Abstract
Retinoic acid signaling is required at several steps during the development of the spinal cord, from the specification of generic properties to the final acquisition of neuronal subtype identities, including its role in trunk neural crest development. These functions are associated with the production of retinoic acid in specific tissues and are highly dependent on context. Here, we review the defects associated with retinoic acid signaling manipulations, mostly in chick and mouse models, trying to separate the different processes where retinoic acid signaling is involved and to highlight common features, such as its ability to promote transitions along the neuronal differentiation cascade.
1. Introduction
The spinal cord is generated from the caudal part of the neural plate at early stages of development. In contrast to other regions of the central nervous system (CNS), such as the brain, the spinal cord develops progressively over a long period of time as the embryonic body axis extends in a head to tail sequence. Initially, the neural plate is induced by signals coming from the underlying hypoblast and the organizer (Hensen’s node in chicken and node in mice) and its derivatives [1]. The early neural plate will then be regionalized to adopt rostral (forebrain) or more caudal fates (midbrain, hindbrain and spinal cord) in response to signals coming from the node and newly formed mesodermal cells that emerge during gastrulation from the primitive streak (PS)/marginal zone [2].
The presumptive spinal cord territory corresponds to the epiblast region located at either side of the primitive streak and the node [3,4,5]. This region, known as the caudal stem zone or caudal lateral epiblast [6], also contributes to paraxial mesoderm for some time and requires further signaling to acquire a complete rostro-caudal (R-C) and dorso-ventral (D-V) repertoire of identities. At the borders between the caudal neural plate and the non-neural ectoderm, the trunk neural crest cell (NCC) territory is specified.
One of the signals involved in several processes in spinal cord development (from early patterning to neurogenesis and neuronal subtype specification) is retinoic acid (RA). In this review, we will discuss how RA signaling is used at different steps reiteratively in the progressive specification and differentiation of both spinal cord and trunk NCCs and how it interacts with other signaling pathways. In particular, we stress how RA counteracts the effect of FGF, which maintains cells in a more multipotent and undifferentiated state, and promotes the transition to subsequent differentiation states.
Several key RA signaling-deficient models have been used and will be discussed in the following sections. Vitamin A (the precursor of RA)-deficient (VAD) embryos are produced from quails reared with a restricted retinoid diet [7] and have undetectable levels of retinoids [8], whereas Raldh2 mouse knock-out mutants have very reduced RA signaling, according to the analysis of RA activity reporters [9,10], and die before midgestation (E10.5). This lethality can be overcome by supplementation with retinoids during the lethality time window [9,11] or through the use of conditional Raldh2−/− mutants [12,13]. Retinoic acid receptor (RAR) agonist and antagonists have also been used in embryo or explant cultures [14], and modified retinoic acid receptors have been used in chicken electroporation experiments, either to activate the pathway with constitutively active forms or to block it with deletions that behave as dominant negatives over the endogenous RAR (DN-RAR) [14].
2. RA Signaling Gradients during Axis Elongation
2.1. Sites of Active RA Signaling during Spinal Cord Elongation: Differential Expression of Key Components of the Pathway
In vertebrates, the primary body axis is generated from head to tail through a similar principle, consisting in the progressive addition of new tissue at the posterior end of the embryo (reviewed in [2,6]). This process requires a highly controlled balance between the maintenance of progenitor pools and the continuous production of cells that form the different body structures. The neck and trunk structures (spinal cord, notochord, somites and lateral plate mesoderm) are progressively generated from the early caudal embryonic region as the extension of the body axis proceeds. Different signaling molecules are involved in the coordinated development of this region, and in particular, RA is an important signal for the spatio-temporal control of caudal development.
RA is synthesized from vitamin A, also known as all-trans-retinol, via two consecutive enzymatic reactions [15]. In the first reaction, all-trans-retinol is oxidized to all-trans-retinaldehyde (retinal). During development, this reaction is controlled mostly by retinoldehydrogenase-10 (Rdh10; [16,17]) that is expressed, amongst others regions, in the floor plate and somites adjacent to the developing spinal cord [18,19]. However, mutations in Rdh10 do not seem to affect spinal cord development, and RA signaling is still retained at the ventral neural tube [17,20], indicating that other retinol dehydrogenases could be acting in the neural tube.
At least two other dehydrogenases are involved in retinal production, Rdhe2 (Sdr16c5) and Cyp1B1 (cytochrome p450 1B1, a monomeric monooxygenase). Rdhe2 is expressed in the notochord, underlying the developing neural tube. In Xenopus embryos, knocking down Rdhe2 results in phenotypes consistent with RA deficiency, such as defects in anterior neural tube closure, disruption of somitogenesis and a curved body axis with a bent tail [21]. Cyp1B1 is expressed at gastrulation stages in the primitive streak and caudal epiblast and later in somites, and it is sufficient to efficiently oxidize retinol to retinal and, subsequently (to a lesser extent), to RA [22]. Furthermore, Cyp1B1 overexpression can alter the R-C and D-V patterning of the hindbrain and spinal cord in a manner that is consistent with the promotion of RA activity [22]. However, it is not clear if Cyp1B1 could be also playing a role in other species apart from the chicken. The expression pattern of Rdhe2 (notochord) and Cyp1B1 (somites) suggests that RA biosynthesis for CNS (at least its first step) may occur in the adjacent axial and paraxial mesoderm.
The second reaction is controlled by three retinaldehyde dehydrogenases that generate RA (Raldh1, Raldh2 and Raldh3), each of which is present in specific spatiotemporal domains during embryogenesis. They are the only enzymes involved in the second step of RA synthesis, at least prior to stage E10.5 of mouse development [9,10]. Raldh2 is the more extensively studied of the three, the earliest expressed and the only one associated with spinal cord development. At gastrulation stages, Raldh2 is expressed transiently in primitive streak and node cells (Figure 1a) and in paraxial mesendoderm underlying the caudal neural plate (future hindbrain and spinal cord) [23,24,25,26]. As somitogenesis is initiated, its expression is progressively activated during axial elongation in the rostral-most presomitic mesoderm (PSM) and is maintained in somites (although, later downregulated in the first four somites; Figure 1b,c) [24,26]. Raldh2−/− mouse mutants die before midgestation (E10.5) from defective heart morphogenesis and exhibit numerous abnormalities, including alterations in hindbrain patterning, motor neuron specification and early forebrain development [9,10,27,28]. Some of these abnormalities can be rescued by transient maternal RA supplementation from E7.5 to E8.5–9.5 [9,11].
Other enzymes involved in the metabolism of RA are members of the Cyp26 family that are capable of degrading RA to inactive forms. Cyp26a1 is expressed in the caudal stem zone and is progressively downregulated as the embryo extends [29,30,31,32]. These complementary expression patterns of Raldh2 and Cyp26a1 allow high RA levels at somitic levels and the maintenance of an RA free caudal-most region.
Both retinol and retinoic acid are normally bound within the cytoplasm to cellular retinol and retinoic acid binding proteins (CRBP, CRABPI and II), which play a role in retinoid traffic and may also work in modulating the availability of retinoic acid, as shown in the zebrafish hindbrain [33]. However, CRBP and CRABP mutant mice do not show overt phenotypes [34,35]. Both types of protein are expressed in the spinal cord in progenitors or in differentiating neurons [36,37,38,39,40], but their roles in spinal cord have not been specifically addressed.
RA binds to nuclear RA receptors (RARα, β, and γ) and peroxisome proliferator-activated receptors (PPAR β/δ), which act as heterodimers with retinoid X receptors (RXRα, β, and γ) to activate transcription of target genes [41,42]. In Hamburger and Hamilton (HH) stage 10 chick embryos, RXRα, RARα and RARβ are expressed in the spinal cord flanked by somites and their expression decline at presomitic levels, while RXRγ and RARγ expression extends more caudally into the caudal neural plate and is also detected in the primitive streak [43,44,45].
Thus, the combination of Raldh2 and RA receptor expression creates an R-C gradient of RA activity in the neural tube and paraxial mesoderm and suggests a paracrine mode of RA action [15], which implies that RA is synthesized in the trunk mesoderm and diffuses to exert transcriptional control in the CNS. This is consistent with RA levels measured by HPLC in chick [46], by genetically encoded probes for RA in zebrafish [47] and with active RA signaling detected in mice with a retinoic acid response element (RARE)-lacZ transgene [48,49], first in the caudal epiblast and then in the developing spinal cord adjacent to somites (Figure 1a–c). At later stages, the cervical/brachial and lumbar spinal cord regions stand out for their high RA pathway activation levels [50] (Figure 1i).
2.2. Mechanisms Regulating the Activation of RA Signaling during Axis Elongation: An RA-FGF-Wnt Gene Regulatory Network
A key feature of spinal cord development is the progressive activation of the RA signaling pathway at somitic levels coupled to downregulation of FGF signaling at presomitic and caudal levels. These pathways have been proposed to constitute a signaling switch that controls the R-C sequence of mesodermal and neural development [45,51]. In addition, the interaction between the RA and FGF pathways is controlled through series of cross-regulations that also involve the Wnt signaling pathway [6].
On the one hand, Fgf8 is expressed as a caudal (high) to rostral (low) gradient in caudal tissues (Figure 1a–c), and the FGF pathway is essential for maintaining an undifferentiated ‘stem’ zone adjacent to the regressing primitive streak [5,52]. In the neural tube, FGF8 is a general repressor of differentiation, and the attenuation of the pathway is required for neural differentiation, including ventral patterning (explained in more detail in Section 3 and Section 4; [45,53]). Additionally, the FGF8 gradient controls the position of somite boundaries in the presomitic mesoderm [51]. One important function of FGF is the blockade or attenuation of RA signaling, as several steps in the RA pathway are influenced by FGF signaling. Thus, FGF4/8 can block the activation of Raldh2 transcription in presomitic mesoderm explant cultures and in whole embryos [45]. FGF4/8 can also downregulate the expression of the RARβ receptor in the spinal cord [54], which may reflect the ability of FGF to interfere with the RA feed-forward loop (as RARβ is also activated in response to RA signals). In addition, the FGF receptor I (FGFRI) is required for the expression of Cyp26a, the RA degrading enzyme in the caudal-most region [55].
This indicates that FGF may restrain RA activity to somitic levels, but downregulation of FGF signaling alone is not sufficient to extend the Raldh2 domain caudally [54,55]. Interestingly, FGF signaling not only blocks Raldh2 expression, but also plays an indirect role in its activation. FGF8 activates Wnt8C in the caudal stem zone, which persists at presomitic levels once Fgf8 expression is extinguished and then activates Raldh2 in the underlying somites [54]. The simultaneous blockade of FGF signaling with the activation of the Wnt canonical pathway can increase the levels of Raldh2 expression [54], but cannot extend its expression caudally, suggesting that other pathways are required for proper Raldh2 expression.
On the other hand, RA can downregulate Fgf8 expression in caudal explants, and the analysis of VAD quail embryos and Raldh2−/− mouse mutants reveals that a lack of RA signaling leads to an anterior expansion of the FGF8 domain along the PSM and to the formation of smaller somites [45,56,57,58]. This role of RA in repressing Fgf8 may be limited to a short period of time when the somitogenesis molecular clock initiates [59]. In conclusion, although the cross-regulation between the two pathways can explain many aspects of the process of axis elongation, the knowledge about the molecular mechanism linking both pathways is very limited, and more focused and high-throughput analysis would be required.
2.3. RA in the Control of Trunk-Tail Transition and Axis Termination
An important aspect to consider in the process of axis elongation is the transition from trunk to tail. Whereas the trunk tissues are generated from all three germ layers and give rise to most of the vital and reproductive organs, the tail is derived from paraxial mesoderm and ectoderm and is basically composed of vertebrae, its associated muscles and caudal spinal cord, lacking motor roots and peripheral ganglia [60]. The trunk-tail transition is associated with a change in the mode of axial extension. Thus, while trunk formation is mainly driven by the PS [61,62]; tail formation is associated with the activity of the tail bud, where axial progenitors from the PS are relocated into a structure named the caudal neural hinge (CNH; [62]). This transition also affects the mechanism of neural tube formation that changes from neural plate folding or primary neurulation to neural tube cavitation in secondary neurulation [6].
Recently, it has been revealed that Gdf11 mutants exhibit delays in the specification of tail structures due to abnormalities observed in the PS to CNH transition [63]. Cyp26a1 expression levels are reduced in the posterior end of Gdf11 mutant embryos at the time of that transition. This could be the origin of an uneven RA distribution along the rostro-caudal axis. Moreover, the inhibition of RA signaling produces a significant reversion of the abnormalities observed in the PS to CNH transition observed in Gdf11 mutants, placing RA signaling as a key regulator of that transition.
Finally, at a specific point during development, body axis elongation ceases, and this must involve the regulated differentiation and/or loss of axial stem and mesoderm progenitor cells. The pathway responsible for that cessation seems to be RA signaling. Exposure to exogenous RA can arrest body elongation by inhibiting the expression of Wnt3a [64,65,66]. More recently, it has been determined that rising retinoid signaling in the late chicken tail bud causes the loss of FGF signaling and, consequently, loss of Brachyury gene expression from the axial stem cell population and presumptive mesoderm cells [39]. These events are followed by local cell death in the tail bud, which can be reduced by the attenuation of RA signaling in an FGF-independent way, and that ultimately leads to the cessation of body elongation. Moreover, at this late stage, FGF signaling in the tail bud no longer opposes retinoid synthesis and activity. However, it is not clear how the RA signaling pathway escapes from the control exerted by FGF signaling [39].
In the case of the mouse embryo, which, in contrast to human or chicken, retains the tail, the mechanism involved in the attenuation of FGF signaling as elongation ceases could be different. While RA is required for caudal regression in chick promoting cell death, recent data suggest that RA is not synthesized in the mouse tail bud [67]. However, the understanding of differences in axis elongation cessation between different species requires further mechanistic analysis. Moreover, it will be interesting to determine if the same signaling dynamics are deployed to arrest the elongation of other axial structures, such as the limb and beak, which are also truncated by an excess of RA [68,69].
3. RA Controlling Early Neurogenesis in Spinal Cord
The initial exposure of the epiblast to RA occurs at the time when neural specification is being stabilized in the caudal neural plate [1]. RA has not been implicated in early neural induction steps in vitro [70] or in vivo, as neural induction occurs in Raldh2−/− embryos [10,49,56]. However, the maintenance of induced neural tissue in caudal regions may have some dependency on retinoid signaling. For instance, Raldh2−/− mutants show early caudal patterning defects, with an expansion of the primitive streak and mesodermal markers at the expense of markers of the prospective neuroepithelium, such as the pan-neural marker Sox2, as early as E7.5 [49]. However, this requirement may be specific to a time window, as explants of presumptive chick spinal cord at gastrulation stages exposed to RAR/RXR inhibitors display normal Sox2 levels [23].
Moreover, RA deficiency in the neural tube is also associated with a reduced number of cells expressing the mitosis marker phospho histone H3 in quail and zebrafish embryos [71,72], indicating that RA deficiency probably causes defects in cell proliferation. Even in vitro, RA increased by 45% the number of BrdU incorporating cells in ventral neural tube explants from brachial levels, suggesting that retinoids increase the number of motor neuron progenitors at limb levels [73].
Once the neural specification step is consolidated, progressive activation of RA signaling in the spinal cord is required for spinal cord differentiation, as shown in VAD quails that have a reduced number of neurons in the spinal cord (Figure 1d) [74]. Using RAR/RXR antagonists in vitro, VAD quails or the Raldh2−/− mutant, a dramatic reduction in early neurogenesis was observed, which included a reduction in the number of cells expressing the proneural genes, Ngn1 and Ngn2, the lateral inhibition gene, Hes5, and the early postmitotic neural markers, Delta1 and NeuroM [45,49]. Moreover, the VAD neural tube possesses very few or no CRABP1+ cells, a group of early-born interneurons [45].
The expression of Delta1 has been shown to be resumed in 14-somite stage Raldh2−/− embryos [49], and the requirement for RA may thus be limited to the early produced neurons labeled with CRABP1 [45]. The requirement of RA to promote neurogenesis could be due to its action at several steps in the sequence, leading to neuronal differentiation. First, RA is required for the expression of Sox1 and Sox3 [75], two important genes for the maintenance and differentiation of progenitors before the onset of neurogenesis [76]. RA is also required for the expression of genes primarily involved in the specification of progenitor domains along the D-V axis but, also with a role promoting neurogenesis (see Section 4). Additionally, a regulatory region in the Ngn2 locus containing two RARE elements required for transgene activity have been identified [77], and thus, there may also be a direct regulation of proneural genes by RA.
Furthermore, stimulation of the RA pathway in prospective spinal cord explants leads to an increase in the number of NeuroM expressing cells, indicating that RA is not only required, but it can drive neurogenesis in the spinal cord [45]. The search for genes activated by RA in the spinal cord identified GDE2, an interesting gene first expressed as cells transition to the postmitotic state and later maintained in differentiating neurons [78]. GDE2 promotes neuronal differentiation acting non-autonomously by downregulating Notch signaling in neighboring progenitor cells, and therefore, part of the function of RA in neurogenesis may be mediated through this indirect mechanism [79].
4. RA in the Acquisition of Dorso-Ventral Identities
The acquisition of neuronal subtype identities along the D-V axis in the spinal cord is initiated at the progenitor stage by the restricted expression of transcription factors in specific domains along this axis. In addition, the identity of neurons derived from a particular D-V domain can be further stabilized and even diversified, either by signaling factors secreted by adjacent tissues, or generated within the neural tissue (including differentiating neurons), or by lateral inhibition mechanisms. RA seems to be playing a role at both progenitor and postmitotic neuron specification stages.
4.1. Ventral Patterning of Progenitors and Acquisition of Motor Neuron Identity
As mentioned above, although all cells of the presumptive spinal cord may have been transiently exposed to RA at gastrulation stages, a subsequent exposure to RA occurs later in a progressive rostral to caudal way. This occurs at the time when the neural tube is patterned along its D-V axis by secreted signals provided by the ventral notochord and floor plate, such as Shh, and by dorsal signals provided by Wnts and BMP. According to the current model, the extracellular gradient of Shh results in the graded distribution of the Gli transcriptional factors within responding cells, with those cells located near the source containing high levels of Gli activators and low Gli repressors and the converse for those further away from the source [80]. As a consequence, Gli responsive genes, such as the homeobox (Nkx2.2, Nkx6.1, Nkx6.2, Irx3, Pax6, Dbx1, Dbx2) and bHLH (Olig2) encoding genes, are expressed in restricted domains. Specific combinations of these transcription factors define the progenitors of four interneuron subtypes and motor neurons (MNs), named p0, p1, p2, pMN and p3, from intermediate to more ventral positions [84] (Figure 1e).
It has been shown that RA is required for the expression of some (but not all) ventral patterning genes. Thus, Olig2 and Pax6 (involved in pMN) are dramatically downregulated in VAD quails [45,81], in chick neural tubes electroporated with DN-RAR or with Cyp26a [82], in Raldh2−/− mutant mice [49,56,83] and in zebrafish embryos treated with DEAB, a Raldh inhibitor that prevents the synthesis of RA [72]. A reduction of Irx3 and Nkx6.2 (involved in p2 [84] and p1 [85] specification, respectively) has been described in VAD quails [45,81], and the expression of Dbx1 and Dbx2 (involved in the specification of p0 and p1 progenitors) can be blocked with RAR/RXR antagonists [86].
RA is required both for the expression of genes that are activated by Shh (Class II, such as Olig2 and Nkx6.2) and of those repressed by Shh signaling (Class I, such as Pax6 and Irx3), and therefore, its activity cannot be explained by the simple action on the Shh pathway. Furthermore, a Shh-dependent gene, such as Nkx6.1, does not require RA, as it is expressed in VAD quails and in Raldh2−/− mutant embryos [45,56], suggesting that RA is acting differently on each ventral gene. Still, part of the requirement of RA on ventral genes could be the consequence of reduced Shh signaling, as Raldh2−/− mutant mice have decreased sensitivity to Shh signals in culture at late gastrulation stages [49] and decreased Shh expression in the floor plate has been described in Raldh2−/− mutants [49] and to a lesser extent in VAD quails [74]. However, decreased Shh has also been associated with over activation of the RA pathway [22], suggesting that the regulation of Shh by RA is complex and may depend on precise levels of RA. In any case, all of these results suggest that the effect of RA loss is not that of a general blockade of Shh signaling.
One interesting possibility is that RA regulates ventral genes through its repressive action on Fgf8 [45,56], as it has been shown that Pax6, Irx3 and Nkx6.2 are repressed by the FGF pathway [53,82,87]. However, other mechanisms must be in place, as Fgf8 expression is not completely expanded rostrally in VAD quails in regions where the expression of ventral genes is affected [45].
Recently, it has been shown that coincident with the transcriptional onset of key differentiation genes, the chromatin around the Pax6 and Irx3 loci undergoes both decompaction and displacement towards the nuclear center [58]. This does not occur in Raldh2−/− mutant embryos, probably due to the persistence of ectopic FGF signaling, as the inhibition of the FGFR signaling in Raldh2−/− mutant embryos restores chromatin decompaction and nuclear positioning of both Pax6 and Irx3 loci. However, Pax6 transcription is not rescued in these conditions, indicating that RA could play two roles, one downregulating FGF signaling to allow for chromatin decompaction of the Pax6 and Irx3 locus and an additional role in the transcriptional activation of Pax6.
In that sense, gene regulatory regions driving Pax6, Nkx6.2 or Olig2 expression in their normal ventral domain have been identified [88], but the presence of functional RARE has not been determined yet. Regulation of ventral genes by RA may also be related to other proteins involved in their transcriptional regulation, such as SoxB1 (Sox1, Sox2 and Sox3) transcription factors [88]. Although Sox2 is expressed in RA-deficient conditions [49], the expression of Sox1 and Sox3 is greatly downregulated in VAD quails, and this may also contribute to the downregulation of many of the ventral patterning genes, although this has not been examined yet [75]. Additionally, as several of the ventral genes are involved in cross-regulatory networks, the effect of RA may be indirect for some of them [84,89].
As discussed in Section 3, RA is required for early neurogenesis and neuronal differentiation, but a requirement for specific neuronal subtypes has also been shown (Figure 1g). A dramatic decrease in Isl1+ and Mnr2+ MNs is observed in VAD quails [81] and in DN-RAR electroporation experiments [82]. This could be a consequence of the RA requirement for Olig2 expression in progenitors, but in fact, RA is also acting after Olig2 to promote the MN fate [82]. RA signaling cooperates with Ngn2 to activate MN specific genes, such as Hb9, and, therefore, promotes MN differentiation. This has been shown to be mediated by the formation of an RAR-Ngn2 complex bound to the regulatory region of Hb9, which upon RA binding, is capable of recruiting cofactors, such as the histone acetyl-transferase CBP and activate transcription [90].
V0, V1 and V2 interneurons (defined by the expression of Evx1/2, En1 and Chx10, respectively) are diminished in RA compromised situations, confirming that RA is required for those neuronal subpopulations [81,82]. In the case of V0, RA seems to be required at two stages; first to control the expression of progenitor proteins and, subsequently, for the specification of V0 interneurons derived from the Dbx domains [86].
A role for RA signaling has also been shown in the most ventral neuron producing domain (p3) that express the progenitor domain marker, Nkx2.2. Progenitors of the subtype p3 require RA to give rise to the spinal cord-specific V3 interneurons instead of the 5HT-expressing neurons (characteristic of hindbrain levels). Experiments with DN-RAR and constitutively active RAR have shown that RA promotes the activity of the Notch receptor within the p3 domain, which, in turn, decreases (but does not eliminate) Ascl1 expression, favoring V3 interneuron identity [91].
Overall, it seems clear that RA signaling is crucial for the development of all ventral neuronal cell types and that its activity may work through different mechanism along the ventral axis.
4.2. Dorsal Patterning of Progenitors
Dorsal patterning of the spinal cord requires the restricted expression of transcription factors regulated by dorsal BMP and Wnt signaling molecules [92,93]. The analysis of VAD quails and Raldh2−/− mutant embryos has shown that RA is important for the expression of dorsal specific genes, such as Pax3, Pax7, Msx2, Wnt1, Wnt3A, BMP and BMP7 [49,81,94], suggesting the role of RA in dorsal progenitor specification. Pax3 and Pax7 are the earliest expressed dorsal-specific transcription factors and are initially present along the R-C axis of the spinal cord primordium [45]. Their downregulation in VAD quails suggests that their expression may require the initial exposure of the spinal cord territory to RA from the early paraxial mesoderm (Figure 1a). The analysis of Pax3 and Pax7 expression following DN-RAR electroporation in the developing neural tube would help ascertain whether RA signaling is required only initially for their onset or also for the maintenance of their expression. Other dorsal genes, such as Msx2, Wnt1 and Wnt3A, are activated in a rostral to caudal progression, and their depletion in VAD quails may reflect either a requirement for somite-derived RA or a consequence of an early requirement (see also Section 5 on the role of RA in a particular dorsal cell population, the trunk neural crest). Interestingly, the expression of many of the dorsal genes (Pax3, Pax7, Msx2, Wnt1, Wnt3A, BMP4, BMP7) in older VAD quails is exclusively downregulated at the rostral half of the spinal cord [81], suggesting that there is a stronger requirement for RA at those levels, which may be related to its role in the establishment of rostro-caudal identities discussed in Section 6. As Wnt and BMP signals are responsible for the expression of most dorsal genes, it would be important to determine which is the most directly affected by RA signaling.
The activation of the RA pathway within spinal cord progenitors is also dynamic and heterogeneous [9], reflecting the existence of restricted sources of RA production and degradation. Raldh2 is expressed in the roof plate of chick at stages HH19–29 [31,73], while the RA degradation enzyme, Cyp26a, is produced from the dorsal neural tube from chick stage HH12-on [31]. In addition, other non-Raldh2 RA sources have been suggested to activate RA signaling at floor plate, ventral and dorsal-intermediate levels [9,11] and may contribute to the RA levels in the neural tube necessary for D-V patterning, although their specific role has not been shown yet.
5. RA in Trunk Neural Crest
Early in development, the embryonic ectoderm becomes subdivided along the mediolateral axis into neural, neural plate border and epidermal territories. From the neural plate border (that will later constitute the dorsal most region of the neural tube), the neural crest cells (NCCs) are specified through a series of steps controlled by different signaling pathways. At the early blastula stage, Wnt and BMP signals interact to block neural fate and to induce epidermal fates [95,96,97]. At the late blastula stage, non-instructive Wnt activity mediates the temporal exposure of epiblast cells to BMP signals that specify neural plate border cells, and a later phase of Wnt activity, corresponding to the late gastrula stage, induces caudal/neural crest character in prospective neural plate border cells [98].
In Xenopus embryos, tissue expressing anterior neural plate border markers, induced by intermediate levels of BMP activity, was transformed into neural crest by posteriorizing signals, such as bFGF, Wnt-8 or RA treatment [97]. This transformation of the anterior neural plate border into NCCs can also be achieved in whole embryos, by RA treatment or by a constitutively active form of RARs [97]. Conversely, neural crest induction in vitro was inhibited by the expression of the dominant negative forms of the FGF receptor, DN-RAR and Wnt signaling effectors [97]. Similarly, in Raldh2−/− mutant embryos, a decrease in the expression of genes involved in dorsal spinal cord specification, such as Msx1 and Pax3, has been observed [49]; and in VAD quails, Pax7 is absent at the neural plate border, and there is a drastic reduction in NCCs markers, such as Snail2 and Sox9 [94]. However, these defects may be due to early caudal patterning alterations, as Raldh2−/− mutant embryos show an expansion of primitive streak and mesodermal markers (Follistatin and Brachyury) at the expense of markers of the prospective neuroepithelium, such as Sox2 [49]. It has been therefore suggested that RA signaling is required for the earliest steps in dorsal and NCC specification during gastrulation, before the RA activity appears in the dorsal neural tube [94], but RA signaling may also contribute at subsequent steps in NCC development.
As mentioned in Section 2.1, when the first somites appear and as the embryo elongates, RA-responsive cells in mouse and in chicken embryos (Figure 1b,c) can be observed in the neural tube adjacent to formed somites. NCCs are a multipotent transient population that, at trunk levels, give rise to sensory neurons and glia, sympathetic neurons and melanocytes, amongst others [99]. To reach that diversity, after being specified, NCCs undergo a process of epithelium to mesenchyme transition (EMT) that confers them the ability to delaminate and migrate away from the dorsal neural tube. The development of trunk NCCs is highly coordinated with the development of adjacent somites, which impose a segmented migration and organization to the trunk NCCs and to the derived peripheral nervous system. Opposite the presomitic mesoderm, NCCs are confined to the dorsal neural tube, whereas NCC emigration begins facing epithelial somites [100,101].
At trunk levels, it has been shown that a BMP-Wnt1 signaling cascade controls NCC emigration from the neural tube [101,102]. The regulation of that cascade is exerted through the caudal (high)-rostral (low) gradient of the BMP inhibitor, Noggin, which, in turn, is controlled by signals coming from the somites [103]. More recently, it has been established that one of the somite signals controlling NCC emigration from the neural tube is RA [94] (Figure 1f). Overexpression of DN-RAR caused a reduction in the number of cells expressing Sox10, and there was a dramatic delay in the emigration of Sox10+ NCCs. However, the initiation of the expression of Snail2 and FoxD3, early markers of NCC specification, was not affected, indicating that RA is required to establish the onset of NCC emigration independently of NCC specification.
Conversely, FGF4/8 maintain dorsal neural tube cells uncommitted with respect to neural crest fate, and an FGF signaling decrease is necessary to promote not only NCC specification (as discussed above), but also subsequent migration from the neural tube [94]. Moreover, in relation to the BMP/Wnt signaling cascade operating in the dorsal neural tube, it has been established that RA signaling activation in the neural tube adjacent to somitic level coincides with the initiation of Wnt1 expression. Whereas RA signaling triggers the initiation of Wnt1 expression in the dorsal neural tube at levels where the NCCs are already specified, FGF signaling prevents the premature expression of Wnt1 [94].
The actual view is that there is a short time window during which the onset of the NCC emigration can be tuned, once NCCs have acquired the essential specification program. That window coincides with the region where FGF and RA gradients collide, and it is required to keep the emergence of peripheral nervous system progenitors in register with the programs of spinal cord neurogenesis and somite development during trunk elongation. It remains to be seen if changes in the onset of emigration (that alter migratory behavior; [94]) will have consequences in the final differentiation of the NCCs.
Once trunk NCCs emigrate from the dorsal neural tube, they will migrate along characteristic pathways to differentiate into a wide variety of derivatives according to their D-V position within the neural tube and the order of emigration [104]. NCCs interact with various extracellular matrix components and acquire specific competences to properly sense the cues they find along their pathways. Amongst the proteins relevant for this process are integrins that form clusters in focal adhesions in migrating cells. Little is known about the possible implications of RA signaling during trunk NCC migration. Recently, it has been established that the motile capacity of multipotent NCCs is governed in part by an RA-controlled dynamic regulation of Nedd9 levels (a member of the β-integrin signaling pathway), which influence cell adhesion and cytoskeleton properties [105]. It has been determined that the Nedd9 promoter contains a RARE site that interacts with the RA receptor [106]. It would be interesting to determine if some of the phenotypes described below for NCC-derived structures in conditions of vitamin A or retinoid insufficiency might be explained by deficiencies in Nedd9 expression [107,108].
RA signaling could also play a role in the terminal differentiation of the NCCs, once they reach their final destination. That could be the case for the neural cells that form the enteric nervous system (ENS). The ENS derives from the NCC precursors that colonize the bowel and differentiate into a network of neurons and glia that control intestinal function. There are several pieces of evidence that RA is essential for normal ENS development. Aganglionosis of the bowel (a Hirschsprung disease-like phenotype due to vagal crest deficiency) has been observed in Raldh2-deficient mice that had been partially “rescued” by treatment of the pregnant mothers with RA [109]. More recently, reduced colon myenteric neuron density and reduced colon myenteric neuron-to-glia ratio was observed in Raldh1−/−, Raldh2+/− and Raldh3+/− mutant mice [110]. Those alterations are unlikely to be due to defective ENS precursor migration, since the Raldh-combined mutant mice have increased enteric neuron progenitor migration into the distal colon compared to wild-type embryos during development.
Finally, the crucial role of RARs in NCC-derived craniofacial structures, which originate from diencephalon to hindbrain levels, has been established for years [111,112,113] and has been discussed in other reviews [114]. The discussion about those aspects is out of the scope of this review chapter.
6. RA and the Acquisition of Rostro-Caudal Identities in the Spinal Cord
6.1. RA and the Specification of Spinal Cord Progenitor Cells
RA has a predominant role in the regionalization of the nervous system along the R-C axis, mostly in the hindbrain and in the spinal cord, although an early role in forebrain patterning has also been described [115,116]. The regionalization of the nervous system is a progressive process initiated at early stages when the neural plate is being established. Initially, most cells in the neural plate express markers that will later get restricted to the forebrain territory (i.e., Otx2), and as development proceeds caudal markers appear. Thus, caudally located cells are initially specified with a forebrain character, and at later stages, they display a midbrain, hindbrain or spinal cord character [2,23,117].
The ability to acquire caudal identities is highly dependent on the interaction of the neural plate with surrounding tissues. Initially, signals from Hensen’s node promote midbrain and hindbrain fates, and later, signals from the presomitic mesoderm promote the expression of spinal cord markers, such as Hoxb8, which is expressed in the presumptive spinal cord with a rostral limit around the level of somites 5–6 [95] and later extends rostrally into the hindbrain [118]. Molecules that promote midbrain, hindbrain and spinal cord identities are FGF, Wnts and RA. The functions of these signals along the R-C axis vary depending on the signaling context (i.e., the competence of the tissue). For instance, FGF is able to promote a midbrain character, whereas a combination of FGF and Wnts promotes a rostral hindbrain character (i.e., rhombomeres r1–5; [95]). More caudal hindbrain (r6–8) and spinal cord characters can be promoted by RA and Wnt signaling [119]. Extensive literature is devoted to the role of RA in hindbrain patterning [44] and will not be analyzed in this review. However, some mechanisms may be shared with spinal cord patterning.
Active RA signaling from the presomitic mesoderm is required for Hoxb8 expression in chick spinal cord explant cultures, and RA in combination with signals from early mesoderm promotes Hoxb8 expression, suggesting a role of RA in spinal cord specification [23,119]. However, as expression of Hoxb8 later extends into the hindbrain in an RA-dependent manner [118], its activation in explants may reflect this second phase of expression. In fact, Hoxb8 is expressed in VAD quails [120] and, similarly, Raldh2−/− embryos and chicken neural tube electroporated with DN-RAR show normal Hoxb8, Hoxb6 and Hoxb7 expression in spinal cord progenitors ([49]; Bel-Vialar, 2002 #5972), suggesting that RA is not required for Hoxb8 expression in the spinal cord. Interestingly, Raldh2−/− embryos show an early decrease in the expression of the caudal specific genes Cdx1 and Hoxb9 (characteristic of caudal spinal cord), confirming an RA requirement in some aspects of spinal cord identity [49]. Normal expression of Cdx1 seems to require direct binding of RAR to a Cdx1 RARE sequence [121]. However, the molecular mechanism responsible for the regulation of Hoxb9 by RA in the spinal cord is not known, but may involve RARE sequences, as has been shown for the expression of Hox genes in the hindbrain [122].
Furthermore, the relationship of Cdx1 and RA may be further complicated. In zebrafish embryos, Cdx1a/4 morpholino treatment causes a posterior shift of the Raldh2 expression domain in the paraxial/lateral mesoderm, resulting in overlapping regions of high FGF and RA signaling in the caudal neural tissue [123]. This alteration is probably behind the ectopic formation of the posterior hindbrain and anterior spinal cord at the caudal neural tube and the severe posterior truncation observed in cdx1a/4 morphants.
The picture that emerges is that the early exposure of the caudal neural plate progenitors to RA is required for the acquisition of some spinal cord properties. Then, an RA-free region has to be established caudally for further caudal spinal cord specification, in order to allow the action of caudalizing signals, such as increasing FGF levels and GDF11 [117], and the expression in early caudal spinal cord progenitors of Hoxc9 (expressed caudally to somite 20) [119]. Finally, the subsequent progressive exposure to RA produced by somites will not alter the established Hox code in spinal cord progenitors [124].
6.2. RA in the Acquisition of Rostro-Caudal Column Identities of Motor Neurons
The heterogeneity of the spinal cord along its R-C axis is functionally relevant. For example, different MN subtypes organized in columns can be specified depending on their position along the R-C axis. Thus, at cervical regions, neurons of the phrenic motor column (PMC) innervating the diaphragm differentiate; at limb forming regions (brachial and lumbar), neurons of the lateral motor column (LMC) form to innervate limbs; and at thoracic levels, neurons of the preganglionic motor column (PGC or Column of Terni in the chick) that innervate sympathetic motor neurons and neurons of the hypaxial motor column (HMC; also known as the lateral medial motor column or MMCL) innervating hypaxial muscles are specified [125] (Figure 1h). These differences along the R-C axis are related to the differential expression of Hox genes, first in progenitors, as shown above, and, subsequently, in the resulting MNs. Whereas, Hoxc5 is expressed in cervical and caudal brachial MN, Hoxc6 is expressed in brachial MNs, Hoxc8 in caudal brachial MN and Hoxc9 in thoracic MN [117]. The acquisition of the different R-C neuronal subtypes within the spinal cord has been related to signals from the Hensen’s node and paraxial mesoderm [73,117,126]. In particular, cervical-level paraxial mesoderm can promote cervical characteristics (Hoxc5) that can be blocked by RAR antagonists [117], suggesting a role for RA in the specification of cervical MNs. Most importantly, removal of Raldh2 from both trunk mesoderm and spinal cord progenitors from stages E10.5 causes a decrease in Hoxc6 and Hoxc8 expression at brachial levels, supporting its requirement for brachial MN differentiation [12]. This change in Hox gene expression may account for some of the changes in motor column programs observed in RA-deficient conditions. Electroporation of DN-RAR in brachial motor neurons results in the loss of LMC columns, defined by their expression of Raldh2 and in the ectopic differentiation of motor neurons characteristic of thoracic regions (PGC; [14]), supporting a role for RA in inducing generic LMC properties. However, the interference with RAR at lumbar levels does not affect generic LMC specification [14], suggesting that other mechanisms regulate the generation of LMC at lumbar regions (see [127]).
RA is not only required, but can also increase the number of Hoxc5 expressing MN in spinal cord explants that have not yet acquired their Hoxc neuronal identities [117]. Moreover, RA alters the fate of MNs derived from thoracic spinal cord explants, increasing the number of MNs that express brachial-specific Hoxc6 [117] and blocking the production of Hoxc9+ thoracic MNs [117]. Furthermore, sustained ectopic activation of RA by electroporation of a constitutively active RAR in thoracic motor neurons impairs the differentiation of PGC and HMC neurons, leading to MN apoptosis [14].
Interestingly, RA is also important within both brachial and lumbar regions for the proper diversification of the LMC neurons into lateral LMC neurons that project to dorsal targets and medial LMC neurons that project ventrally (Figure 1i). All LMC neurons express initially Isl1, but this is downregulated in the lateral LMC neurons as they initiate Lim1 expression. RA favors lateral LMC fates, as it promotes Lim1 expression and downregulates Isl1 [73], which, in turn, control Ephrin production, which allows cells to differentially project to their targets [128]. Raldh2−/− mutants rescued from early lethality by RA supplementation from E7.5 to 8.5 show changes in the MN fates at brachial levels compatible with a change of fate from lateral to medial LMC when analyzed at E12.5 [83]. Specific removal of Raldh2 from paraxial mesoderm, which results in a 50% decrease in RA levels in the spinal cord, only slightly decreases MN production, but provokes a large reduction in the number of lateral LMC both at brachial and lumbar levels [13].
Both activities of RA in the specification of brachial LMC and in the subsequent diversification of LMCs into lateral and medial populations could be exerted through the control of Nolz transcription, which is activated by retinoids [129]. This transcription factor specifies LMC identity by inducing the expression of the postmitotic LMC determinant Hoxc6 and later contributes to specification of lateral LMC identity through Lim1 induction [129].
An additional role for RA in the maintenance and survival of both medial and lateral LMCs and in axonal projections has also been suggested. The specific removal of Raldh2 from MNs results in a decrease of medial and lateral LMC neurons and abnormal axonal projections to the limb [13].
These multiple requirements for RA in MN differentiation could be related to the dynamic Raldh2 pattern of expression (Figure 1h,i). At the time of MN generation, Raldh2 is expressed in somites, from where it promotes the specification of MN progenitors (Figure 1c,e) and their progression to differentiation (Figure 1g). Moreover, Raldh2 is especially high at the cervical/brachial levels, where it promotes cervical/brachial versus thoracic MN specification [14]. Finally, the expression of Raldh2 increases in lumbar paraxial mesoderm levels and also is expressed in brachial and lumbar LMC MNs, resulting in high signaling levels that promote the diversification of LMC neurons into lateral and medial fates [14] and the survival of all LMC neurons.
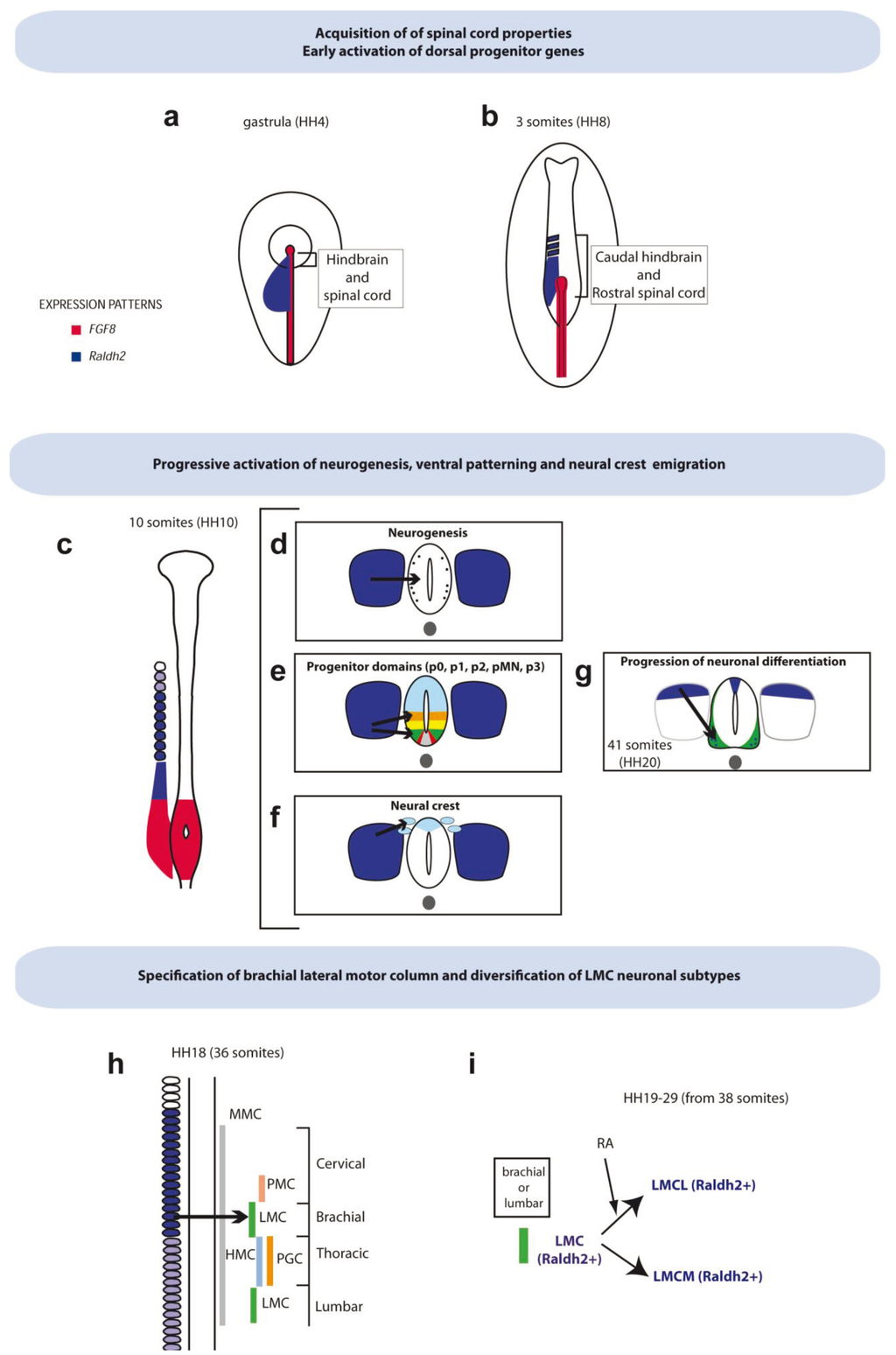
Figure 1.
Retinoic acid (RA) functions in spinal cord development. At gastrulation (a) and early somite (b) chick stages, Raldh2 is expressed in the paraxial mesoderm underlying the presumptive hindbrain and spinal cord. RA is involved in the acquisition of spinal cord properties. At these stages, it may also allow the activation of dorsal-specific genes. (c,d–f) Around the 10-somite stage, Raldh2 is produced in the somites (at lower levels in s1–4) and RA is involved in several processes shown in the cross sections (d–f), such as early neurogenesis (d), establishment of ventral progenitor domains (e) and neural crest emigration (f). (g) At the 40-somite stage, the expression of Raldh2 is restricted to the dermomyotome component of somites, and it is initiated in the roof plate and in motor neuros (MNs) of the lateral motor column (LMC) at the brachial and lumbar levels. Around these stages, RA is involved in the progression of neuronal differentiation. (h) Around the 36-somite stage, Raldh2 is highly expressed in somites at cervical/brachial levels. RA is involved here in the specification of brachial MN and, in particular, in the LMC MNs. (i) At around the 40-somite stage, Raldh2 is expressed specifically in LMC MNs and continues to be expressed in somites. RA promotes the diversification of LMC neurons, promoting a lateral LMC fate. HH, Hamburger and Hamilton; MMC, medial motor column; PMC, phrenic motor column; HMC, hypaxial motor column; PGC, preganglionic motor column.
7. Conclusions
The actual view in the field is that RA signaling has essential and diverse roles during spinal cord development, from early specification and patterning of neural progenitors to the progression to the differentiation and diversification of neuronal subtypes. For that reason, a careful consideration of the most appropriate RA experimental manipulation procedure is needed to reach precise conclusions about RA-specific roles and mechanisms of action. For instance, the requirement of RA for the expression of dorsal patterning genes may be the result of a defective spinal cord specification or reflect a later role in specification or maintenance of dorsal identity.
Given the complexity of RA activity, several questions remain open in the field. An important challenge is to determine the sequence of direct gene activation events attributed to changes in RA signaling levels. Several studies have led to novel insights into the interplay between RA and other signaling pathways (such as FGF, Hedgehog, TGF) in several developing systems. However, better knowledge of the immediate early RA target genes is necessary to clarify whether there are common regulatory networks, rather than specific gene targets, for each system.
It will be important to further improve live imaging tools to accurately monitor endogenous RA activity gradients across embryonic development at the cellular level [47]. These tools, in combination with the identification of the mechanisms controlling the expression of enzymes involved in RA production and degradation, as well as of the positive and negative feedback loops, should provide a better understanding of the dynamics and fine-tuning of RA signaling levels throughout development.
Finally, RA has been shown to promote specific MN fates in embryonic stem cells [70] and constitutes an important reagent for exploring the disease sensitivity of motor neuron subtypes in vitro [130,131]. The advances in the understanding of the function of RA during normal neurogenesis and the acquisition of neuronal subtype identities will provide essential information for the field of neurodegenerative and regenerative medicine and may contribute to the establishment of treatments for damaged or aged human spinal cord, based on the introduction of differentiated cells produced in vitro from embryonic stem cells or induced pluripotent stem cells.
Acknowledgements
This work was supported by a grant from the Spanish government to Ruth Diez del Corral and Aixa V. Morales (BFU2011-29490).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stern, C.D. Neural induction: Old problem, new findings, yet more questions. Development 2005, 132, 2007–2021. [Google Scholar] [CrossRef]
- Stern, C.D.; Charite, J.; Deschamps, J.; Duboule, D.; Durston, A.J.; Kmita, M.; Nicolas, J.F.; Palmeirim, I.; Smith, J.C.; Wolpert, L. Head-tail patterning of the vertebrate embryo: One, two or many unresolved problems? Int. J. Dev. Biol. 2006, 50, 3–15. [Google Scholar] [CrossRef]
- Tam, P.P.; Beddington, R.S. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development 1987, 99, 109–126. [Google Scholar]
- Brown, J.M.; Storey, K.G. A region of the vertebrate neural plate in which neighbouring cells can adopt neural or epidermal cell fates. Curr. Biol. 2000, 10, 869–872. [Google Scholar] [CrossRef]
- Mathis, L.; Kulesa, P.M.; Fraser, S.E. Fgf receptor signalling is required to maintain neural progenitors during Hensen’s node progression. Nat. Cell. Biol. 2001, 3, 559–566. [Google Scholar] [CrossRef]
- Wilson, V.; Olivera-Martinez, I.; Storey, K.G. Stem cells, signals and vertebrate body axis extension. Development 2009, 136, 1591–1604. [Google Scholar] [CrossRef]
- Dersch, H.; Zile, M.H. Induction of normal cardiovascular development in the vitamin A-deprived quail embryo by natural retinoids. Dev. Biol. 1993, 160, 424–433. [Google Scholar] [CrossRef]
- Dong, D.; Zile, M.H. Endogenous retinoids in the early avian embryo. Biochem. Biophys. Res. Commun. 1995, 217, 1026–1031. [Google Scholar] [CrossRef]
- Mic, F.A.; Haselbeck, R.J.; Cuenca, A.E.; Duester, G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of raldh2 null mutant mice. Development 2002, 129, 2271–2282. [Google Scholar]
- Niederreither, K.; Subbarayan, V.; Dolle, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post- implantation development. Nat. Genet. 1999, 21, 444–448. [Google Scholar] [CrossRef]
- Niederreither, K.; Vermot, J.; Fraulob, V.; Chambon, P.; Dolle, P. Retinaldehyde dehydrogenase 2 (raldh2)-independent patterns of retinoic acid synthesis in the mouse embryo. Proc. Natl. Acad. Sci. USA 2002, 99, 16111–16116. [Google Scholar] [CrossRef]
- Vermot, J.; Schuhbaur, B.; Le Mouellic, H.; McCaffery, P.; Garnier, J.M.; Hentsch, D.; Brulet, P.; Niederreither, K.; Chambon, P.; Dolle, P.; et al. Retinaldehyde dehydrogenase 2 and hoxc8 are required in the murine brachial spinal cord for the specification of lim1+ motoneurons and the correct distribution of islet1+ motoneurons. Development 2005, 132, 1611–1621. [Google Scholar] [CrossRef]
- Ji, S.J.; Zhuang, B.; Falco, C.; Schneider, A.; Schuster-Gossler, K.; Gossler, A.; Sockanathan, S. Mesodermal and neuronal retinoids regulate the induction and maintenance of limb innervating spinal motor neurons. Dev. Biol. 2006, 297, 249–261. [Google Scholar] [CrossRef]
- Sockanathan, S.; Perlmann, T.; Jessell, T.M. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron 2003, 40, 97–111. [Google Scholar] [CrossRef]
- Duester, G. Retinoic acid synthesis and signalling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef]
- Sandell, L.L.; Lynn, M.L.; Inman, K.E.; McDowell, W.; Trainor, P.A. Rdh10 oxidation of vitamin a is a critical control step in synthesis of retinoic acid during mouse embryogenesis. PLoS ONE 2012, 7, e30698. [Google Scholar]
- Sandell, L.L.; Sanderson, B.W.; Moiseyev, G.; Johnson, T.; Mushegian, A.; Young, K.; Rey, J.P.; Ma, J.X.; Staehling-Hampton, K.; Trainor, P.A. Rdh10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007, 21, 1113–1124. [Google Scholar] [CrossRef]
- Cammas, L.; Romand, R.; Fraulob, V.; Mura, C.; Dolle, P. Expression of the murine retinol dehydrogenase 10 (rdh10) gene correlates with many sites of retinoid signalling during embryogenesis and organ differentiation. Dev. Dyn. 2007, 236, 2899–2908. [Google Scholar] [CrossRef]
- Reijntjes, S.; Zile, M.H.; Maden, M. The expression of stra6 and rdh10 in the avian embryo and their contribution to the generation of retinoid signatures. Int. J. Dev. Biol. 2010, 54, 1267–1275. [Google Scholar] [CrossRef]
- Rhinn, M.; Schuhbaur, B.; Niederreither, K.; Dolle, P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc. Natl. Acad. Sci. USA 2011, 108, 16687–16692. [Google Scholar]
- Belyaeva, O.V.; Lee, S.A.; Adams, M.K.; Chang, C.; Kedishvili, N.Y. Short chain dehydrogenase/reductase rdhe2 is a novel retinol dehydrogenase essential for frog embryonic development. J. Biol. Chem. 2012, 287, 9061–9071. [Google Scholar]
- Chambers, D.; Wilson, L.; Maden, M.; Lumsden, A. Raldh-independent generation of retinoic acid during vertebrate embryogenesis by cyp1b1. Development 2007, 134, 1369–1383. [Google Scholar] [CrossRef]
- Muhr, J.; Graziano, E.; Wilson, S.; Jessell, T.M.; Edlund, T. Convergent inductive signals specify midbrain, hindbrain, and spinal cord identity in gastrula stage chick embryos. Neuron 1999, 23, 689–702. [Google Scholar] [CrossRef]
- Swindell, E.C.; Thaller, C.; Sockanathan, S.; Petkovich, M.; Jessell, T.M.; Eichele, G. Complementary domains of retinoic acid production and degradation in the early chick embryo. Dev. Biol. 1999, 216, 282–296. [Google Scholar] [CrossRef]
- Berggren, K.; McCaffery, P.; Drager, U.; Forehand, C.J. Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, raldh-2. Dev. Biol. 1999, 210, 288–304. [Google Scholar] [CrossRef]
- Niederreither, K.; McCaffery, P.; Drager, U.C.; Chambon, P.; Dolle, P. Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (raldh-2) gene during mouse development. Mech. Dev. 1997, 62, 67–78. [Google Scholar] [CrossRef]
- Niederreither, K.; Vermot, J.; Schuhbaur, B.; Chambon, P.; Dolle, P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development 2000, 127, 75–85. [Google Scholar]
- Ribes, V.; Wang, Z.; Dolle, P.; Niederreither, K. Retinaldehyde dehydrogenase 2 (raldh2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling fgf and sonic hedgehog signaling. Development 2006, 133, 351–361. [Google Scholar]
- Fujii, H.; Sato, T.; Kaneko, S.; Gotoh, O.; Fujii-Kuriyama, Y.; Osawa, K.; Kato, S.; Hamada, H. Metabolic inactivation of retinoic acid by a novel p450 differentially expressed in developing mouse embryos. EMBO J. 1997, 16, 4163–4173. [Google Scholar] [CrossRef]
- Reijntjes, S.; Gale, E.; Maden, M. Generating gradients of retinoic acid in the chick embryo: Cyp26c1 expression and a comparative analysis of the cyp26 enzymes. Dev. Dyn. 2004, 230, 509–517. [Google Scholar] [CrossRef]
- Blentic, A.; Gale, E.; Maden, M. Retinoic acid signalling centres in the avian embryo identified by sites of expression of synthesising and catabolising enzymes. Dev. Dyn. 2003, 227, 114–127. [Google Scholar] [CrossRef]
- Sirbu, I.O.; Gresh, L.; Barra, J.; Duester, G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development 2005, 132, 2611–2622. [Google Scholar] [CrossRef]
- Cai, A.Q.; Radtke, K.; Linville, A.; Lander, A.D.; Nie, Q.; Schilling, T.F. Cellular retinoic acid-binding proteins are essential for hindbrain patterning and signal robustness in zebrafish. Development 2012, 139, 2150–2155. [Google Scholar] [CrossRef]
- Lampron, C.; Rochette-Egly, C.; Gorry, P.; Dolle, P.; Mark, M.; Lufkin, T.; LeMeur, M.; Chambon, P. Mice deficient in cellular retinoic acid binding protein ii (crabpii) or in both crabpi and crabpii are essentially normal. Development 1995, 121, 539–548. [Google Scholar]
- Ghyselinck, N.B.; Bavik, C.; Sapin, V.; Mark, M.; Bonnier, D.; Hindelang, C.; Dierich, A.; Nilsson, C.B.; Hakansson, H.; Sauvant, P.; et al. Cellular retinol-binding protein i is essential for vitamin a homeostasis. EMBO J. 1999, 18, 4903–4914. [Google Scholar] [CrossRef]
- Maden, M.; Ong, D.E.; Summerbell, D.; Chytil, F. The role of retinoid-binding proteins in the generation of pattern in the developing limb, the regenerating limb and the nervous system. Development 1989, 107, 109–119. [Google Scholar]
- Perez-Castro, A.V.; Toth-Rogler, L.E.; Wei, L.N.; Nguyen-Huu, M.C. Spatial and temporal pattern of expression of the cellular retinoic acid-binding protein and the cellular retinol-binding protein during mouse embryogenesis. Proc. Natl. Acad. Sci. USA 1989, 86, 8813–8817. [Google Scholar]
- Ruberte, E.; Friederich, V.; Morriss-Kay, G.; Chambon, P. Differential distribution patterns of crabp i and crabp ii transcripts during mouse embryogenesis. Development 1992, 115, 973–987. [Google Scholar]
- Olivera-Martinez, I.; Harada, H.; Halley, P.A.; Storey, K.G. Loss of fgf-dependent mesoderm identity and rise of endogenous retinoid signalling determine cessation of body axis elongation. PLoS Biol. 2012, 10, e1001415. [Google Scholar] [CrossRef]
- Shiga, T.; Gaur, V.P.; Yamaguchi, K.; Oppenheim, R.W. The development of interneurons in the chick embryo spinal cord following in vivo treatment with retinoic acid. J. Comp. Neurol. 1995, 360, 463–474. [Google Scholar] [CrossRef]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 451–480. [Google Scholar] [CrossRef]
- Schug, T.T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 2007, 129, 723–733. [Google Scholar]
- Dolle, P. Developmental expression of retinoic acid receptors (RARs). Nucl. Recept. Signal. 2009, 7, e006. [Google Scholar]
- Rhinn, M.; Dolle, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef]
- Diez del Corral, R.; Olivera-Martinez, I.; Goriely, A.; Gale, E.; Maden, M.; Storey, K.G. Opposing fgf and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 2003, 40, 65–79. [Google Scholar] [CrossRef]
- Maden, M.; Sonneveld, E.; van der Saag, P.T.; Gale, E. The distribution of endogenous retinoic acid in the chick embryo: Implications for developmental mechanisms. Development 1998, 125, 4133–4144. [Google Scholar]
- Shimozono, S.; Iimura, T.; Kitaguchi, T.; Higashijima, S.; Miyawaki, A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature 2013, 496, 363–366. [Google Scholar] [CrossRef]
- Rossant, J.; Zirngibl, R.; Cado, D.; Shago, M.; Giguere, V. Expression of a retinoic acid response element-hsplacz transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991, 5, 1333–1344. [Google Scholar] [CrossRef]
- Ribes, V.; Le Roux, I.; Rhinn, M.; Schuhbaur, B.; Dolle, P. Early mouse caudal development relies on crosstalk between retinoic acid, shh and fgf signalling pathways. Development 2009, 136, 665–676. [Google Scholar] [CrossRef]
- Colbert, M.C.; Rubin, W.W.; Linney, E.; LaMantia, A.S. Retinoid signaling and the generation of regional and cellular diversity in the embryonic mouse spinal cord. Dev. Dyn. 1995, 204, 1–12. [Google Scholar] [CrossRef]
- Dubrulle, J.; McGrew, M.J.; Pourquie, O. Fgf signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal hox gene activation. Cell 2001, 106, 219–232. [Google Scholar] [CrossRef]
- Akai, J.; Halley, P.A.; Storey, K.G. Fgf-dependent notch signaling maintains the spinal cord stem zone. Genes Dev. 2005, 19, 2877–2887. [Google Scholar] [CrossRef]
- Diez del Corral, R.; Breitkreuz, D.N.; Storey, K.G. Onset of neuronal differentiation is regulated by paraxial mesoderm and requires attenuation of fgf signalling. Development 2002, 129, 1681–1691. [Google Scholar]
- Olivera-Martinez, I.; Storey, K.G. Wnt signals provide a timing mechanism for the fgf-retinoid differentiation switch during vertebrate body axis extension. Development 2007, 134, 2125–2135. [Google Scholar] [CrossRef]
- Wahl, M.B.; Deng, C.; Lewandoski, M.; Pourquie, O. Fgf signaling acts upstream of the notch and wnt signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development 2007, 134, 4033–4041. [Google Scholar] [CrossRef]
- Molotkova, N.; Molotkov, A.; Sirbu, I.O.; Duester, G. Requirement of mesodermal retinoic acid generated by raldh2 for posterior neural transformation. Mech. Dev. 2005, 122, 145–155. [Google Scholar] [CrossRef]
- Vermot, J.; Gallego Llamas, J.; Fraulob, V.; Niederreither, K.; Chambon, P.; Dolle, P. Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science 2005, 308, 563–566. [Google Scholar] [CrossRef]
- Patel, N.S.; Rhinn, M.; Semprich, C.I.; Halley, P.A.; Dolle, P.; Bickmore, W.A.; Storey, K.G. Fgf signalling regulates chromatin organisation during neural differentiation via mechanisms that can be uncoupled from transcription. PLoS Genet. 2013, 9, e1003614. [Google Scholar] [CrossRef]
- Sirbu, I.O.; Duester, G. Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nat. Cell Biol. 2006, 8, 271–277. [Google Scholar] [CrossRef]
- Afonso, N.D.; Catala, M. Sonic hedgehog and retinoic acid are not sufficient to induce motoneuron generation in the avian caudal neural tube. Dev. Biol. 2005, 279, 356–367. [Google Scholar] [CrossRef]
- Psychoyos, D.; Stern, C.D. Restoration of the organizer after radical ablation of Hensen’s node and the anterior primitive streak in the chick embryo. Development 1996, 122, 3263–3273. [Google Scholar]
- Wilson, V.; Beddington, R.S. Cell fate and morphogenetic movement in the late mouse primitive streak. Mech. Dev. 1996, 55, 79–89. [Google Scholar] [CrossRef]
- Jurberg, A.D.; Aires, R.; Varela-Lasheras, I.; Novoa, A.; Mallo, M. Switching axial progenitors from producing trunk to tail tissues in vertebrate embryos. Dev. Cell 2013, 25, 451–462. [Google Scholar] [CrossRef]
- Kessel, M.; Gruss, P. Homeotic transformations of murine vertebrae and concomitant alteration of hox codes induced by retinoic acid. Cell 1991, 67, 89–104. [Google Scholar] [CrossRef]
- Shum, A.S.; Poon, L.L.; Tang, W.W.; Koide, T.; Chan, B.W.; Leung, Y.C.; Shiroishi, T.; Copp, A.J. Retinoic acid induces down-regulation of wnt-3a, apoptosis and diversion of tail bud cells to a neural fate in the mouse embryo. Mech. Dev. 1999, 84, 17–30. [Google Scholar] [CrossRef]
- Takada, S.; Stark, K.L.; Shea, M.J.; Vassileva, G.; McMahon, J.A.; McMahon, A.P. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994, 8, 174–189. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Zhao, X.; Duester, G. Uncoupling of retinoic acid signaling from tailbud development before termination of body axis extension. Genesis 2011, 49, 776–783. [Google Scholar]
- Brown, J.M.; Robertson, K.E.; Wedden, S.E.; Tickle, C. Alterations in msx 1 and msx 2 expression correlate with inhibition of outgrowth of chick facial primordia induced by retinoic acid. Anat. Embryol. Berl. 1997, 195, 203–207. [Google Scholar] [CrossRef]
- Tickle, C.; Crawley, A.; Farrar, J. Retinoic acid application to chick wing buds leads to a dose-dependent reorganization of the apical ectodermal ridge that is mediated by the mesenchyme. Development 1989, 106, 691–705. [Google Scholar]
- Wichterle, H.; Lieberam, I.; Porter, J.A.; Jessell, T.M. Directed differentiation of embryonic stem cells into motor neurons. Cell 2002, 110, 385–397. [Google Scholar] [CrossRef]
- Wilson, L.; Gale, E.; Maden, M. The role of retinoic acid in the morphogenesis of the neural tube. J. Anat. 2003, 203, 357–368. [Google Scholar] [CrossRef]
- England, S.; Batista, M.F.; Mich, J.K.; Chen, J.K.; Lewis, K.E. Roles of hedgehog pathway components and retinoic acid signalling in specifying zebrafish ventral spinal cord neurons. Development 2011, 138, 5121–5134. [Google Scholar] [CrossRef]
- Sockanathan, S.; Jessell, T.M. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell 1998, 94, 503–514. [Google Scholar] [CrossRef]
- Maden, M.; Gale, E.; Kostetskii, I.; Zile, M. Vitamin a-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996, 6, 417–426. [Google Scholar] [CrossRef]
- Stavridis, M.P.; Collins, B.J.; Storey, K.G. Retinoic acid orchestrates fibroblast growth factor signalling to drive embryonic stem cell differentiation. Development 2010, 137, 881–890. [Google Scholar] [CrossRef]
- Guth, S.I.; Wegner, M. Having it both ways: Sox protein function between conservation and innovation. Cell. Mol. Life Sci. 2008, 65, 3000–3018. [Google Scholar] [CrossRef]
- Ribes, V.; Stutzmann, F.; Bianchetti, L.; Guillemot, F.; Dolle, P.; Le Roux, I. Combinatorial signalling controls neurogenin2 expression at the onset of spinal neurogenesis. Dev. Biol. 2008, 321, 470–481. [Google Scholar] [CrossRef]
- Rao, M.; Sockanathan, S. Transmembrane protein gde2 induces motor neuron differentiation in vivo. Science 2005, 309, 2212–2215. [Google Scholar] [CrossRef]
- Sabharwal, P.; Lee, C.; Park, S.; Rao, M.; Sockanathan, S. Gde2 regulates subtype-specific motor neuron generation through inhibition of notch signaling. Neuron 2011, 71, 1058–1070. [Google Scholar] [CrossRef]
- Dessaud, E.; McMahon, A.P.; Briscoe, J. Pattern formation in the vertebrate neural tube: A sonic hedgehog morphogen-regulated transcriptional network. Development 2008, 135, 2489–2503. [Google Scholar] [CrossRef]
- Wilson, L.; Gale, E.; Chambers, D.; Maden, M. Retinoic acid and the control of dorsoventral patterning in the avian spinal cord. Dev. Biol. 2004, 269, 433–446. [Google Scholar] [CrossRef]
- Novitch, B.G.; Wichterle, H.; Jessell, T.M.; Sockanathan, S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 2003, 40, 81–95. [Google Scholar] [CrossRef]
- Paschaki, M.; Lin, S.C.; Wong, R.L.; Finnell, R.H.; Dolle, P.; Niederreither, K. Retinoic acid-dependent signaling pathways and lineage events in the developing mouse spinal cord. PLoS ONE 2012, 7, e32447. [Google Scholar]
- Briscoe, J.; Pierani, A.; Jessell, T.M.; Ericson, J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 2000, 101, 435–445. [Google Scholar] [CrossRef]
- Vallstedt, A.; Muhr, J.; Pattyn, A.; Pierani, A.; Mendelsohn, M.; Sander, M.; Jessell, T.M.; Ericson, J. Different levels of repressor activity assign redundant and specific roles to nkx6 genes in motor neuron and interneuron specification. Neuron 2001, 31, 743–755. [Google Scholar] [CrossRef]
- Pierani, A.; Brenner-Morton, S.; Chiang, C.; Jessell, T.M. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell 1999, 97, 903–915. [Google Scholar] [CrossRef]
- Bertrand, N.; Medevielle, F.; Pituello, F. Fgf signalling controls the timing of pax6 activation in the neural tube. Development 2000, 127, 4837–4843. [Google Scholar]
- Oosterveen, T.; Kurdija, S.; Alekseenko, Z.; Uhde, C.W.; Bergsland, M.; Sandberg, M.; Andersson, E.; Dias, J.M.; Muhr, J.; Ericson, J. Mechanistic differences in the transcriptional interpretation of local and long-range shh morphogen signaling. Dev. Cell 2012, 23, 1006–1019. [Google Scholar] [CrossRef]
- Balaskas, N.; Ribeiro, A.; Panovska, J.; Dessaud, E.; Sasai, N.; Page, K.M.; Briscoe, J.; Ribes, V. Gene regulatory logic for reading the sonic hedgehog signaling gradient in the vertebrate neural tube. Cell 2012, 148, 273–284. [Google Scholar] [CrossRef]
- Lee, S.; Lee, B.; Lee, J.W.; Lee, S.K. Retinoid signaling and neurogenin2 function are coupled for the specification of spinal motor neurons through a chromatin modifier cbp. Neuron 2009, 62, 641–654. [Google Scholar] [CrossRef]
- Jacob, J.; Kong, J.; Moore, S.; Milton, C.; Sasai, N.; Gonzalez-Quevedo, R.; Terriente, J.; Imayoshi, I.; Kageyama, R.; Wilkinson, D.G.; et al. Retinoid acid specifies neuronal identity through graded expression of ascl1. Curr. Biol. 2013, 23, 412–418. [Google Scholar] [CrossRef]
- Le Dreau, G.; Marti, E. Dorsal-ventral patterning of the neural tube: A tale of three signals. Dev. Neurobiol. 2012, 72, 1471–1481. [Google Scholar] [CrossRef]
- Zhuang, B.; Sockanathan, S. Dorsal-ventral patterning: A view from the top. Curr. Opin. Neurobiol. 2006, 16, 20–24. [Google Scholar] [CrossRef]
- Martinez-Morales, P.L.; Diez Del Corral, R.; Olivera-Martinez, I.; Quiroga, A.C.; Das, R.M.; Barbas, J.A.; Storey, K.G.; Morales, A.V. Fgf and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. J. Cell. Biol. 2011, 194, 489–503. [Google Scholar] [CrossRef]
- Nordstrom, U.; Jessell, T.M.; Edlund, T. Progressive induction of caudal neural character by graded wnt signaling. Nat. Neurosci. 2002, 5, 525–532. [Google Scholar] [CrossRef]
- Patthey, C.; Gunhaga, L.; Edlund, T. Early development of the central and peripheral nervous systems is coordinated by wnt and bmp signals. PLoS ONE 2008, 3, e1625. [Google Scholar] [CrossRef]
- Villanueva, S.; Glavic, A.; Ruiz, P.; Mayor, R. Posteriorization by fgf, wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 2002, 241, 289–301. [Google Scholar] [CrossRef]
- Patthey, C.; Edlund, T.; Gunhaga, L. Wnt-regulated temporal control of bmp exposure directs the choice between neural plate border and epidermal fate. Development 2009, 136, 73–83. [Google Scholar] [CrossRef]
- Le Douarin, N.; Kalcheim, C. The Neural Crest; Cambridge University Press: Cambridge, UK, 1999; p. 445. [Google Scholar]
- Teillet, M.A.; Kalcheim, C.; Le Douarin, N.M. Formation of the dorsal root ganglia in the avian embryo: Segmental origin and migratory behavior of neural crest progenitor cells. Dev. Biol. 1987, 120, 329–347. [Google Scholar] [CrossRef]
- Sela-Donenfeld, D.; Kalcheim, C. Regulation of the onset of neural crest migration by coordinated activity of bmp4 and noggin in the dorsal neural tube. Development 1999, 126, 4749–4762. [Google Scholar]
- Burstyn-Cohen, T.; Stanleigh, J.; Sela-Donenfeld, D.; Kalcheim, C. Canonical wnt activity regulates trunk neural crest delamination linking bmp/noggin signaling with g1/s transition. Development 2004, 131, 5327–5339. [Google Scholar] [CrossRef]
- Sela-Donenfeld, D.; Kalcheim, C. Inhibition of noggin expression in the dorsal neural tube by somitogenesis: A mechanism for coordinating the timing of neural crest emigration. Development 2000, 127, 4845–4854. [Google Scholar]
- Krispin, S.; Nitzan, E.; Kassem, Y.; Kalcheim, C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development 2010, 137, 585–595. [Google Scholar] [CrossRef]
- Aquino, J.B.; Lallemend, F.; Marmigere, F.; Adameyko, I.I.; Golemis, E.A.; Ernfors, P. The retinoic acid inducible Cas-family signaling protein nedd9 regulates neural crest cell migration by modulating adhesion and actin dynamics. Neuroscience 2009, 162, 1106–1119. [Google Scholar] [CrossRef]
- Merrill, R.A.; See, A.W.; Wertheim, M.L.; Clagett-Dame, M. Crk-associated substrate (Cas) family member, nedd9, is regulated in human neuroblastoma cells and in the embryonic hindbrain by all-trans retinoic acid. Dev. Dyn. 2004, 231, 564–575. [Google Scholar] [CrossRef]
- Dickman, E.D.; Thaller, C.; Smith, S.M. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development 1997, 124, 3111–3121. [Google Scholar]
- Lohnes, D.; Mark, M.; Mendelsohn, C.; Dolle, P.; Decimo, D.; LeMeur, M.; Dierich, A.; Gorry, P.; Chambon, P. Developmental roles of the retinoic acid receptors. J. Steroid Biochem. Mol. Biol. 1995, 53, 475–486. [Google Scholar] [CrossRef]
- Niederreither, K.; Vermot, J.; Le Roux, I.; Schuhbaur, B.; Chambon, P.; Dolle, P. The regional pattern of retinoic acid synthesis by raldh2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 2003, 130, 2525–2534. [Google Scholar] [CrossRef]
- Wright-Jin, E.C.; Grider, J.R.; Duester, G.; Heuckeroth, R.O. Retinaldehyde dehydrogenase enzymes regulate colon enteric nervous system structure and function. Dev. Biol. 2013, 381, 28–37. [Google Scholar] [CrossRef]
- Lohnes, D.; Mark, M.; Mendelsohn, C.; Dolle, P.; Dierich, A.; Gorry, P.; Gansmuller, A.; Chambon, P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development 1994, 120, 2723–2748. [Google Scholar]
- Ghyselinck, N.B.; Dupe, V.; Dierich, A.; Messaddeq, N.; Garnier, J.M.; Rochette-Egly, C.; Chambon, P.; Mark, M. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int. J. Dev. Biol. 1997, 41, 425–447. [Google Scholar]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 2009, 7, e002. [Google Scholar]
- Minoux, M.; Rijli, F.M. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 2010, 137, 2605–2621. [Google Scholar] [CrossRef]
- Halilagic, A.; Ribes, V.; Ghyselinck, N.B.; Zile, M.H.; Dolle, P.; Studer, M. Retinoids control anterior and dorsal properties in the developing forebrain. Dev. Biol. 2007, 303, 362–375. [Google Scholar] [CrossRef]
- Halilagic, A.; Zile, M.H.; Studer, M. A novel role for retinoids in patterning the avian forebrain during presomite stages. Development 2003, 130, 2039–2050. [Google Scholar] [CrossRef]
- Liu, J.P.; Laufer, E.; Jessell, T.M. Assigning the positional identity of spinal motor neurons: Rostrocaudal patterning of hox-c expression by fgfs, gdf11, and retinoids. Neuron 2001, 32, 997–1012. [Google Scholar] [CrossRef]
- Oosterveen, T.; Niederreither, K.; Dolle, P.; Chambon, P.; Meijlink, F.; Deschamps, J. Retinoids regulate the anterior expression boundaries of 5' hoxb genes in posterior hindbrain. Embo J. 2003, 22, 262–269. [Google Scholar] [CrossRef]
- Nordstrom, U.; Maier, E.; Jessell, T.M.; Edlund, T. An early role for wnt signaling in specifying neural patterns of cdx and hox gene expression and motor neuron subtype identity. PLoS Biol. 2006, 4, e252. [Google Scholar] [CrossRef]
- Delfino-Machin, M.; Lunn, J.S.; Breitkreuz, D.N.; Akai, J.; Storey, K.G. Specification and maintenance of the spinal cord stem zone. Development 2005, 132, 4273–4283. [Google Scholar] [CrossRef]
- Houle, M.; Prinos, P.; Iulianella, A.; Bouchard, N.; Lohnes, D. Retinoic acid regulation of cdx1: An indirect mechanism for retinoids and vertebral specification. Mol. Cell. Biol. 2000, 20, 6579–6586. [Google Scholar] [CrossRef]
- Alexander, T.; Nolte, C.; Krumlauf, R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu. Rev. Cell Dev. Biol. 2009, 25, 431–456. [Google Scholar] [CrossRef]
- Shimizu, T.; Bae, Y.K.; Hibi, M. Cdx-hox code controls competence for responding to fgfs and retinoic acid in zebrafish neural tissue. Development 2006, 133, 4709–4719. [Google Scholar] [CrossRef]
- Bel-Vialar, S.; Itasaki, N.; Krumlauf, R. Initiating hox gene expression: In the early chick neural tube differential sensitivity to fgf and ra signaling subdivides the hoxb genes in two distinct groups. Development 2002, 129, 5103–5115. [Google Scholar]
- Philippidou, P.; Dasen, J.S. Hox genes: Choreographers in neural development, architects of circuit organization. Neuron 2013, 80, 12–34. [Google Scholar] [CrossRef]
- Ensini, M.; Tsuchida, T.N.; Belting, H.G.; Jessell, T.M. The control of rostrocaudal pattern in the developing spinal cord: Specification of motor neuron subtype identity is initiated by signals from paraxial mesoderm. Development 1998, 125, 969–982. [Google Scholar]
- Misra, M.; Shah, V.; Carpenter, E.; McCaffery, P.; Lance-Jones, C. Restricted patterns of hoxd10 and hoxd11 set segmental differences in motoneuron subtype complement in the lumbosacral spinal cord. Dev. Biol. 2009, 330, 54–72. [Google Scholar] [CrossRef]
- Kania, A.; Jessell, T.M. Topographic motor projections in the limb imposed by lim homeodomain protein regulation of ephrin-a:Epha interactions. Neuron 2003, 38, 581–596. [Google Scholar] [CrossRef]
- Ji, S.J.; Periz, G.; Sockanathan, S. Nolz1 is induced by retinoid signals and controls motoneuron subtype identity through distinct repressor activities. Development 2009, 136, 231–240. [Google Scholar] [CrossRef]
- Amoroso, M.W.; Croft, G.F.; Williams, D.J.; O’Keeffe, S.; Carrasco, M.A.; Davis, A.R.; Roybon, L.; Oakley, D.H.; Maniatis, T.; Henderson, C.E.; et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J. Neurosci. 2013, 33, 574–586. [Google Scholar] [CrossRef]
- Peljto, M.; Wichterle, H. Programming embryonic stem cells to neuronal subtypes. Curr. Opin. Neurobiol. 2011, 21, 43–51. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).