Activity-Dependent Increases in Quantal Size at the Drosophila NMJ
Abstract
1. Introduction
2. Materials and Methods
3. Results
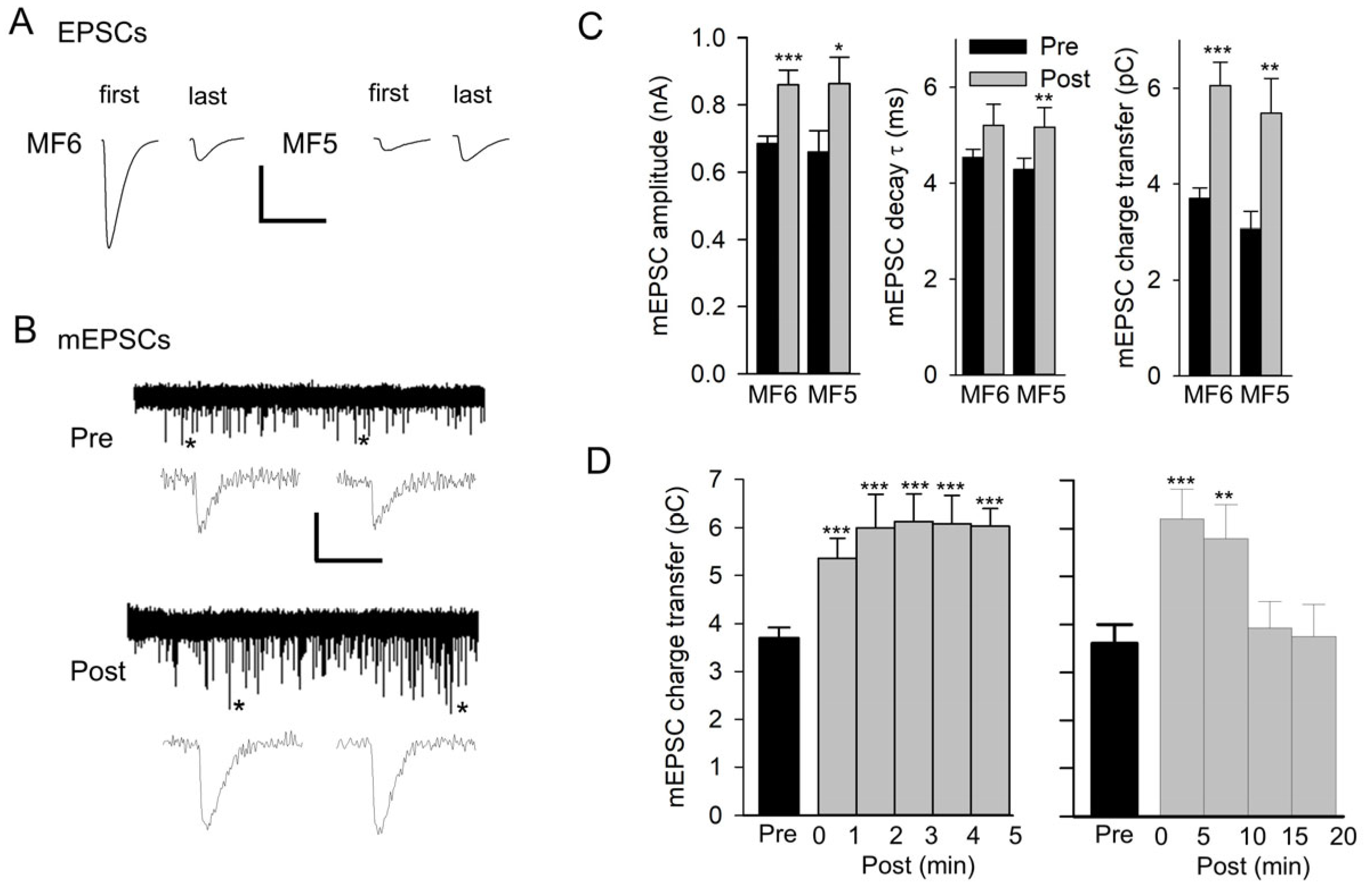
3.1. Nerve Stimulation Produces Potentiation of mEPSCs
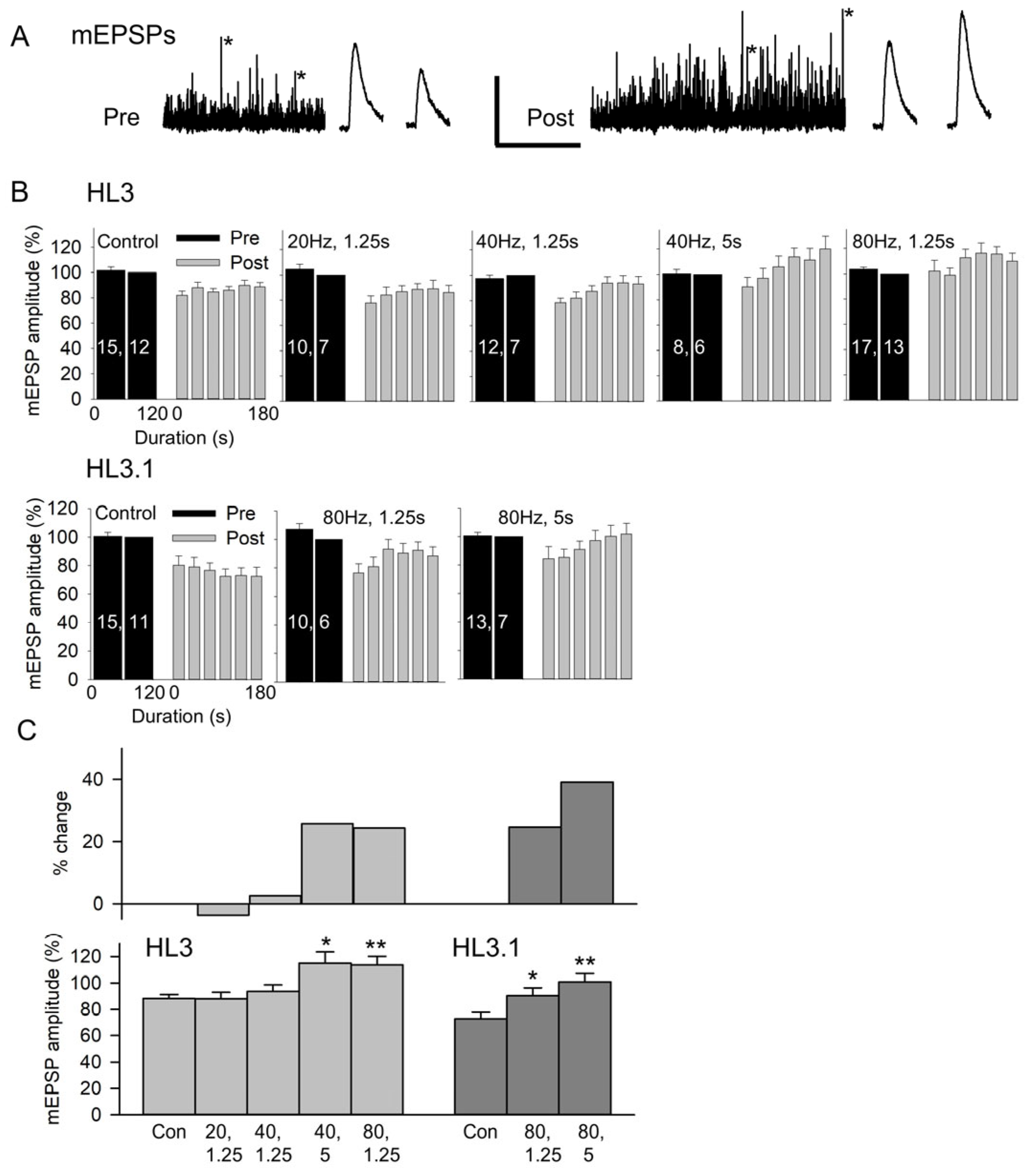
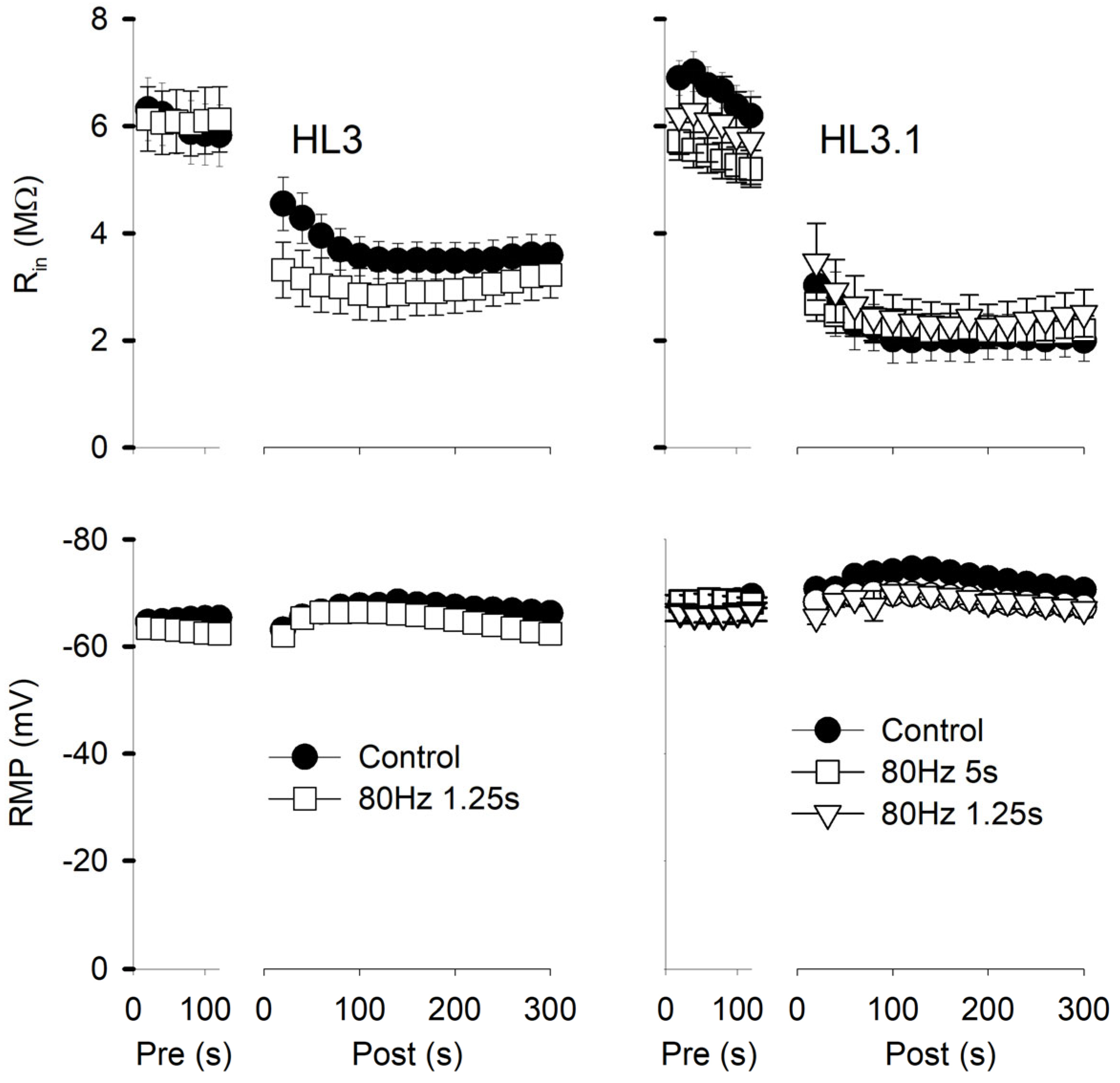
3.2. Brief High-Frequency Stimulation Produces Potentiation of mEPSPs
3.3. mEPSP Amplitude Appears Potentiated by In Vivo Activity
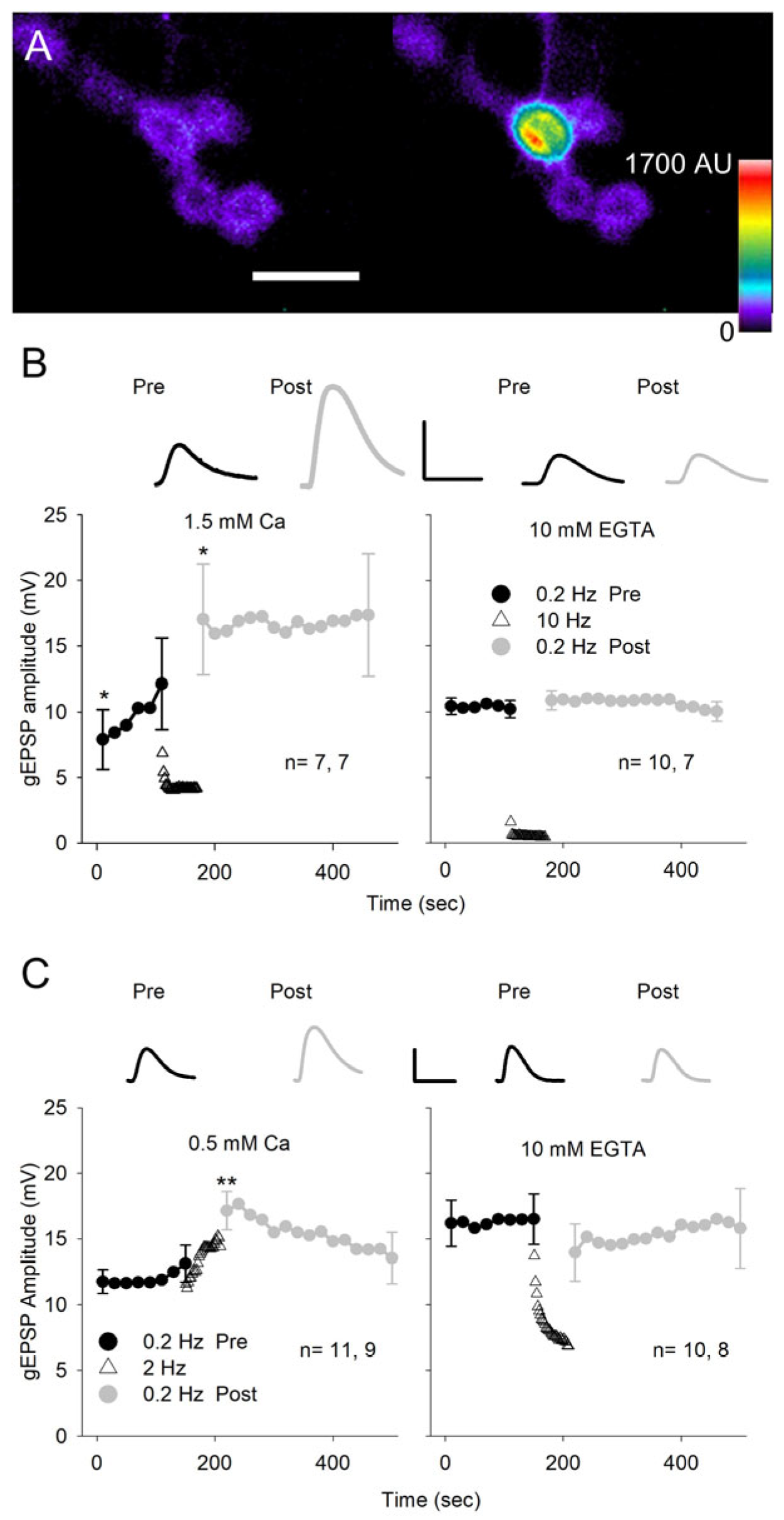
3.4. Potentiation of Quantal Ca2+ Signals
3.5. The Potentiation of Quantal Size Is Dependent on Increased Postsynaptic Ca2+ Concentration
3.6. Activation of GluRs Produces a Ca2+-Dependent Increase in Glutamate Sensitivity
4. Discussion
4.1. An Activity-Dependent Increase in Quantal Size Was Demonstrated by Electrophysiological Measurements
4.2. The Activity-Dependent Increase in Quantal Size Was Examined with Optophysiology
4.3. The Potentiation of mEPSP Amplitude Is Dependent upon an Increase in Postsynaptic Ca2+
4.4. Glutamate Iontophoresis Produced a Ca2+-Dependent Increase in Glutamate Sensitivity
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, K.P.; Littleton, J.T. Transmission, Development, and Plasticity of Synapses. Genetics 2015, 201, 345–375. [Google Scholar] [CrossRef]
- Keshishian, H.; Broadie, K.; Chiba, A.; Bate, M. The drosophila neuromuscular junction: A model system for studying synaptic development and function. Annu. Rev. Neurosci. 1996, 19, 545–575. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.W.; Muller, M. Homeostatic control of presynaptic neurotransmitter release. Annu. Rev. Physiol. 2015, 77, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Atwood, H.L.; Wojtowicz, J.M. Short-term and long-term plasticity and physiological differentiation of crustacean motor synapses. Int. Rev. Neurobiol. 1986, 28, 275–362. [Google Scholar]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Manabe, T.; Renner, P.; Nicoll, R.A. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature 1992, 355, 50–55. [Google Scholar] [CrossRef]
- Lisman, J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc. Natl. Acad. Sci. USA 1989, 86, 9574–9578. [Google Scholar] [CrossRef]
- Mizuno, T.; Kanazawa, I.; Sakurai, M. Differential induction of LTP and LTD is not determined solely by instantaneous calcium concentration: An essential involvement of a temporal factor. Eur. J. Neurosci. 2001, 14, 701–708. [Google Scholar] [CrossRef]
- Van der Kloot, W.; Van der Kloot, T.E. Activity increases quantal size at the frog neuromuscular junction. Experientia 1985, 41, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Vrbová, G.; Wareham, A.C. Effects of nerve activity on the postsynaptic membrane of skeletal muscle. Brain Res. 1976, 118, 371–382. [Google Scholar] [CrossRef]
- Desai, S.A.; Lnenicka, G.A. Characterization of postsynaptic Ca2+ signals at the Drosophila larval NMJ. J. Neurophysiol. 2011, 106, 710–721. [Google Scholar] [CrossRef]
- Guerrero, G.; Reiff, D.F.; Agarwal, G.; Ball, R.W.; Borst, A.; Goodman, C.S.; Isacoff, E.Y. Heterogeneity in synaptic transmission along a Drosophila larval motor axon. Nat. Neurosci. 2005, 8, 1188–1196. [Google Scholar] [CrossRef]
- Melom, J.E.; Akbergenova, Y.; Gavornik, J.P.; Littleton, J.T. Spontaneous and evoked release are independently regulated at individual active zones. J. Neurosci. 2013, 33, 17253–17263. [Google Scholar] [CrossRef]
- Barber, C.F.; Jorquera, R.A.; Melom, J.E.; Littleton, J.T. Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains. J. Cell Biol. 2009, 187, 295–310. [Google Scholar] [CrossRef]
- Gertner, D.M.; Desai, S.; Lnenicka, G.A. Synaptic excitation is regulated by the postsynaptic dSK channel at the Drosophila larval NMJ. J. Neurophysiol. 2014, 111, 2533–2543. [Google Scholar] [CrossRef]
- Powers, A.S.; Grizzaffi, J.; Lnenicka, G.A. Increased postsynaptic Ca(2+) reduces mini frequency at the Drosophila larval NMJ. Synapse 2017, 71, 10. [Google Scholar] [CrossRef]
- Yoshihara, M.; Adolfsen, B.; Galle, K.T.; Littleton, J.T. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science 2005, 310, 858–863. [Google Scholar] [CrossRef] [PubMed]
- DiAntonio, A. Glutamate receptors at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 2006, 75, 165–179. [Google Scholar] [PubMed]
- Petersen, S.A.; Fetter, R.D.; Noordermeer, J.N.; Goodman, C.S.; DiAntonio, A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron 1997, 19, 1237–1248. [Google Scholar] [CrossRef]
- Hoang, B.; Chiba, A. Single-cell analysis of Drosophila larval neuromuscular synapses. Dev. Biol. 2001, 229, 55–70. [Google Scholar] [CrossRef]
- Akbergenova, Y.; Cunningham, K.L.; Zhang, Y.V.; Weiss, S.; Littleton, J.T. Characterization of developmental and molecular factors underlying release heterogeneity at Drosophila synapses. eLife 2018, 7, e38268. [Google Scholar] [CrossRef]
- Stewart, B.A.; Atwood, H.L.; Renger, J.J.; Wang, J.; Wu, C.F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A 1994, 175, 179–191. [Google Scholar] [CrossRef]
- Feng, Y.; Ueda, A.; Wu, C.F. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J. Neurogenet. 2004, 18, 377–402. [Google Scholar] [CrossRef]
- He, T.; Lnenicka, G.A. Ca(2+) buffering at a drosophila larval synaptic terminal. Synapse 2011, 65, 687–693. [Google Scholar] [CrossRef]
- Powers, A.S.; Grizzaffi, J.; Ribchester, R.; Lnenicka, G.A. Regulation of quantal currents determines synaptic strength at neuromuscular synapses in larval Drosophila. Pflug. Arch. 2016, 468, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Lnenicka, G.A.; Keshishian, H. Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J. Neurobiol. 2000, 43, 186–197. [Google Scholar] [CrossRef]
- Lnenicka, G.A.; Grizzaffi, J.; Lee, B.; Rumpal, N. Ca2+ dynamics along identified synaptic terminals in Drosophila larvae. J. Neurosci. 2006, 26, 12283–12293. [Google Scholar] [CrossRef] [PubMed]
- Newman, Z.L.; Hoagland, A.; Aghi, K.; Worden, K.; Levy, S.L.; Son, J.H.; Lee, L.P.; Isacoff, E.Y. Input-Specific Plasticity and Homeostasis at the Drosophila Larval Neuromuscular Junction. Neuron 2017, 93, 1388–1404.e10. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Liley, A.W. The quantal components of the mammalian end-plate potential. J. Physiol. 1956, 133, 571–587. [Google Scholar] [CrossRef]
- Steinert, J.R.; Kuromi, H.; Hellwig, A.; Knirr, M.; Wyatt, A.W.; Kidokoro, Y.; Schuster, C.M. Experience-dependent formation and recruitment of large vesicles from reserve pool. Neuron 2006, 50, 723–733. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Chouhan, A.K.; Zhang, J.; Zinsmaier, K.E.; Macleod, G.T. Presynaptic mitochondria in functionally different motor neurons exhibit similar affinities for Ca2+ but exert little influence as Ca2+ buffers at nerve firing rates in situ. J. Neurosci. 2010, 30, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Onishi, M.; Jan, L.Y.; Jan, Y.N. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc. Natl. Acad. Sci. USA 2007, 104, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.A.; Kennedy, M.J.; Goold, C.P.; Marek, K.W.; Davis, G.W. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron 2006, 52, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Li, X.; Dickman, D. Disparate Postsynaptic Induction Mechanisms Ultimately Converge to Drive the Retrograde Enhancement of Presynaptic Efficacy. Cell Rep. 2017, 21, 2339–2347. [Google Scholar] [CrossRef]
- Nair, A.G.; Muttathukunnel, P.; Müller, M. Distinct molecular pathways govern presynaptic homeostatic plasticity. Cell Rep. 2021, 37, 110105. [Google Scholar] [CrossRef]
- Frank, C.A.; James, T.D.; Müller, M. Homeostatic control of Drosophila neuromuscular junction function. Synapse 2020, 74, e22133. [Google Scholar] [CrossRef]
- Genç, Ö.; Davis, G.W. Target-wide Induction and Synapse Type-Specific Robustness of Presynaptic Homeostasis. Curr. Biol. CB 2019, 29, 3863–3873.e2. [Google Scholar] [CrossRef]
- Peled, E.S.; Newman, Z.L.; Isacoff, E.Y. Evoked and spontaneous transmission favored by distinct sets of synapses. Curr. Biol. CB 2014, 24, 484–493. [Google Scholar] [CrossRef]
- Dudel, J.; Kuffler, S.W. Mechanism of facilitation at the crayfish neuromuscular junction. J. Physiol. 1961, 155, 530–542. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Stewart, B.A. The influence of postsynaptic structure on missing quanta at the Drosophila neuromuscular junction. BMC Neurosci. 2016, 17, 53. [Google Scholar] [CrossRef]
- Li, X.; Goel, P.; Chen, C.; Angajala, V.; Chen, X.; Dickman, D.K. Synapse-specific and compartmentalized expression of presynaptic homeostatic potentiation. eLife 2018, 7, e34338. [Google Scholar] [CrossRef]
- Aponte-Santiago, N.A.; Ormerod, K.G.; Akbergenova, Y.; Littleton, J.T. Synaptic Plasticity Induced by Differential Manipulation of Tonic and Phasic Motoneurons in Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 6270–6288. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Ballard, S.L.; Ganetzky, B. Analysis of synaptic growth and function in Drosophila with an extended larval stage. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 13776–13786. [Google Scholar] [CrossRef]
- Benke, T.A.; Luthi, A.; Isaac, J.T.; Collingridge, G.L. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 1998, 393, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Shi, S.H.; Esteban, J.A.; Piccini, A.; Poncer, J.C.; Malinow, R. Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science 2000, 287, 2262–2267. [Google Scholar] [CrossRef]
- Broadie, K.; Bate, M. Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron 1993, 11, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Marrus, S.B.; DiAntonio, A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr. Biol. 2004, 14, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Hallermann, S.; Kittel, R.J.; Khorramshahi, O.; Frolich, A.M.; Quentin, C.; Rasse, T.M.; Mertel, S.; Heckmann, M.; Sigrist, S.J. Activity-dependent site-specific changes of glutamate receptor composition in vivo. Nat. Neurosci. 2008, 11, 659–666. [Google Scholar] [CrossRef]
- Ljaschenko, D.; Ehmann, N.; Kittel, R.J. Hebbian plasticity guides maturation of glutamate receptor fields in vivo. Cell Rep. 2013, 3, 1407–1413. [Google Scholar] [CrossRef]
- Sigrist, S.J.; Reiff, D.F.; Thiel, P.R.; Steinert, J.R.; Schuster, C.M. Experience-dependent strengthening of Drosophila neuromuscular junctions. J. Neurosci. 2003, 23, 6546–6556. [Google Scholar] [CrossRef]
- Patterson, M.A.; Szatmari, E.M.; Yasuda, R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 15951–15956. [Google Scholar] [CrossRef]
- Penn, A.C.; Zhang, C.L.; Georges, F.; Royer, L.; Breillat, C.; Hosy, E.; Petersen, J.D.; Humeau, Y.; Choquet, D. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 2017, 549, 384–388. [Google Scholar] [CrossRef]
- Kristensen, A.S.; Jenkins, M.A.; Banke, T.G.; Schousboe, A.; Makino, Y.; Johnson, R.C.; Huganir, R.; Traynelis, S.F. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat. Neurosci. 2011, 14, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.H.; Popova, E.; Thomas, U.; Griffith, L.C.; Budnik, V. Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell 1999, 98, 353–363. [Google Scholar] [CrossRef]
- Haghighi, A.P.; McCabe, B.D.; Fetter, R.D.; Palmer, J.E.; Hom, S.; Goodman, C.S. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron 2003, 39, 255–267. [Google Scholar] [CrossRef]
- Kazama, H.; Morimoto-Tanifuji, T.; Nose, A. Postsynaptic activation of calcium/calmodulin-dependent protein kinase II promotes coordinated pre- and postsynaptic maturation of Drosophila neuromuscular junctions. Neuroscience 2003, 117, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Hell, J.W. CaMKII: Claiming center stage in postsynaptic function and organization. Neuron 2014, 81, 249–265. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powers, A.S.; Gajic, P.; Rittereiser, E.; Dasrat, K.; Lnenicka, G.A. Activity-Dependent Increases in Quantal Size at the Drosophila NMJ. J. Dev. Biol. 2025, 13, 38. https://doi.org/10.3390/jdb13040038
Powers AS, Gajic P, Rittereiser E, Dasrat K, Lnenicka GA. Activity-Dependent Increases in Quantal Size at the Drosophila NMJ. Journal of Developmental Biology. 2025; 13(4):38. https://doi.org/10.3390/jdb13040038
Chicago/Turabian StylePowers, Andrew S., Petar Gajic, Ethan Rittereiser, Kavindra Dasrat, and Gregory A. Lnenicka. 2025. "Activity-Dependent Increases in Quantal Size at the Drosophila NMJ" Journal of Developmental Biology 13, no. 4: 38. https://doi.org/10.3390/jdb13040038
APA StylePowers, A. S., Gajic, P., Rittereiser, E., Dasrat, K., & Lnenicka, G. A. (2025). Activity-Dependent Increases in Quantal Size at the Drosophila NMJ. Journal of Developmental Biology, 13(4), 38. https://doi.org/10.3390/jdb13040038




