Nature 2015, 519, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Zorn, A.M.; Wells, J.M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell. Dev. Biol. 2009, 25, 221–251. [Google Scholar] [CrossRef] [PubMed]
- Warga, R.M.; Nusslein-Volhard, C. Origin and development of the zebrafish endoderm. Development 1999, 126, 827–838. [Google Scholar] [PubMed]
- Rodaway, A.; Takeda, H.; Koshida, S.; Broadbent, J.; Price, B.; Smith, J.C.; Patient, R.; Holder, N. Induction of the mesendoderm in the zebrafish germ ring by yolk cell-derived tgf-beta family signals and discrimination of mesoderm and endoderm by fgf. Development 1999, 126, 3067–3078. [Google Scholar] [PubMed]
- Lemaire, P.; Darras, S.; Caillol, D.; Kodjabachian, L. A role for the vegetally expressed xenopus gene mix.1 in endoderm formation and in the restriction of mesoderm to the marginal zone. Development 1998, 125, 2371–2380. [Google Scholar] [PubMed]
- Maduro, M.F.; Meneghini, M.D.; Bowerman, B.; Broitman-Maduro, G.; Rothman, J.H. Restriction of mesendoderm to a single blastomere by the combined action of skn-1 and a gsk-3beta homolog is mediated by med-1 and -2 in c. Elegans. Mol. Cell. 2001, 7, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Technau, U.; Scholz, C.B. Origin and evolution of endoderm and mesoderm. Int. J. Dev. Biol. 2003, 47, 531–539. [Google Scholar] [PubMed]
- Shen, M.M. Nodal signaling: Developmental roles and regulation. Development 2007, 134, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Schier, A.F. The zebrafish nodal signal squint functions as a morphogen. Nature 2001, 411, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Kruithof-de Julio, M.; Alvarez, M.J.; Galli, A.; Chu, J.; Price, S.M.; Califano, A.; Shen, M.M. Regulation of extra-embryonic endoderm stem cell differentiation by nodal and cripto signaling. Development 2011, 138, 3885–3895. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Brown, K.; Legros, S.; Foley, A.C. Nodal mutant extraembryonic endoderm (xen) stem cells upregulate markers for the anterior visceral endoderm and impact the timing of cardiac differentiation in mouse embryoid bodies. Biol Open 2012, 1, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Zorn, A.M.; Wells, J.M. Molecular basis of vertebrate endoderm development. Int Rev. Cytol 2007, 259, 49–111. [Google Scholar] [PubMed]
- Chiu, W.T.; Charney Le, R.; Blitz, I.L.; Fish, M.B.; Li, Y.; Biesinger, J.; Xie, X.; Cho, K.W. Genome-wide view of tgfbeta/foxh1 regulation of the early mesendoderm program. Development 2014, 141, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Yashiro, K.; Takaoka, K.; Yamamoto, M.; Hamada, H. Removal of maternal retinoic acid by embryonic cyp26 is required for correct nodal expression during early embryonic patterning. Genes Dev. 2009, 23, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wakamiya, M.; Shea, M.J.; Albrecht, U.; Behringer, R.R.; Bradley, A. Requirement for wnt3 in vertebrate axis formation. Nat. Genet. 1999, 22, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.; Lu, C.C.; Norris, D.P.; Rodriguez, T.A.; Beddington, R.S.; Robertson, E.J. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 2001, 411, 965–969. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Cata, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Niakan, K.K.; Schrode, N.; Cho, L.T.; Hadjantonakis, A.K. Derivation of extraembryonic endoderm stem (xen) cells from mouse embryos and embryonic stem cells. Nat. Protoc. 2013, 8, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Krawetz, R.; Kelly, G.M. Wnt6 induces the specification and epithelialization of F9 embryonal carcinoma cells to primitive endoderm. Cell. Signal. 2008, 20, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Adamson, E.D. Evidence for the existence of an early common biochemical pathway in the differentiation of f9 cells into visceral or parietal endoderm: Modulation by cyclic amp. Dev. Biol. 1986, 114, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 451–480. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 2009, 7, e002. [Google Scholar] [PubMed]
- Farjo, K.M.; Moiseyev, G.; Nikolaeva, O.; Sandell, L.L.; Trainor, P.A.; Ma, J.X. Rdh10 is the primary enzyme responsible for the first step of embryonic vitamin a metabolism and retinoic acid synthesis. Dev. Biol. 2011, 357, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Schuhbaur, B.; Niederreither, K.; Dolle, P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc. Natl. Acad. Sci. USA 2011, 108, 16687–16692. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.L.; Lynn, M.L.; Inman, K.E.; McDowell, W.; Trainor, P.A. Rdh10 oxidation of vitamin a is a critical control step in synthesis of retinoic acid during mouse embryogenesis. PLoS One 2012, 7, e30698. [Google Scholar] [CrossRef] [PubMed]
- Strate, I.; Min, T.H.; Iliev, D.; Pera, E.M. Retinol dehydrogenase 10 is a feedback regulator of retinoic acid signalling during axis formation and patterning of the central nervous system. Development 2009, 136, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Hernandez, R.E.; Waxman, J.S.; Yelon, D.; Moens, C.B. Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev. Biol. 2010, 338, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sandell, L.L.; Trainor, P.A.; Koentgen, F.; Duester, G. Alcohol and aldehyde dehydrogenases: Retinoid metabolic effects in mouse knockout models. Biochim. Biophys. Acta 2012, 1821, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.; Leung, C.Y.; Tang, W.W.; Choi, H.L.; Leung, Y.C.; McCaffery, P.J.; Wang, C.C.; Woolf, A.S.; Shum, A.S. A paradoxical teratogenic mechanism for retinoic acid. Proc. Natl. Acad. Sci. USA 2012, 109, 13668–13673. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Fraulob, V.; Garnier, J.M.; Chambon, P.; Dolle, P. Differential expression of retinoic acid-synthesizing (raldh) enzymes during fetal development and organ differentiation in the mouse. Mech. Dev. 2002, 110, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Subbarayan, V.; Dolle, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999, 21, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Vermot, J.; Messaddeq, N.; Schuhbaur, B.; Chambon, P.; Dolle, P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 2001, 128, 1019–1031. [Google Scholar]
- Niederreither, K.; Vermot, J.; Schuhbaur, B.; Chambon, P.; Dolle, P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development 2002, 129, 3563–3574. [Google Scholar]
- Niederreither, K.; Dolle, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Molotkov, A.; Manabe, S.; Donmoyer, C.M.; Deltour, L.; Foglio, M.H.; Cuenca, A.E.; Blaner, W.S.; Lipton, S.A.; Duester, G. Targeted disruption of aldh1a1 (raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol. Cell. Biol. 2003, 23, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Dupe, V.; Matt, N.; Garnier, J.M.; Chambon, P.; Mark, M.; Ghyselinck, N.B. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl. Acad. Sci. USA 2003, 100, 14036–14041. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, C.; Brade, T.; Duester, G. Retinoic acid functions as a key gabaergic differentiation signal in the basal ganglia. PLoS Biol. 2011, 9, e1000609. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.; Wilson, L.; Maden, M.; Lumsden, A. Raldh-independent generation of retinoic acid during vertebrate embryogenesis by cyp1b1. Development 2007, 134, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Lampert, J.M.; Holzschuh, J.; Hessel, S.; Driever, W.; Vogt, K.; von Lintig, J. Provitamin a conversion to retinal via the beta,beta-carotene-15,15'-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 2003, 130, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Wassef, L.; Chung, S.; Jiang, H.; Wyss, A.; Blaner, W.S.; Quadro, L. Beta-carotene and its cleavage enzyme beta-carotene-15,15'-oxygenase (cmoi) affect retinoid metabolism in developing tissues. FASEB J. 2011, 25, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Hessel, S.; Eichinger, A.; Isken, A.; Amengual, J.; Hunzelmann, S.; Hoeller, U.; Elste, V.; Hunziker, W.; Goralczyk, R.; Oberhauser, V.; et al. Cmo1 deficiency abolishes vitamin a production from beta-carotene and alters lipid metabolism in mice. J. Biol. Chem. 2007, 282, 33553–33561. [Google Scholar] [CrossRef] [PubMed]
- Molotkov, A.; Molotkova, N.; Duester, G. Retinoic acid generated by raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev. Dyn. 2005, 232, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Pennimpede, T.; Cameron, D.A.; MacLean, G.A.; Li, H.; Abu-Abed, S.; Petkovich, M. The role of cyp26 enzymes in defining appropriate retinoic acid exposure during embryogenesis. Birth Defects Res. A Clin Mol. Teratol. 2010, 88, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Zolfaghari, R. Cytochrome p450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Meno, C.; Fujii, H.; Nishino, J.; Shiratori, H.; Saijoh, Y.; Rossant, J.; Hamada, H. The retinoic acid-inactivating enzyme cyp26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001, 15, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Abu-Abed, S.; Dolle, P.; Metzger, D.; Beckett, B.; Chambon, P.; Petkovich, M. The retinoic acid-metabolizing enzyme, cyp26a1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001, 15, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.E.; Putzke, A.P.; Myers, J.P.; Margaretha, L.; Moens, C.B. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development 2007, 134, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Tibbles, L.; Wiley, M.J. A comparative study of the effects of retinoic acid given during the critical period for inducing spina bifida in mice and hamsters. Teratology 1988, 37, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Thaller, C.; Eichele, G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature 1987, 327, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.Q.; Radtke, K.; Linville, A.; Lander, A.D.; Nie, Q.; Schilling, T.F. Cellular retinoic acid-binding proteins are essential for hindbrain patterning and signal robustness in zebrafish. Development 2012, 139, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Lampron, C.; Rochette-Egly, C.; Gorry, P.; Dolle, P.; Mark, M.; Lufkin, T.; LeMeur, M.; Chambon, P. Mice deficient in cellular retinoic acid binding protein ii (crabpii) or in both crabpi and crabpii are essentially normal. Development 1995, 121, 539–548. [Google Scholar] [PubMed]
- Ghyselinck, N.B.; Bavik, C.; Sapin, V.; Mark, M.; Bonnier, D.; Hindelang, C.; Dierich, A.; Nilsson, C.B.; Hakansson, H.; Sauvant, P.; et al. Cellular retinol-binding protein i is essential for vitamin a homeostasis. EMBO J. 1999, 18, 4903–4914. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Jacobs, H.; Marwarha, G.; Gely-Pernot, A.; O’Byrne, S.M.; DeSantis, D.; Klopfenstein, M.; Feret, B.; Dennefeld, C.; Blaner, W.S.; et al. The stra6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin a homeostasis in tissues other than the eye. J. Biol. Chem. 2013, 288, 24528–24539. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Mark, M.; Jacobs, H.; Klopfenstein, M.; Hu, J.; Lloyd, M.; Habib, S.; Tosha, C.; Radu, R.A.; Ghyselinck, N.B.; et al. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, stra6. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.Y.; Pasutto, F.; Bardakjian, T.M.; Wilson, M.J.; Watson, G.; Schneider, A.; Mackey, D.A.; Grigg, J.R.; Zenker, M.; Jamieson, R.V. A puzzle over several decades: Eye anomalies with fras1 and stra6 mutations in the same family. Clin. Genet. 2013, 83, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Pasutto, F.; Sticht, H.; Hammersen, G.; Gillessen-Kaesbach, G.; Fitzpatrick, D.R.; Nurnberg, G.; Brasch, F.; Schirmer-Zimmermann, H.; Tolmie, J.L.; Chitayat, D.; et al. Mutations in stra6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet. 2007, 80, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Al Tanoury, Z.; Piskunov, A.; Rochette-Egly, C. Vitamin a and retinoid signaling: Genomic and nongenomic effects. J. Lipid Res. 2013, 54, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Moutier, E.; Ye, T.; Choukrallah, M.A.; Urban, S.; Osz, J.; Chatagnon, A.; Delacroix, L.; Langer, D.; Rochel, N.; Moras, D.; et al. Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J. Biol. Chem. 2012, 287, 26328–26341. [Google Scholar] [CrossRef] [PubMed]
- Mahony, S.; Mazzoni, E.O.; McCuine, S.; Young, R.A.; Wichterle, H.; Gifford, D.K. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 2011, 12, R2. [Google Scholar] [CrossRef] [PubMed]
- Al Tanoury, Z.; Gaouar, S.; Piskunov, A.; Ye, T.; Urban, S.; Jost, B.; Keime, C.; Davidson, I.; Dierich, A.; Rochette-Egly, C. Phosphorylation of the retinoic acid receptor rargamma2 is crucial for the neuronal differentiation of mouse embryonic stem cells. J. Cell Sci. 2014, 127, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Al Tanoury, Z.; Piskunov, A.; Andriamoratsiresy, D.; Gaouar, S.; Lutzing, R.; Ye, T.; Jost, B.; Keime, C.; Rochette-Egly, C. Genes involved in cell adhesion and signaling: A new repertoire of retinoic acid receptor target genes in mouse embryonic fibroblasts. J. Cell Sci. 2014, 127, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Delacroix, L.; Moutier, E.; Altobelli, G.; Legras, S.; Poch, O.; Choukrallah, M.A.; Bertin, I.; Jost, B.; Davidson, I. Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Mol. Cell. Biol. 2010, 30, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.G.; Lunyak, V.V.; Glass, C.K. Sensors and signals: A coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006, 20, 1405–1428. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Kittler, R.; White, K.P. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell 2009, 137, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.; Gudas, L.J. Epigenetic regulatory mechanisms distinguish retinoic acid-mediated transcriptional responses in stem cells and fibroblasts. J. Biol. Chem. 2010, 285, 14534–14548. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jordan, N.; Melton, D.; Grapin-Botton, A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 2003, 259, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pollet, N.; Niehrs, C.; Pieler, T. Increased xraldh2 activity has a posteriorizing effect on the central nervous system of xenopus embryos. Mech. Dev. 2001, 101, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; McEwan, J.; Beck, C.W. Analysis of the expression of retinoic acid metabolising genes during xenopus laevis organogenesis. Gene Expr. Patterns 2011, 11, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Deimling, S.J.; D’Alessandro, N.E.; Zhao, L.; Possmayer, F.; Drysdale, T.A. Retinoic acid is a key regulatory switch determining the difference between lung and thyroid fates in xenopus laevis. BMC Dev. Biol. 2011, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Pera, E.M.; Acosta, H.; Gouignard, N.; Climent, M.; Arregi, I. Active signals, gradient formation and regional specificity in neural induction. Exp. Cell Res. 2014, 321, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ribes, V.; Le Roux, I.; Rhinn, M.; Schuhbaur, B.; Dolle, P. Early mouse caudal development relies on crosstalk between retinoic acid, shh and fgf signalling pathways. Development 2009, 136, 665–676. [Google Scholar] [CrossRef]
- Aulehla, A.; Pourquie, O. Signaling gradients during paraxial mesoderm development. Cold Spring Harb. Perspect. Biol. 2010, 2, a000869. [Google Scholar] [CrossRef] [PubMed]
- Horb, M.E.; Slack, J.M. Endoderm specification and differentiation in xenopus embryos. Dev. Biol. 2001, 236, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Deimling, S.J.; Drysdale, T.A. Retinoic acid regulates anterior-posterior patterning within the lateral plate mesoderm of xenopus. Mech. Dev. 2009, 126, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Deimling, S.J.; Drysdale, T.A. Fgf is required to regulate anterior-posterior patterning in the xenopus lateral plate mesoderm. Mech. Dev. 2011, 128, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.T.; Shah, R.; Jamrich, M. Function and regulation of foxf1 during xenopus gut development. Development 2004, 131, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cao, Y.; Qian, J.; Shao, F.; Niederreither, K.; Cardoso, W.V. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Invest. 2010, 120, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dolle, P.; Cardoso, W.V.; Niederreither, K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 2006, 297, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.; Ghyselinck, N.B.; Wendling, O.; Chambon, P.; Mark, M. Stage-dependent responses of the developing lung to retinoic acid signaling. Int. J. Dev. Biol. 2000, 44, 457–462. [Google Scholar] [PubMed]
- Mendelsohn, C.; Lohnes, D.; Decimo, D.; Lufkin, T.; LeMeur, M.; Chambon, P.; Mark, M. Function of the retinoic acid receptors (rars) during development (ii). Multiple abnormalities at various stages of organogenesis in rar double mutants. Development 1994, 120, 2749–2771. [Google Scholar] [PubMed]
- Graham, A.; Smith, A. Patterning the pharyngeal arches. BioEssays 2001, 23, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Retinoic acid signalling in the development of branchial arches. Curr. Opin. Genet. Dev. 2004, 14, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Vermot, J.; Le Roux, I.; Schuhbaur, B.; Chambon, P.; Dolle, P. The regional pattern of retinoic acid synthesis by raldh2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 2003, 130, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Kopinke, D.; Sasine, J.; Swift, J.; Stephens, W.Z.; Piotrowski, T. Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification. Dev. Dyn. 2006, 235, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Maden, M.; Gale, E.; Kostetskii, I.; Zile, M. Vitamin a-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996, 6, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Dupe, V.; Ghyselinck, N.B.; Wendling, O.; Chambon, P.; Mark, M. Key roles of retinoic acid receptors alpha and beta in the patterning of the caudal hindbrain, pharyngeal arches and otocyst in the mouse. Development 1999, 126, 5051–5059. [Google Scholar] [PubMed]
- Matt, N.; Ghyselinck, N.B.; Wendling, O.; Chambon, P.; Mark, M. Retinoic acid-induced developmental defects are mediated by rarbeta/rxr heterodimers in the pharyngeal endoderm. Development 2003, 130, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Wendling, O.; Dennefeld, C.; Chambon, P.; Mark, M. Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development 2000, 127, 1553–1562. [Google Scholar] [PubMed]
- Graham, A.; Okabe, M.; Quinlan, R. The role of the endoderm in the development and evolution of the pharyngeal arches. J. Anat. 2005, 207, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Mulder, G.B.; Manley, N.; Maggio-Price, L. Retinoic acid-induced thymic abnormalities in the mouse are associated with altered pharyngeal morphology, thymocyte maturation defects, and altered expression of hoxa3 and pax1. Teratology 1998, 58, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Begemann, G.; Schilling, T.F.; Rauch, G.J.; Geisler, R.; Ingham, P.W. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 2001, 128, 3081–3094. [Google Scholar] [PubMed]
- Trainor, P.A.; Krumlauf, R. Hox genes, neural crest cells and branchial arch patterning. Curr. Opin. Cell Biol. 2001, 13, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Nolte, C.; Jinks, T.; Wang, X.; Martinez Pastor, M.T.; Krumlauf, R. Shadow enhancers flanking the hoxb cluster direct dynamic hox expression in early heart and endoderm development. Dev. Biol. 2013, 383, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Ryckebusch, L.; Bertrand, N.; Mesbah, K.; Bajolle, F.; Niederreither, K.; Kelly, R.G.; Zaffran, S. Decreased levels of embryonic retinoic acid synthesis accelerate recovery from arterial growth delay in a mouse model of digeorge syndrome. Circ. Res. 2010, 106, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Yu, J.K.; Holland, N.D.; Escriva, H.; Laudet, V.; Holland, L.Z. Retinoic acid signaling acts via hox1 to establish the posterior limit of the pharynx in the chordate amphioxus. Development 2005, 132, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Dickman, E.D.; Thaller, C.; Smith, S.M. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development 1997, 124, 3111–3121. [Google Scholar] [PubMed]
- Desai, T.J.; Chen, F.; Lu, J.; Qian, J.; Niederreither, K.; Dolle, P.; Chambon, P.; Cardoso, W.V. Distinct roles for retinoic acid receptors alpha and beta in early lung morphogenesis. Dev. Biol 2006, 291, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Desai, T.J.; Qian, J.; Niederreither, K.; Lu, J.; Cardoso, W.V. Inhibition of tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 2007, 134, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.J.; Malpel, S.; Flentke, G.R.; Smith, S.M.; Cardoso, W.V. Retinoic acid selectively regulates fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev. Biol 2004, 273, 402–415. [Google Scholar] [CrossRef]
- Malpel, S.; Mendelsohn, C.; Cardoso, W.V. Regulation of retinoic acid signaling during lung morphogenesis. Development 2000, 127, 3057–3067. [Google Scholar] [PubMed]
- Wilson, J.G.; Roth, C.B.; Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin a deficiency. Effects of restoration of vitamin a at various times during gestation. Am. J. Anat. 1953, 92, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B.L. Fibroblast growth factor 10 (fgf10) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124, 4867–4878. [Google Scholar] [PubMed]
- Vermot, J.; Pourquie, O. Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature 2005, 435, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Vilhais-Neto, G.C.; Maruhashi, M.; Smith, K.T.; Vasseur-Cognet, M.; Peterson, A.S.; Workman, J.L.; Pourquie, O. Rere controls retinoic acid signalling and somite bilateral symmetry. Nature 2010, 463, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Garnaas, M.K.; Cutting, C.C.; Meyers, A.; Kelsey, P.B., Jr.; Harris, J.M.; North, T.E.; Goessling, W. Rargb regulates organ laterality in a zebrafish model of right atrial isomerism. Dev. Biol. 2012, 372, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, K.; Schmitt, C.; Sablyak, A.; Yoder, J.A.; Nascone-Yoder, N. Role for retinoid signaling in left-right asymmetric digestive organ morphogenesis. Dev. Dyn. 2006, 235, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, R.; Babovic, N.; Lteif, A.; Eidem, B.; Kirmani, S.; Olson, T.; Babovic-Vuksanovic, D. Vitamin a deficiency in an infant with pagod syndrome. Am. J. Med. Genet. A 2009, 149A, 2241–2247. [Google Scholar] [CrossRef]
- Golzio, C.; Martinovic-Bouriel, J.; Thomas, S.; Mougou-Zrelli, S.; Grattagliano-Bessieres, B.; Bonniere, M.; Delahaye, S.; Munnich, A.; Encha-Razavi, F.; Lyonnet, S.; et al. Matthew-wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene stra6. Am. J. Hum. Genet. 2007, 80, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Marquez, H.; Kim, Y.K.; Qian, J.; Shao, F.; Fine, A.; Cruikshank, W.W.; Quadro, L.; Cardoso, W.V. Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J. Clin. Invest. 2014, 124, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Gallego-Llamas, J.; Ribes, V.; Kedinger, M.; Niederreither, K.; Chambon, P.; Dolle, P.; Gradwohl, G. Dorsal pancreas agenesis in retinoic acid-deficient raldh2 mutant mice. Dev. Biol. 2005, 284, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Stafford, D.; White, R.J.; Kinkel, M.D.; Linville, A.; Schilling, T.F.; Prince, V.E. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development 2006, 133, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, M.; Loffler, K.A.; Edfalk, S.; Selander, L.; Dahl, U.; Ricordi, C.; Jeon, J.; Correa-Medina, M.; Diez, J.; Edlund, H. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One 2008, 3, e2841. [Google Scholar] [CrossRef] [PubMed]
- Kinkel, M.D.; Sefton, E.M.; Kikuchi, Y.; Mizoguchi, T.; Ward, A.B.; Prince, V.E. Cyp26 enzymes function in endoderm to regulate pancreatic field size. Proc. Natl. Acad. Sci. USA 2009, 106, 7864–7869. [Google Scholar] [CrossRef] [PubMed]
- Stafford, D.; Hornbruch, A.; Mueller, P.R.; Prince, V.E. A conserved role for retinoid signaling in vertebrate pancreas development. Dev. Genes Evol. 2004, 214, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Alexa, K.; Choe, S.K.; Hirsch, N.; Etheridge, L.; Laver, E.; Sagerstrom, C.G. Maternal and zygotic aldh1a2 activity is required for pancreas development in zebrafish. PLoS One 2009, 4, e8261. [Google Scholar] [CrossRef] [PubMed]
- Trasino, S.E.; Benoit, Y.D.; Gudas, L.J. Vitamin a deficiency causes hyperglycemia and loss of pancreatic beta-cell mass. J. Biol. Chem. 2015, 290, 1456–1473. [Google Scholar] [CrossRef] [PubMed]
- Shiota, G.; Kanki, K. Retinoids and their target genes in liver functions and diseases. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 33–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Korzh, V.; Gong, Z. Localized rbp4 expression in the yolk syncytial layer plays a role in yolk cell extension and early liver development. BMC Dev. Biol. 2007, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Grapin-Botton, A. Antero-posterior patterning of the vertebrate digestive tract: 40 years after Nicole le Douarin’s PhD thesis. Inte. J. Dev. Biol. 2005, 49, 335–347. [Google Scholar] [CrossRef]
- Spencer-Dene, B.; Sala, F.G.; Bellusci, S.; Gschmeissner, S.; Stamp, G.; Dickson, C. Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b-mediated signaling. Gastroenterology 2006, 130, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Zeynali, B.; Dixon, K.E. Effects of retinoic acid on the endoderm in xenopus embryos. Dev. Genes Evol. 1998, 208, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Pitera, J.E.; Smith, V.V.; Woolf, A.S.; Milla, P.J. Embryonic gut anomalies in a mouse model of retinoic acid-induced caudal regression syndrome: Delayed gut looping, rudimentary cecum, and anorectal anomalies. Am. J. Pathol. 2001, 159, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.; Ledon-Rettig, C.; Infante, C.; Everly, A.; Hanken, J.; Nascone-Yoder, N. Developmental origins of a novel gut morphology in frogs. Evol. Dev. 2013, 15, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, S.W.; Langston, A.W.; Gudas, L.J. A conserved retinoic acid responsive element in the murine hoxb-1 gene is required for expression in the developing gut. Development 1998, 125, 3235–3246. [Google Scholar] [PubMed]
- Wright-Jin, E.C.; Grider, J.R.; Duester, G.; Heuckeroth, R.O. Retinaldehyde dehydrogenase enzymes regulate colon enteric nervous system structure and function. Dev. Biol. 2013, 381, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Heuckeroth, R.O. Retinoic acid regulates murine enteric nervous system precursor proliferation, enhances neuronal precursor differentiation, and reduces neurite growth in vitro. Dev. Biol. 2008, 320, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Nadauld, L.D.; Shelton, D.N.; Chidester, S.; Yost, H.J.; Jones, D.A. The zebrafish retinol dehydrogenase, rdh1l, is essential for intestinal development and is regulated by the tumor suppressor adenomatous polyposis coli. J. Biol. Chem. 2005, 280, 30490–30495. [Google Scholar] [CrossRef] [PubMed]
- Nadauld, L.D.; Sandoval, I.T.; Chidester, S.; Yost, H.J.; Jones, D.A. Adenomatous polyposis coli control of retinoic acid biosynthesis is critical for zebrafish intestinal development and differentiation. J. Biol. Chem. 2004, 279, 51581–51589. [Google Scholar] [CrossRef] [PubMed]
- Vezina, C.M.; Allgeier, S.H.; Fritz, W.A.; Moore, R.W.; Strerath, M.; Bushman, W.; Peterson, R.E. Retinoic acid induces prostatic bud formation. Dev. Dyn. 2008, 237, 1321–1333. [Google Scholar] [CrossRef]
- Thomson, A.A.; Cunha, G.R. Prostatic growth and development are regulated by fgf10. Development 1999, 126, 3693–3701. [Google Scholar] [PubMed]
- Strickland, S.; Mahdavi, V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell 1978, 15, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gachelin, G. Experimental teratocarcinoma in mice: A model system for the study of the relationship between cellular surface antigens and embryonic differentiation. Bull. Cancer 1976, 63, 95–110. (In French) [Google Scholar] [PubMed]
- Hogan, B.L.; Taylor, A.; Adamson, E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature 1981, 291, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Roach, S.; Schmid, W.; Pera, M.F. Hepatocytic transcription factor expression in human embryonal carcinoma and yolk sac carcinoma cell lines: Expression of hnf-3 alpha in models of early endodermal cell differentiation. Exp. Cell Res. 1994, 215, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Capo-Chichi, C.D.; Rula, M.E.; Smedberg, J.L.; Vanderveer, L.; Parmacek, M.S.; Morrisey, E.E.; Godwin, A.K.; Xu, X.X. Perception of differentiation cues by gata factors in primitive endoderm lineage determination of mouse embryonic stem cells. Dev. Biol. 2005, 286, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Bielinska, M.; Wilson, D.B. Induction of yolk sac endoderm in gata-4-deficient embryoid bodies by retinoic acid. Mech. Dev. 1997, 65, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, J.; Smuga-Otto, K.; Tian, S.; Yu, J.; Stewart, R.; Thomson, J.A. Protein kinase c mediated extraembryonic endoderm differentiation of human embryonic stem cells. Stem Cells 2012, 30, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Papalopulu, N.; Lovell-Badge, R.; Krumlauf, R. The expression of murine hox-2 genes is dependent on the differentiation pathway and displays a collinear sensitivity to retinoic acid in f9 cells and xenopus embryos. Nucleic Acids Res. 1991, 19, 5497–5506. [Google Scholar] [CrossRef]
- Mummery, C.L.; Feyen, A.; Freund, E.; Shen, S. Characteristics of embryonic stem cell differentiation: A comparison with two embryonal carcinoma cell lines. Cell Differ. Dev. 1990, 30, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Bielinska, M.; Narita, N.; Wilson, D.B. Distinct roles for visceral endoderm during embryonic mouse development. Int. J. Dev. Biol. 1999, 43, 183–205. [Google Scholar] [PubMed]
- Rochette-Egly, C.; Chambon, P. F9 embryocarcinoma cells: A cell autonomous model to study the functional selectivity of rars and rxrs in retinoid signaling. Histol. Histopathol. 2001, 16, 909–922. [Google Scholar] [PubMed]
- Evain, D.; Binet, E.; Anderson, W.B. Alterations in calcitonin and parathyroid hormone responsiveness of adenylate cyclase in f9 embryonal carcinoma cells treated with retinoic acid and dibutyryl cyclic amp. J. Cell. Phys. 1981, 109, 453–459. [Google Scholar] [CrossRef]
- Strickland, S.; Smith, K.K.; Marotti, K.R. Hormonal induction of differentiation in teratocarcinoma stem cells: Generation of parietal endoderm by retinoic acid and dibutyryl camp. Cell 1980, 21, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Evain-Brion, D.; Binet, E.; Donnadieu, M.; Laurent, P.; Anderson, W.B. Production of immunoreactive calcitonin and parathyroid hormone by embryonal carcinoma cells: Alteration with retinoic acid-induced differentiation. Dev. Biol. 1984, 104, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Campisi, J.; Wang, S.Y.; Gudas, L.J. Butyrate inhibits the retinoic acid-induced differentiation of f9 teratocarcinoma stem cells. Dev. Biol. 1984, 105, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Sugimoto, E.; Kitagawa, Y. Reversible interconversion between primitive endoderm- and parietal endoderm-like f9 cells demonstrated by mrnas expression. J. Biochem. 1987, 102, 385–392. [Google Scholar] [PubMed]
- Hadadeh, O.; Barruet, E.; Peiretti, F.; Verdier, M.; Bernot, D.; Hadjal, Y.; Yazidi, C.E.; Robaglia-Schlupp, A.; de Paula, A.M.; Negre, D.; et al. The plasminogen activation system modulates differently adipogenesis and myogenesis of embryonic stem cells. PLoS ONE 2012, 7, e49065. [Google Scholar] [CrossRef] [PubMed]
- Solter, D.; Shevinsky, L.; Knowles, B.B.; Strickland, S. The induction of antigenic changes in a teratocarcinoma stem cell line (f9) by retinoic acid. Dev. Biol. 1979, 70, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Oshima, R.G.; Adamson, E.D. Epithelial layer formation in differentiating aggregates of f9 embryonal carcinoma cells. J. Cell Biol. 1983, 96, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Adamson, E. Localization and synthesis of alphafoetoprotein in post-implantation mouse embryos. J. Embryol. Exp. Morphol. 1978, 43, 289–313. [Google Scholar] [PubMed]
- Harris, T.M.; Childs, G. Global gene expression patterns during differentiation of f9 embryonal carcinoma cells into parietal endoderm. Funct. Integr. Genomics 2002, 2, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Sangster-Guity, N.; Yu, L.M.; McCormick, P. Molecular profiling of embryonal carcinoma cells following retinoic acid or histone deacetylase inhibitor treatment. Cancer Biol. Ther. 2004, 3, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Eifert, C.; Sangster-Guity, N.; Yu, L.-M.; Chittur, S.V.; Perez, A.V.; Tine, J.A.; McCormick, P.J. Global gene expression profiles associated with retinoic acid-induced differentiation of embryonal carcinoma cells. Mo. Reprod. Dev. 2006, 73, 796–824. [Google Scholar] [CrossRef]
- Wang, S.Y.; LaRosa, G.J.; Gudas, L.J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic amp in cultured mouse teratocarcinoma cells. Dev. Biol. 1985, 107, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Soprano, D.R.; Teets, B.W.; Soprano, K.J. Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam. Horm. 2007, 75, 69–95. [Google Scholar] [PubMed]
- Alonso, A.; Breuer, B.; Steuer, B.; Fischer, J. The F9-EC cell line as a model for the analysis of differentiation. Int. J. Dev. Biol. 1991, 35, 389–397. [Google Scholar] [PubMed]
- Su, D.; Gudas, L.J. Gene expression profiling elucidates a specific role for rargamma in the retinoic acid-induced differentiation of f9 teratocarcinoma stem cells. Biochem. Pharmacol. 2008, 75, 1129–1160. [Google Scholar] [CrossRef] [PubMed]
- Astigiano, S.; Sherman, M.I.; Abarzua, P. Regulation and patterns of endogenous and exogenous gene expression during differentiation of embryonal carcinoma cells. Environ. Health Perspect 1989, 80, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.B.; Watkins, S.C.; Gudas, L.J. Gene expression in visceral endoderm: A comparison of mutant and wild-type f9 embryonal carcinoma cell differentiation. J. Cell Biol. 1990, 110, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Ikuma, S.; Kiyota, M.; Setoyama, C.; Shimada, K. Isolation and characterization of the cdnas corresponding to mrnas abundant in undifferentiated mouse embryonal teratocarcinoma stem cells, but not in differentiated mouse parietal endoderm cells. J. Biochem. 1986, 100, 1185–1192. [Google Scholar] [PubMed]
- Sleigh, M.J. Gene expression and differentiation in f9 mouse embryonal carcinoma cells. Biochem. Soc. Symp. 1989, 55, 1–12. [Google Scholar] [PubMed]
- Hu, L.; Gudas, L.J. Cyclic amp analogs and retinoic acid influence the expression of retinoic acid receptor alpha, beta, and gamma mrnas in f9 teratocarcinoma cells. Mol. Cell. Biol. 1990, 10, 391–396. [Google Scholar] [PubMed]
- Boylan, J.F.; Lufkin, T.; Achkar, C.C.; Taneja, R.; Chambon, P.; Gudas, L.J. Targeted disruption of retinoic acid receptor alpha (rar alpha) and rar gamma results in receptor-specific alterations in retinoic acid-mediated differentiation and retinoic acid metabolism. Mol. Cell. Biol. 1995, 15, 843–851. [Google Scholar] [PubMed]
- Mendoza-Parra, M.A.; Walia, M.; Sankar, M.; Gronemeyer, H. Dissecting the retinoid-induced differentiation of f9 embryonal stem cells by integrative genomics. Mol. Syst. Biol. 2011, 7, 538. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Tilghman, S.M. Induction of alpha-fetoprotein synthesis in differentiating f9 teratocarcinoma cells is accompanied by a genome-wide loss of DNA methylation. Mol. Cell. Biol. 1984, 4, 898–907. [Google Scholar] [PubMed]
- Gudas, L.J. Retinoids induce stem cell differentiation via epigenetic changes. Semin. Cell. Dev. Biol. 2013, 24, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.E.; Grabel, L.B. The role of cell interactions in the differentiation of teratocarcinoma-derived parietal and visceral endoderm. Dev. Biol. 1988, 129, 124–139. [Google Scholar] [CrossRef] [PubMed]
- LaMonica, K.; Bass, M.; Grabel, L. The planar cell polarity pathway directs parietal endoderm migration. Dev. Biol. 2009, 330, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Wiles, M.V. Isolation of differentially expressed human cdna clones: Similarities between mouse and human embryonal carcinoma cell differentiation. Development 1988, 104, 403–413. [Google Scholar] [PubMed]
- Chuykin, I.; Schulz, H.; Guan, K.; Bader, M. Activation of the pthrp/adenylate cyclase pathway promotes differentiation of rat xen cells into parietal endoderm, whereas wnt/beta-catenin signaling promotes differentiation into visceral endoderm. J. Cell Sci. 2013, 126, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Moerkamp, A.T.; Paca, A.; Goumans, M.J.; Kunath, T.; Kruithof, B.P.; Kruithof-de Julio, M. Extraembryonic endoderm cells as a model of endoderm development. Dev. Growth Differ. 2013, 55, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hosler, B.A.; LaRosa, G.J.; Grippo, J.F.; Gudas, L.J. Expression of rex-1, a gene containing zinc finger motifs, is rapidly reduced by retinoic acid in f9 teratocarcinoma cells. Mol. Cell. Biol. 1989, 9, 5623–5629. [Google Scholar]
- Vasios, G.W.; Gold, J.D.; Petkovich, M.; Chambon, P.; Gudas, L.J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin b1 gene. Proc. Natl. Acad. Sci. USA 1989, 86, 9099–9103. [Google Scholar] [CrossRef]
- Kogai, T.; Liu, Y.Y.; Richter, L.L.; Mody, K.; Kagechika, H.; Brent, G.A. Retinoic acid induces expression of the thyroid hormone transporter, monocarboxylate transporter 8 (mct8). J. Biol. Chem. 2010, 285, 27279–27288. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Capo-chichi, C.D.; He, J.; Smedberg, J.L.; Yang, D.H.; Prowse, A.H.; Godwin, A.K.; Hamilton, T.C.; Xu, X.X. Disabled-2 mediates c-fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J Biol. Chem. 2001, 276, 47303–47310. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Cho, S.Y.; Lee, S.H.; Park, S.S. Differential expression of mouse disabled 2 gene in retinoic acid-treated f9 embryonal carcinoma cells and early mouse embryos. Mol. Cells 1999, 9, 179–184. [Google Scholar] [PubMed]
- Karperien, M.; Farih-Sips, H.; Hendriks, J.A.; Lanske, B.; Papapoulos, S.E.; Abou-Samra, A.B.; Löwik, C.W.; Defize, L.H. Identification of a retinoic acid-inducible element in the murine pth/pthrp (parathyroid hormone/parathyroid hormone-related peptide) receptor gene. Mol. Endocrinol. 1999, 13, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, M.H.; Wolthuis, R.M.; Bos, J.L.; Defize, L.H. The ras/erk pathway induces primitive endoderm but prevents parietal endoderm differentiation of f9 embryonal carcinoma cells. J. Biol. Chem. 1999, 274, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.D.; Strewler, G.J.; King, K.L.; Nissenson, R.A. Expression of a parathyroid hormone-like protein and its receptor during differentiation of embryonal carcinoma cells. Mol. Endocrinol. 1990, 4, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Van de Stolpe, A.; Karperien, M.; Lowik, C.W.; Juppner, H.; Segre, G.V.; Abou-Samra, A.B.; de Laat, S.W.; Defize, L.H. Parathyroid hormone-related peptide as an endogenous inducer of parietal endoderm differentiation. J. Cell Biol. 1993, 120, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Vilardaga, J.P.; Romero, G.; Friedman, P.A.; Gardella, T.J. Molecular basis of parathyroid hormone receptor signaling and trafficking: A family b gpcr paradigm. Cell. Mol. Life Sci. 2011, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Smedberg, J.L.; Smith, E.R.; Capo-Chichi, C.D.; Frolov, A.; Yang, D.H.; Godwin, A.K.; Xu, X.X. Ras/mapk pathway confers basement membrane dependence upon endoderm differentiation of embryonic carcinoma cells. J. Biol. Chem. 2002, 277, 40911–40918. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Smedberg, J.L.; Rula, M.E.; Hamilton, T.C.; Xu, X.X. Disassociation of mapk activation and c-fos expression in f9 embryonic carcinoma cells following retinoic acid-induced endoderm differentiation. J. Biol. Chem. 2001, 276, 32094–32100. [Google Scholar] [CrossRef] [PubMed]
- Karperien, M.; van Dijk, T.B.; Hoeijmakers, T.; Cremers, F.; Abou-Samra, A.B.; Boonstra, J.; de Laat, S.W.; Defize, L.H. Expression pattern of parathyroid hormone/parathyroid hormone related peptide receptor mrna in mouse postimplantation embryos indicates involvement in multiple developmental processes. Mech. Dev. 1994, 47, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Karperien, M.; Lanser, P.; de Laat, S.W.; Boonstra, J.; Defize, L.H. Parathyroid hormone related peptide mrna expression during murine postimplantation development: Evidence for involvement in multiple differentiation processes. Int. J. Dev. Biol. 1996, 40, 599–608. [Google Scholar] [PubMed]
- Verheijen, M.H.; Defize, L.H. Signals governing extraembryonic endoderm formation in the mouse: Involvement of the type 1 parathyroid hormone-related peptide (pthrp) receptor, p21ras and cell adhesion molecules. Int. J. Dev. Biol. 1999, 43, 711–721. [Google Scholar] [PubMed]
- Yoshida-Koide, U.; Matsuda, T.; Saikawa, K.; Nakanuma, Y.; Yokota, T.; Asashima, M.; Koide, H. Involvement of ras in extraembryonic endoderm differentiation of embryonic stem cells. Biochem. Biophys. Res. Commun. 2004, 313, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Veltmaat, J.M.; Orelio, C.C.; Ward-van Oostwaard, D.; van Rooijen, M.A.; Mummery, C.L.; Defize, L.H. Snail is an immediate early target gene of parathyroid hormone related peptide signaling in parietal endoderm formation. Int. J. Dev. Biol. 2000, 44, 297–307. [Google Scholar] [PubMed]
- Doble, B.W.; Woodgett, J.R. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs 2007, 185, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Krawetz, R.; Kelly, G.M. Coordinate Gα13 and Wnt6-β-catenin signaling in F9 embryonal carcinoma cells is required for primitive endoderm differentiation. Biochem. Cell Biol. 2009, 87, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Krawetz, R.; MacKenzie, M.J.; Sun, Q.; Walton, P.A.; Kelly, G.M. Galpha13 activation rescues moesin-depletion induced apoptosis in F9 teratocarcinoma cells. Exp. Cell Res. 2006, 312, 3224–3240. [Google Scholar] [CrossRef] [PubMed]
- Krawetz, R.J.; Taiani, J.; Greene, A.; Kelly, G.M.; Rancourt, D.E. Inhibition of rho kinase regulates specification of early differentiation events in P19 embryonal carcinoma stem cells. PLoS ONE 2011, 6, e26484. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Davis, R.J.; Malbon, C.C. C-jun amino-terminal kinase is regulated by galpha12/galpha13 and obligate for differentiation of p19 embryonal carcinoma cells by retinoic acid. J. Biol. Chem. 1997, 272, 24468–24474. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Wang, H.Y.; Malbon, C.C. Ku80 is required but not sufficient for galpha13-mediated endodermal differentiation in p19 embryonic carcinoma cells. Biochem. Biophys. Res. Commun. 2004, 323, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Malbon, C.C.; Wang, H.Y. G alpha 13 signals via p115rhogef cascades regulating jnk1 and primitive endoderm formation. J. Biol. Chem. 2004, 279, 54896–54904. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Kanungo, J.; Malbon, C.C. Expression of galpha 13 (q226l) induces p19 stem cells to primitive endoderm via mekk1, 2, or 4. J. Biol. Chem. 2002, 277, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- Bikkavilli, R.K.; Feigin, M.E.; Malbon, C.C. P38 mitogen-activated protein kinase regulates canonical wnt-beta-catenin signaling by inactivation of gsk3beta. J. Cell Sci. 2008, 121, 3598–3607. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Nagafuchi, A.; Kikuchi, A. Retinoic acid induces discrete wnt-signaling-dependent differentiation in f9 cells. Biochem. Biophys. Res. Commun. 2009, 390, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zhao, L.; Yin, F.; Lan, R.; Li, L.; Zhang, X.; Zhang, H.; Yang, B. Tcf3 inhibits f9 embryonal carcinoma growth by the down-regulation of oct4. Oncol. Rep. 2011, 26, 893–899. [Google Scholar] [PubMed]
- Sandieson, L.; Hwang, J.T.; Kelly, G.M. Redox regulation of canonical Wnt signaling affects extraembryonic endoderm formation. Stem Cells Dev. 2014, 23, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, S.; Winer, J.; Williams, M.; Polakis, P. A blockade in wnt signaling is activated following the differentiation of f9 teratocarcinoma cells. Exp. Cell Res. 2004, 292, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Prunier, C.; Howe, P.H. The inhibitory effects of disabled-2 (dab2) on wnt signaling are mediated through axin. Oncogene 2008, 27, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Arceci, R.J.; King, A.A.; Simon, M.C.; Orkin, S.H.; Wilson, D.B. Mouse gata-4: A retinoic acid-inducible gata-binding transcription factor expressed in endodermally derived tissues and heart. Mol. Cell. Biol. 1993, 13, 2235–2246. [Google Scholar] [PubMed]
- Koutsourakis, M.; Langeveld, A.; Patient, R.; Beddington, R.; Grosveld, F. The transcription factor gata6 is essential for early extraembryonic development. Development 1999, 126, 723–732. [Google Scholar] [PubMed]
- Shimoda, M.; Kanai-Azuma, M.; Hara, K.; Miyazaki, S.; Kanai, Y.; Monden, M.; Miyazaki, J. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J. Cell Sci. 2007, 120, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.B.; Pan, J.; Zhang, C.; Huang, S.Y. Sox17 facilitates the differentiation of mouse embryonic stem cells into primitive and definitive endoderm in vitro. Dev. Growth Differ. 2008, 50, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Niakan, K.K.; Ji, H.; Maehr, R.; Vokes, S.A.; Rodolfa, K.T.; Sherwood, R.I.; Yamaki, M.; Dimos, J.T.; Chen, A.E.; Melton, D.A.; et al. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 2010, 24, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Seguin, C.A.; Draper, J.S.; Nagy, A.; Rossant, J. Establishment of endoderm progenitors by sox transcription factor expression in human embryonic stem cells. Cell Stem Cell 2008, 3, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Hayashi, Y.; Emoto, T.; Weber, C.N.; Sekiguchi, K. Sox7 plays crucial roles in parietal endoderm differentiation in f9 embryonal carcinoma cells through regulating gata-4 and gata-6 expression. Mol. Cell. Biol. 2004, 24, 10492–10503. [Google Scholar] [CrossRef] [PubMed]
- Barbacci, E.; Reber, M.; Ott, M.O.; Breillat, C.; Huetz, F.; Cereghini, S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development 1999, 126, 4795–4805. [Google Scholar] [PubMed]
- Jacob, A.; Budhiraja, S.; Qian, X.; Clevidence, D.; Costa, R.H.; Reichel, R.R. Retinoic acid-mediated activation of hnf-3 alpha during ec stem cell differentiation. Nucleic Acids Res. 1994, 22, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Reichel, R.R.; Budhiraja, S.; Jacob, A. Delayed activation of hnf-3 beta upon retinoic acid-induced teratocarcinoma cell differentiation. Exp. Cell Res. 1994, 214, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Manova, K.; Weinstein, D.C.; Duncan, S.A.; Plump, A.S.; Prezioso, V.R.; Bachvarova, R.F.; Darnell, J.E., Jr. Disruption of the hnf-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994, 8, 2466–2477. [Google Scholar] [CrossRef]
- Cho, S.Y.; Jeon, J.W.; Lee, S.H.; Park, S.S. P67 isoform of mouse disabled 2 protein acts as a transcriptional activator during the differentiation of F9 cells. Biochem. J. 2000, 352 Pt 3, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Bae, S.H.; Choi, Y.J.; Kim, K.H.; Park, S.S. Feed-back regulation of disabled-2 (dab2) p96 isoform for gata-4 during differentiation of f9 cells. Biochem. Biophys. Res. Commun. 2012, 421, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, R.I.; Jitianu, C.; Cleaver, O.; Shaywitz, D.A.; Lamenzo, J.O.; Chen, A.E.; Golub, T.R.; Melton, D.A. Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev. Biol. 2007, 304, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, E.E.; Tang, Z.; Sigrist, K.; Lu, M.M.; Jiang, F.; Ip, H.S.; Parmacek, M.S. Gata6 regulates hnf4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998, 12, 3579–3590. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.K.; Kelly, G.M. Gata6 and Foxa2 regulate Wnt6 expression during extraembryonic endoderm formation. Stem Cells Dev. 2012, 21, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Tam, P.P. Definitive endoderm of the mouse embryo: Formation, cell fates, and morphogenetic function. Dev. Dyn. 2006, 235, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.P.; Kanai-Azuma, M.; Kanai, Y. Early endoderm development in vertebrates: Lineage differentiation and morphogenetic function. Curr. Opin. Genet. Dev. 2003, 13, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kunath, T.; Arnaud, D.; Uy, G.D.; Okamoto, I.; Chureau, C.; Yamanaka, Y.; Heard, E.; Gardner, R.L.; Avner, P.; Rossant, J. Imprinted x-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development 2005, 132, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J. Stem cells and lineage development in the mammalian blastocyst. Reprod. Fertil. Dev. 2007, 19, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Shimosato, D.; Shiki, M.; Niwa, H. Extra-embryonic endoderm cells derived from es cells induced by gata factors acquire the character of xen cells. BMC Dev. Biol. 2007, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.T.; Wamaitha, S.E.; Tsai, I.J.; Artus, J.; Sherwood, R.I.; Pedersen, R.A.; Hadjantonakis, A.K.; Niakan, K.K. Conversion from mouse embryonic to extra-embryonic endoderm stem cells reveals distinct differentiation capacities of pluripotent stem cell states. Development 2012, 139, 2866–2877. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Legros, S.; Artus, J.; Doss, M.X.; Khanin, R.; Hadjantonakis, A.K.; Foley, A. A comparative analysis of extra-embryonic endoderm cell lines. PLoS One 2010, 5, e12016. [Google Scholar] [CrossRef] [PubMed]
- Senner, C.E.; Krueger, F.; Oxley, D.; Andrews, S.; Hemberger, M. DNA methylation profiles define stem cell identity and reveal a tight embryonic-extraembryonic lineage boundary. Stem Cells 2012, 30, 2732–2745. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.R.; Grapin-Botton, A. Patterning and shaping the endoderm in vivo and in culture. Curr. Opin. Genetics Dev. 2012, 22, 347–353. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; Segel, M.; van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Kwon, H.J.; Shin, J.O.; Lee, J.M.; Cho, S.W.; Tickle, C.; Jung, H.S. Retinoic acid signaling and the initiation of mammary gland development. Dev. Biol. 2012, 365, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Frenz, D.A.; Liu, W.; Cvekl, A.; Xie, Q.; Wassef, L.; Quadro, L.; Niederreither, K.; Maconochie, M.; Shanske, A. Retinoid signaling in inner ear development: A “goldilocks” phenomenon. Am. J. Med. Genetics. A 2010, 152A, 2947–2961. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, G.M.; Drysdale, T.A. Retinoic Acid and the Development of the Endoderm. J. Dev. Biol. 2015, 3, 25-56. https://doi.org/10.3390/jdb3020025
Kelly GM, Drysdale TA. Retinoic Acid and the Development of the Endoderm. Journal of Developmental Biology. 2015; 3(2):25-56. https://doi.org/10.3390/jdb3020025
Chicago/Turabian StyleKelly, Gregory M., and Thomas A. Drysdale. 2015. "Retinoic Acid and the Development of the Endoderm" Journal of Developmental Biology 3, no. 2: 25-56. https://doi.org/10.3390/jdb3020025
APA StyleKelly, G. M., & Drysdale, T. A. (2015). Retinoic Acid and the Development of the Endoderm. Journal of Developmental Biology, 3(2), 25-56. https://doi.org/10.3390/jdb3020025





