Vasa, Piwi, and Pl10 Expression during Sexual Maturation and Asexual Reproduction in the Annelid Pristina longiseta
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Material and Fixation
2.2. Semi-Thin Sections Preparation
2.3. Gene Cloning and Phylogenetic Analysis
2.4. Whole-Mount In Situ Hybridization
2.5. Data Visualization
3. Results
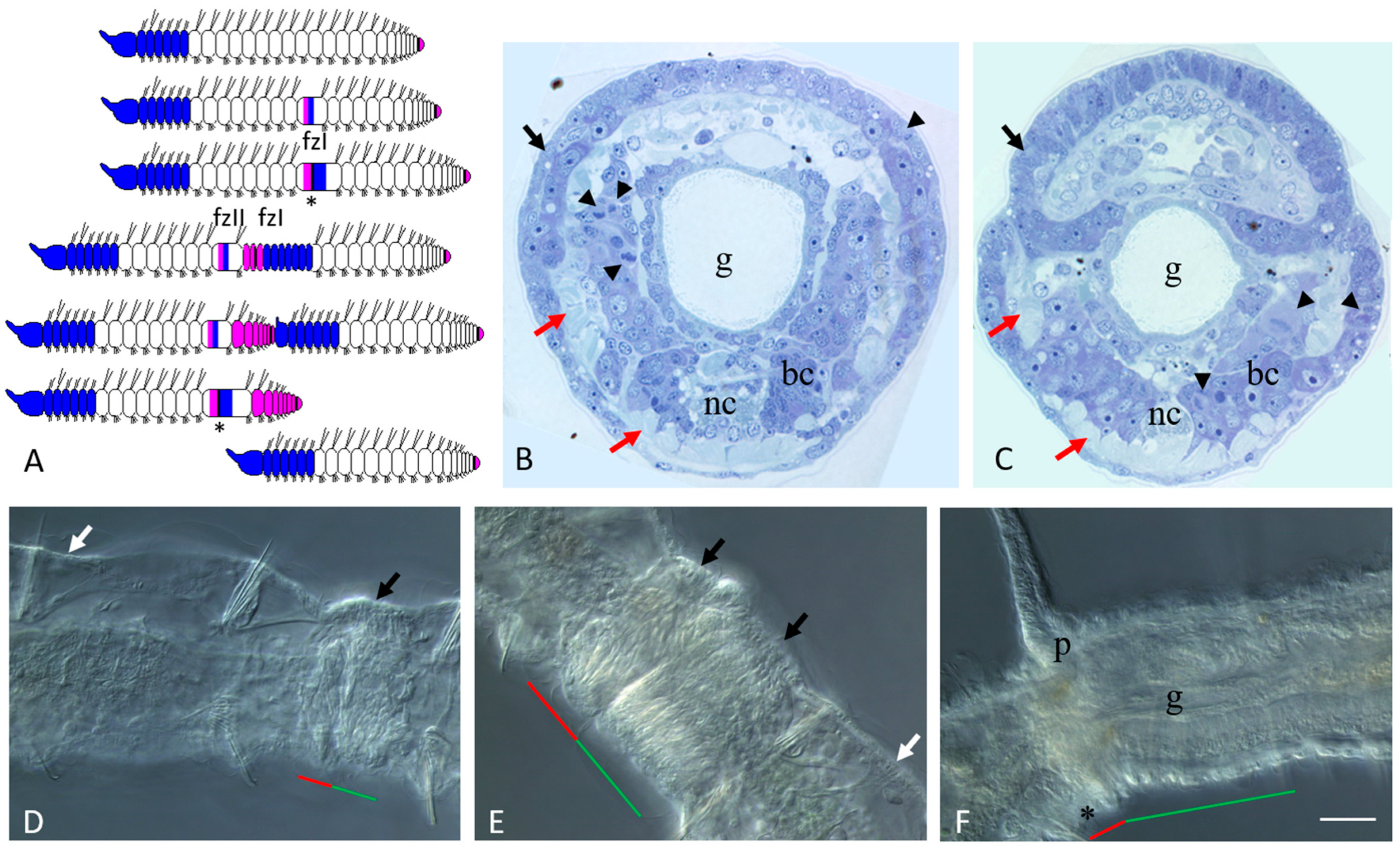
3.1. Asexual Reproduction in Pristina longiseta
3.2. Sexually Matured Form of Pristina longiseta
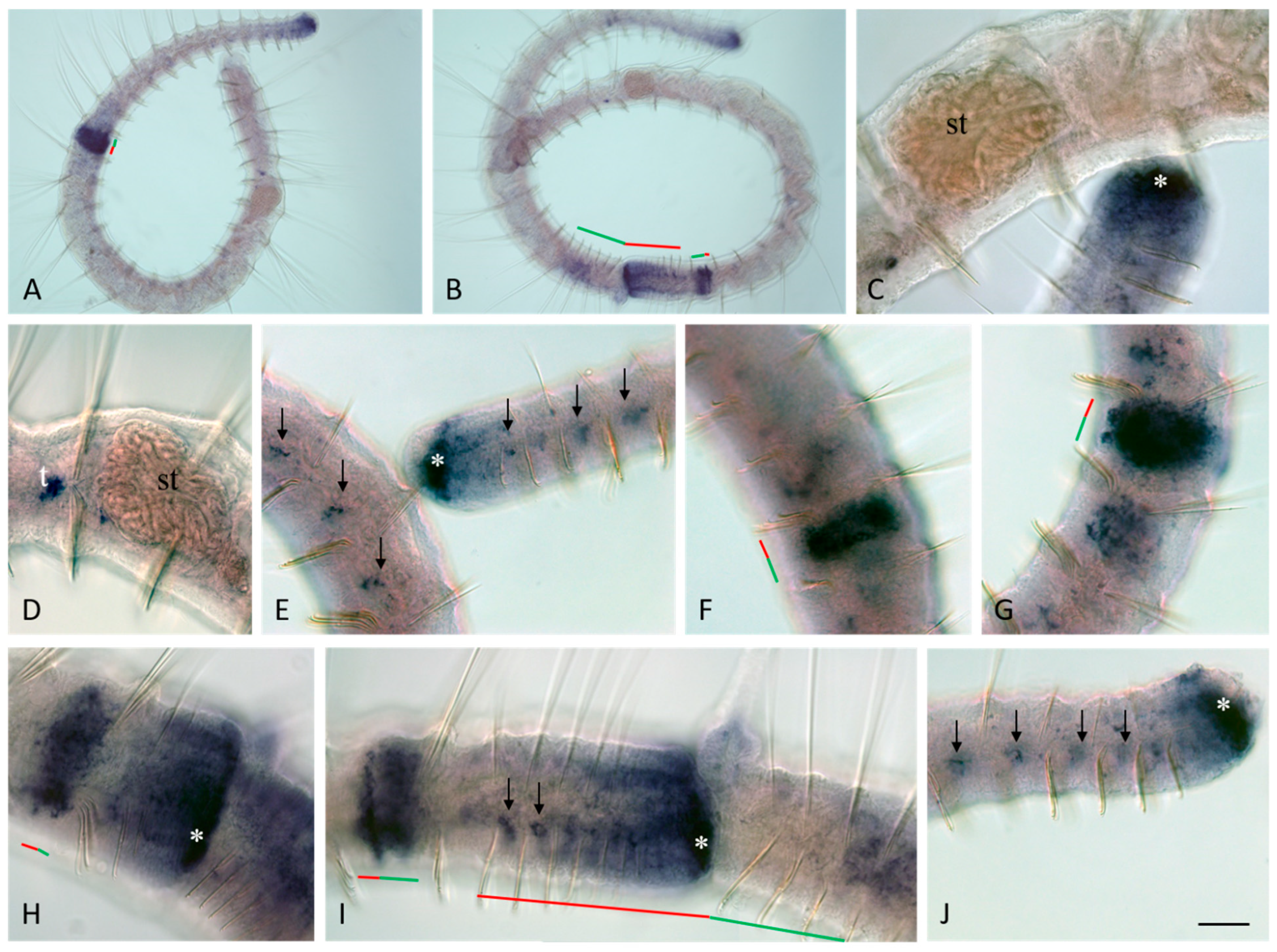
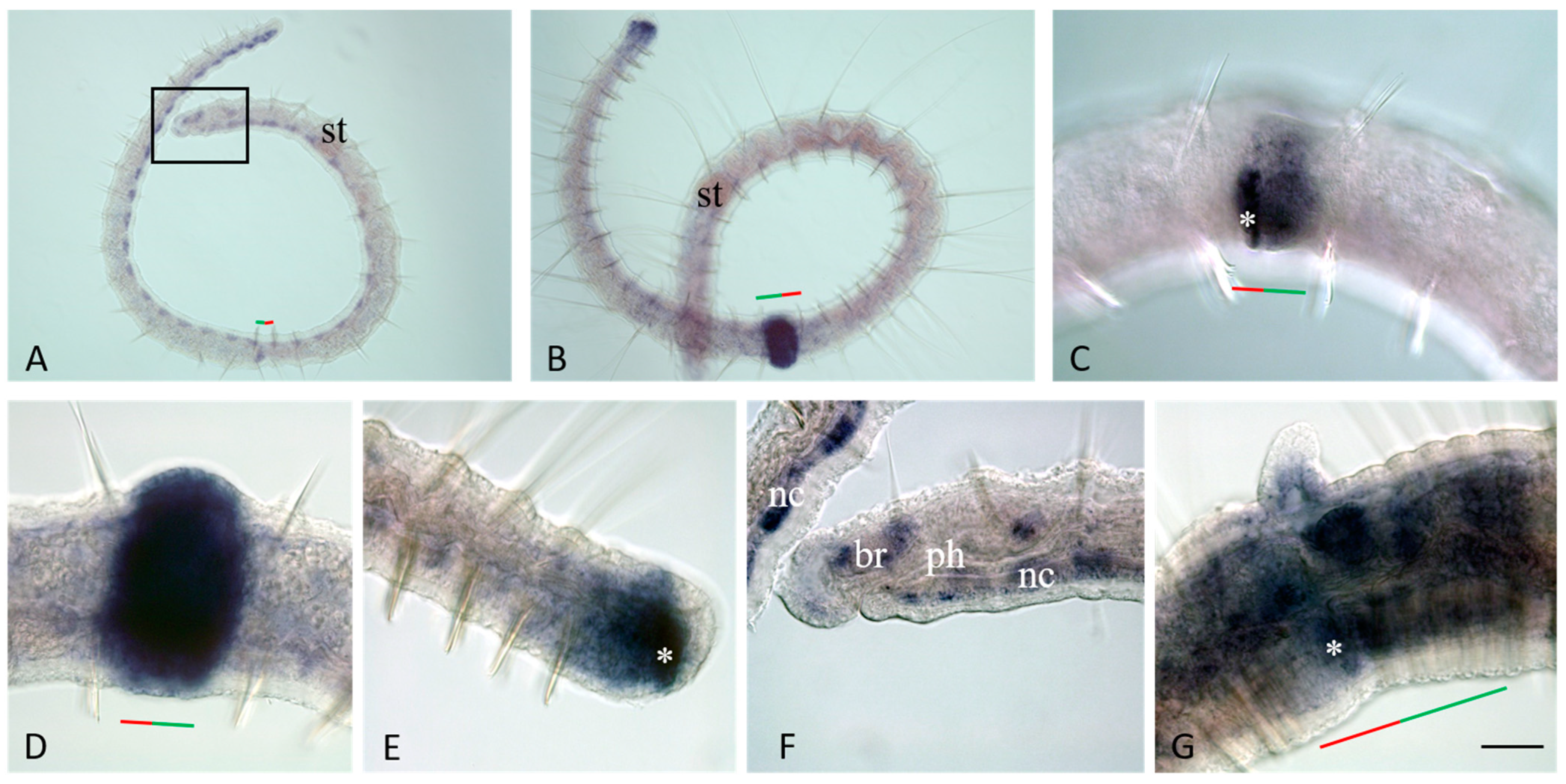
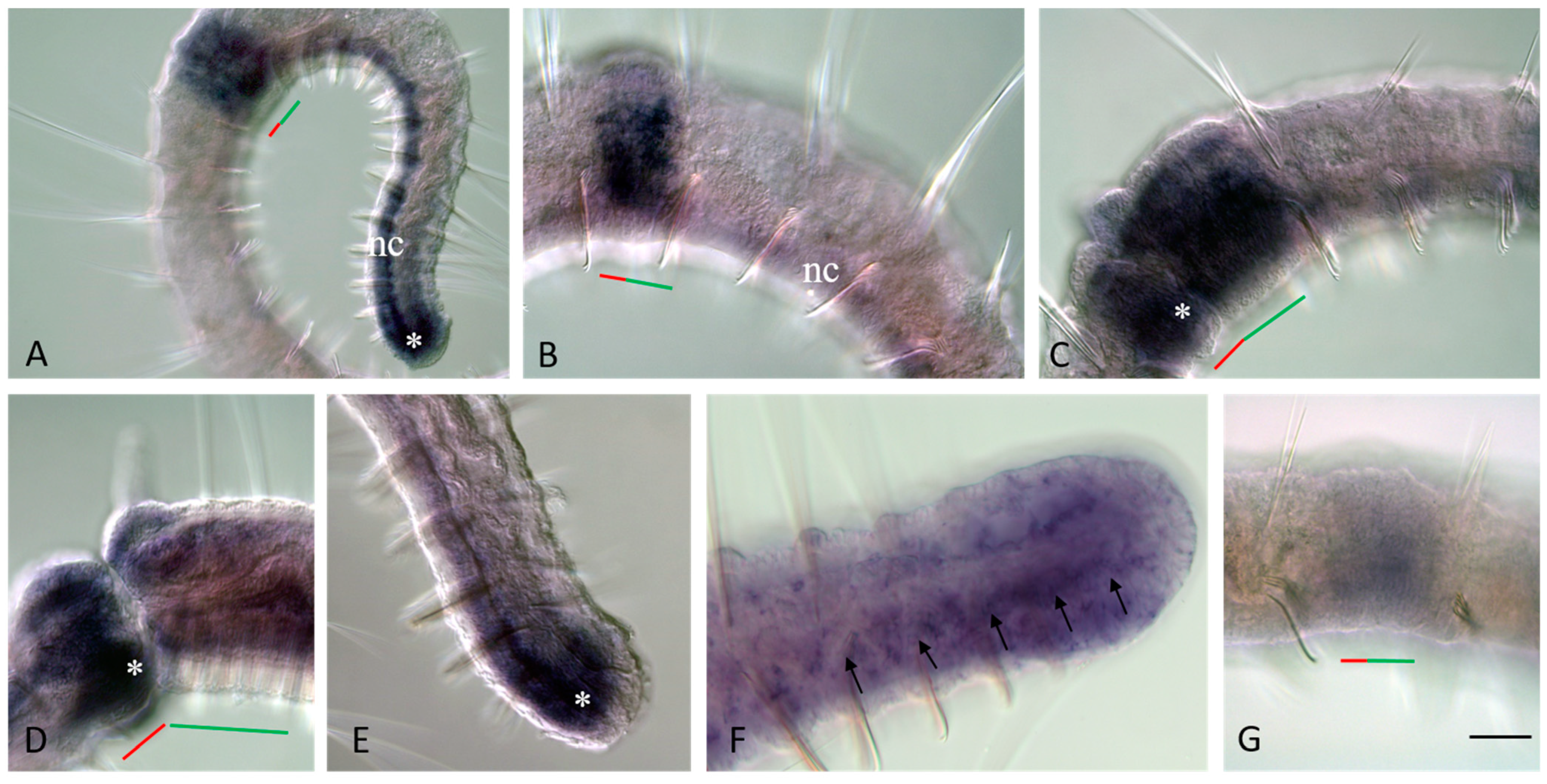
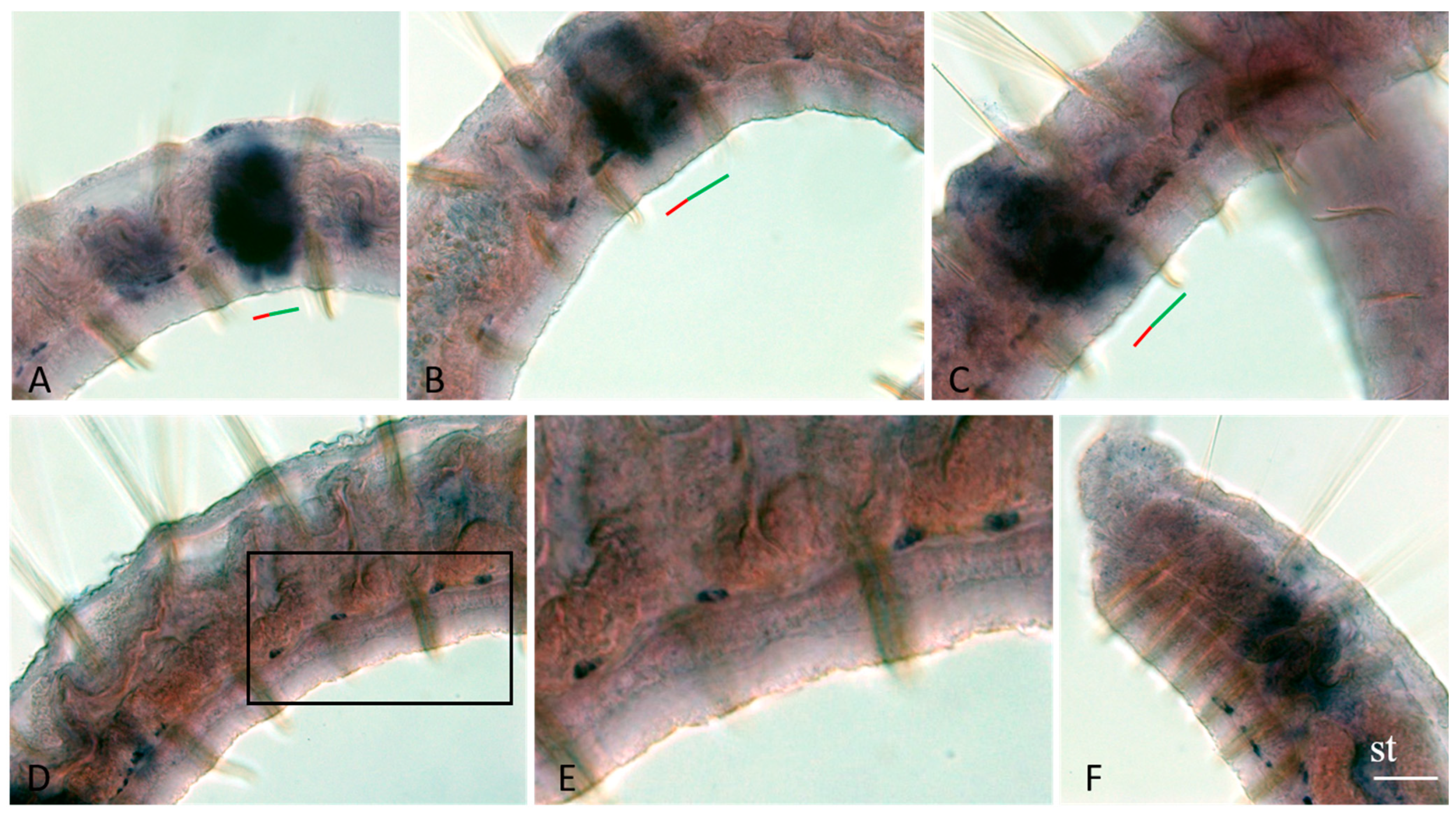
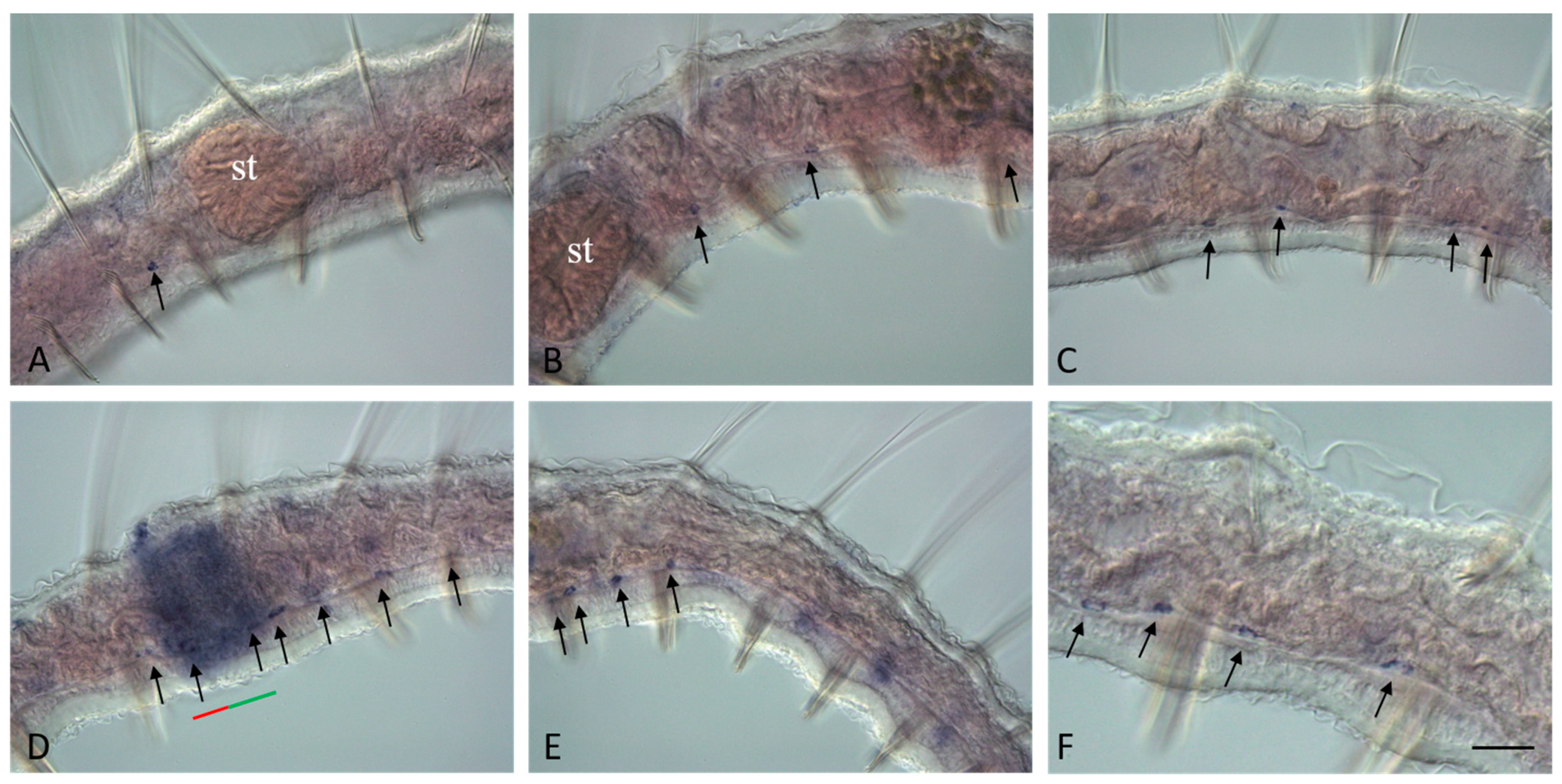
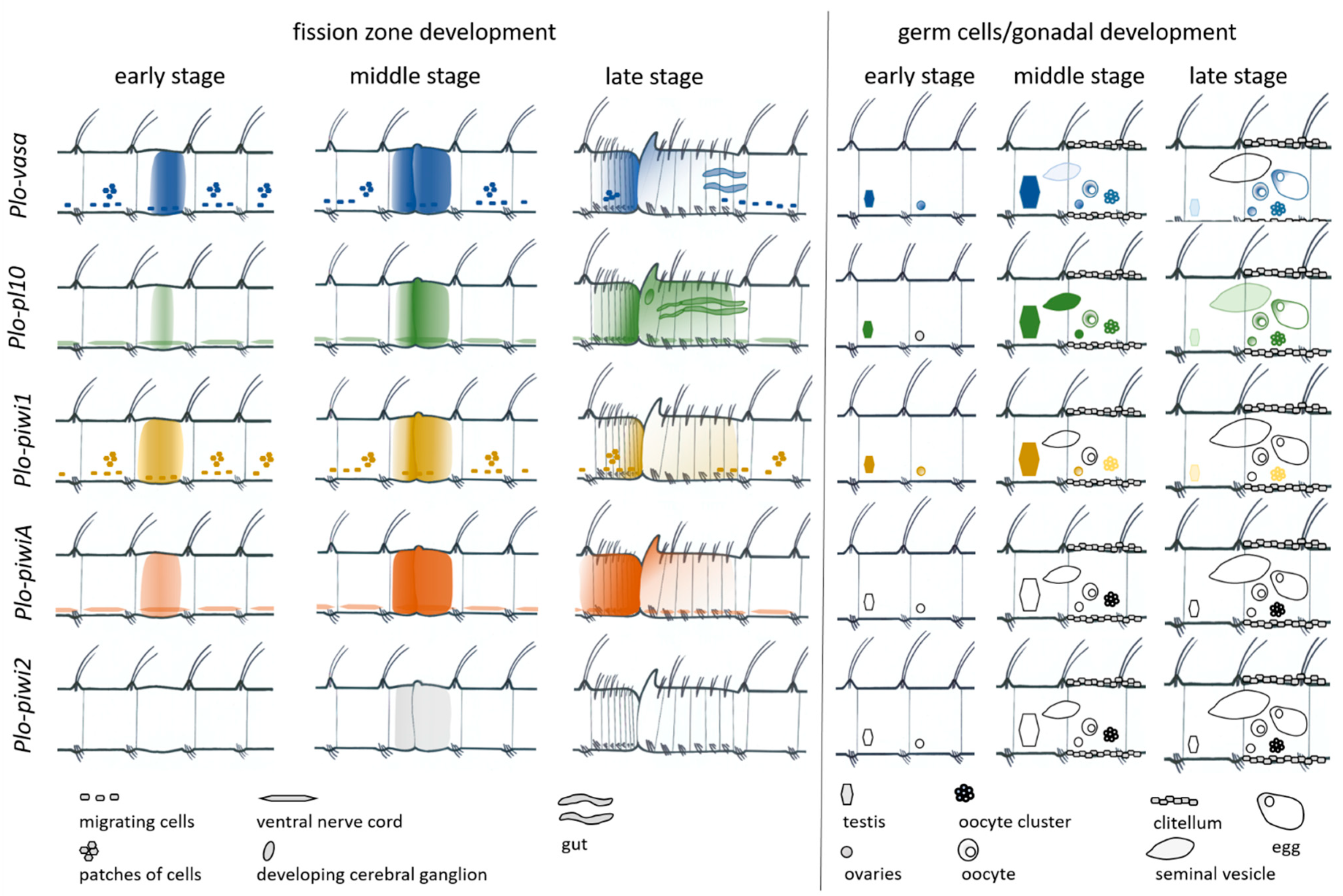
3.3. Vasa, Piwi, and Pl10 Homolog Expression in Growing Adults and Asexually Reproducing Pristina longiseta
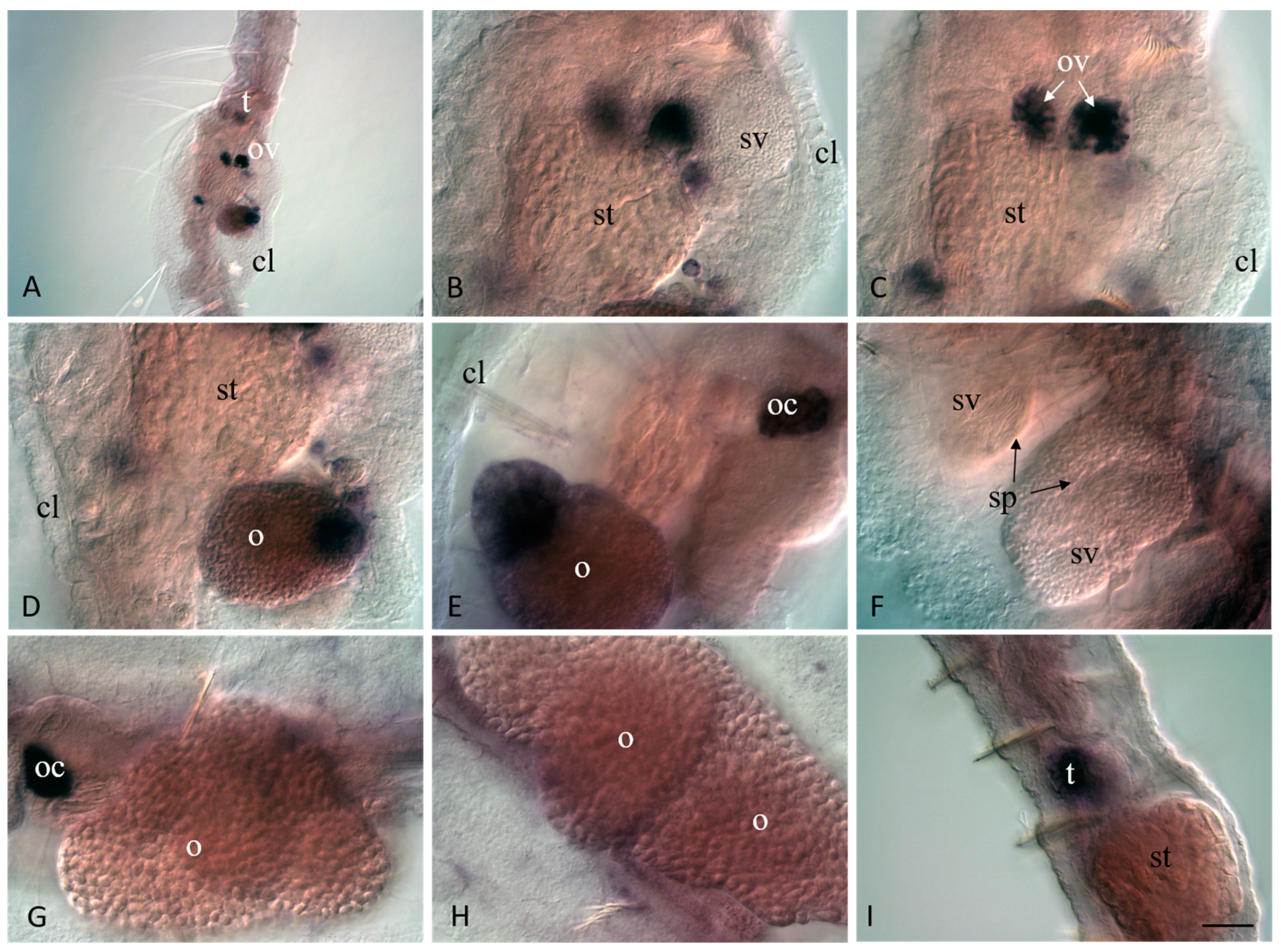
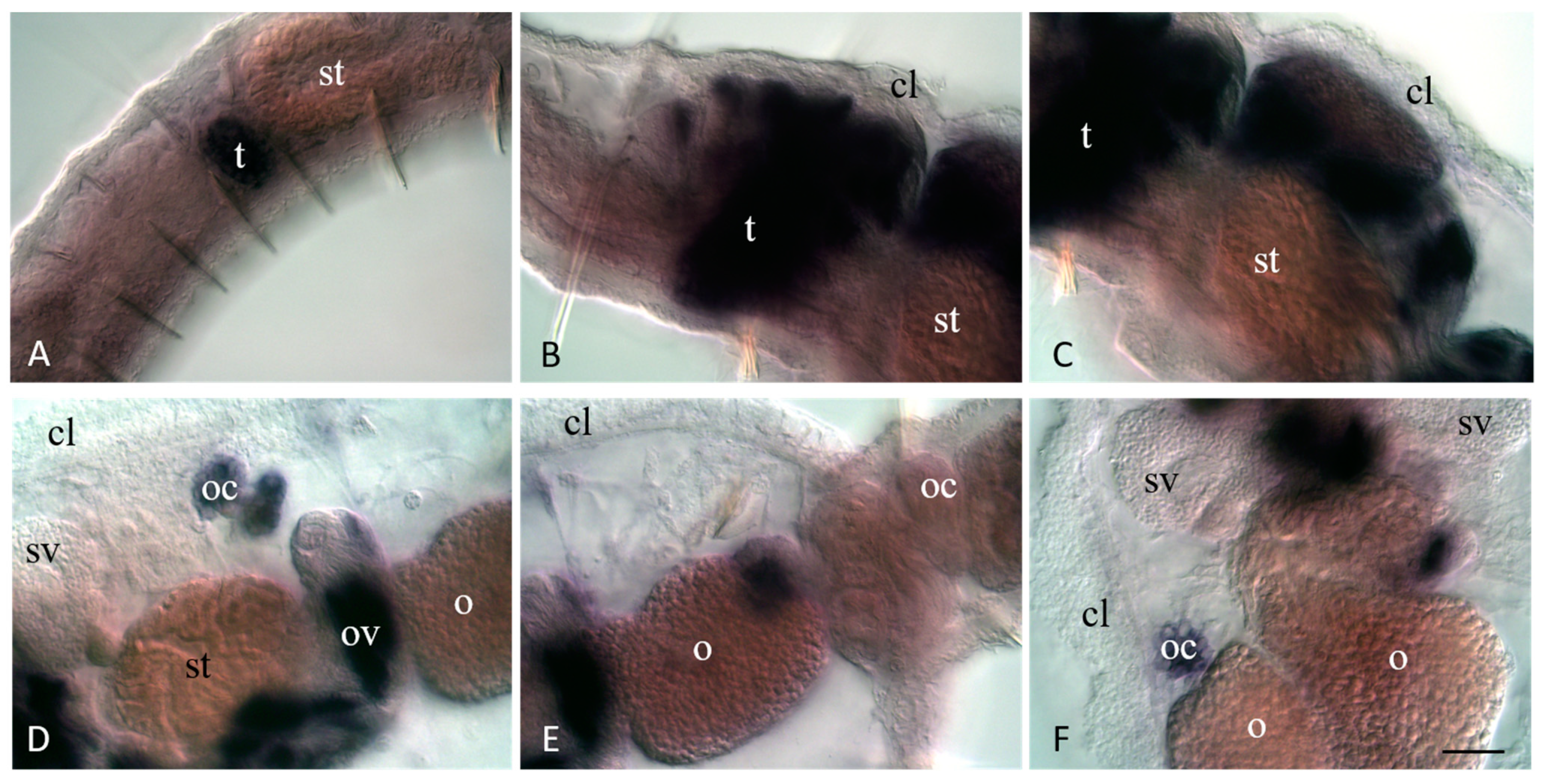
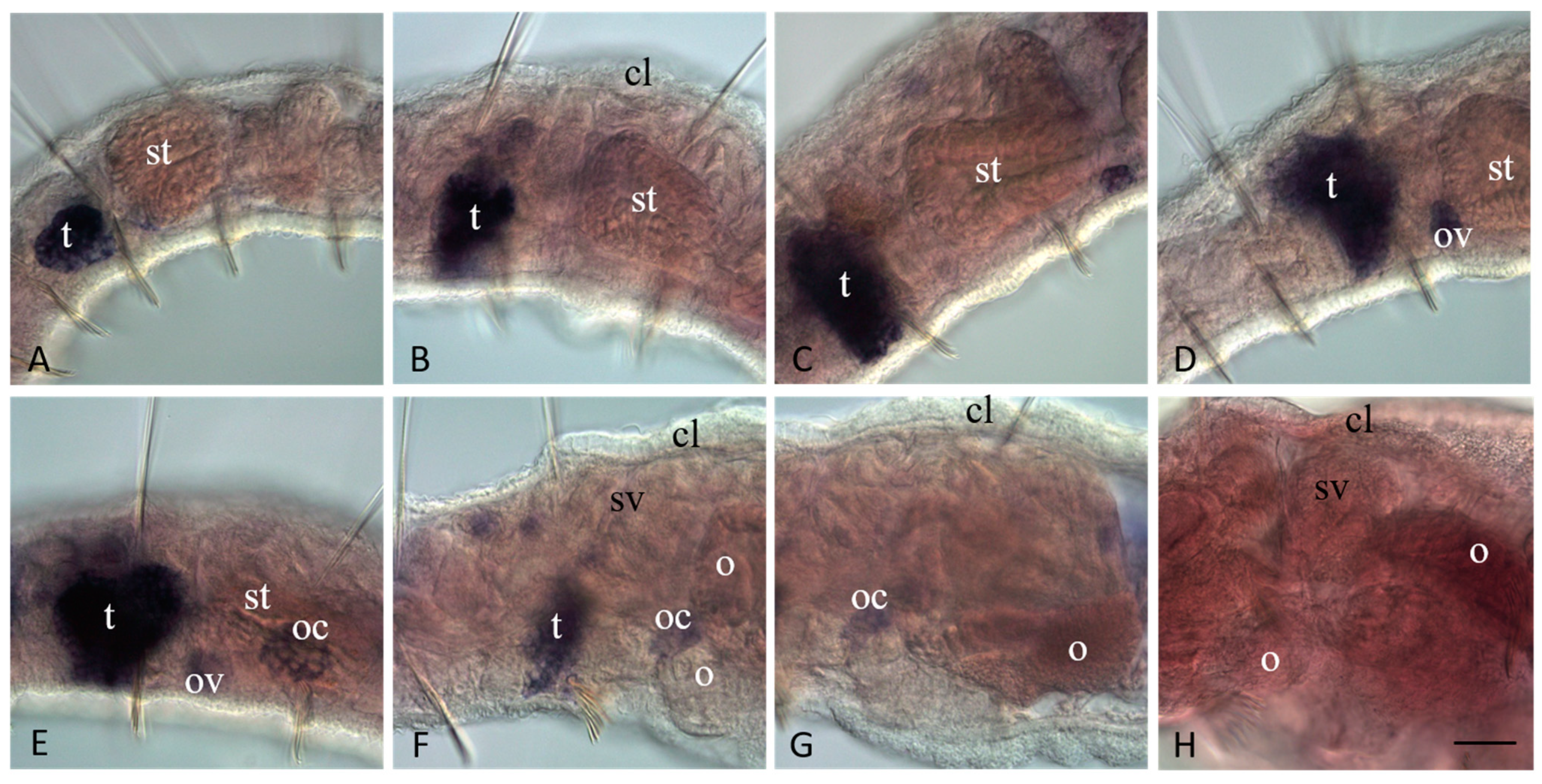
3.4. Vasa, Piwi, and Pl10 Homolog Expression in Sexually Matured Pristina longiseta
4. Discussion
4.1. Reproductive Strategies in Pristina longiseta
4.2. Vasa, Piwi, and Pl10 Homolog Expression in the Context of Reproductive Strategies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barresi, M.; Gilbert, S. Developmental Biology, 13th ed.; Oxford University Press: New York, NY, USA, 2023; 880p. [Google Scholar]
- Brusca, R.C.; Brusca, G.J. Invertebrates, 2nd ed.; Sinauer Associates: Sunderland, UK, 2003; 936p. [Google Scholar]
- Berrill, N.J. Regeneraton and Budding in Worms. Biol. Rev. 1952, 27, 401–438. [Google Scholar] [CrossRef]
- Vorontsova, M.A.; Liosner, L.D. Asexual Propagation and Regeneration; Pergamon Press: London, UK, 1960; 489p. [Google Scholar]
- Ivanova-Kazas, O.M. Asexual Reproduction of Animals; LGU Publ.: Leningrad, Russia, 1977; 273p. (In Russian) [Google Scholar]
- Kostyuchenko, R.P.; Kozin, V.V.; Kupriashova, E.E. Regeneration and asexual reproduction in annelids: Cells, genes, and evolution. Biol. Bull. 2016, 43, 185–194. [Google Scholar] [CrossRef]
- Ribeiro, R.P.; Bleidorn, C.; Aguado, M.T. Regeneration mechanisms in Syllidae (Annelida). Regeneration 2018, 5, 26–42. [Google Scholar] [CrossRef]
- Stelzer, C.-P.; Lehtonen, J. Diapause and maintenance of facultative sexual reproductive strategies. Philos. Trans. R. Soc. B 2016, 371, 20150536. [Google Scholar] [CrossRef]
- Ram, Y.; Hadany, L. Condition-dependent sex: Who does it, when and why? Phil. Trans. R. Soc. B 2016, 371, 20150539. [Google Scholar] [CrossRef]
- Best, J.B.; Goodman, A.B.; Pigon, A. Fissioning in planarians: Control by the brain. Science 1969, 164, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.P.; Benham-Pyle, B.W.; Lange, J.J.; Wood, C.J.; Alvarado, A.S. Wnt and TGFβ coordinate growth and patterning to regulate size-dependent behaviour. Nature 2019, 572, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.; Zhang, F.; Chi, X.; Sun, S. Relationship between asexual reproduction of Aurelia coerulea polyps and jellyfish blooms under the influence of temperature dynamics in winter and spring. Front. Mar. Sci. 2022, 9, 888656. [Google Scholar] [CrossRef]
- Tökölyi, J.; Gergely, R.; Miklós, M. Seasonal variation in sexual readiness in a facultatively sexual freshwater cnidarian with diapausing eggs. Ecosphere 2021, 12, e03713. [Google Scholar] [CrossRef]
- Al-Shaer, L.; Leach, W.; Baban, N.; Yagodich, M.; Gibson, M.C.; Layden, M.J. Environmental and molecular regulation of asexual reproduction in the sea anemone Nematostella vectensis. R. Soc. Open Sci. 2023, 10, 230152. [Google Scholar] [CrossRef] [PubMed]
- Parish, J. Reproductive ecology of Naididae (Oligochaeta). Hydrobiologia 1981, 83, 115–123. [Google Scholar] [CrossRef]
- Juliano, C.E.; Swartz, S.Z.; Wessel, G.M. A conserved germline multipotency program. Development 2010, 137, 4113–4126. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Liu, W.-S. The molecular evolution of PL10 homologs. BMC Evol. Biol. 2010, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Fierro-Constaín, L.; Schenkelaars, Q.; Gazave, E.; Haguenauer, A.; Rocher, C.; Ereskovsky, A.; Borchiellini, C.; Renard, E. The conservation of the germline multipotency program, from sponges to vertebrates: A stepping stone to understanding the somatic and germline origins. Genome Biol. Evol. 2017, 9, 474–488. [Google Scholar] [CrossRef]
- Milani, L.; Pecci, A.; Ghiselli, F.; Passamonti, M.; Bettini, S.; Franceschini, V.; Maurizii, M.G. VASA expression suggests shared germ line dynamics in bivalve molluscs. Histochem. Cell Biol. 2017, 148, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.E.; Popratiloff, A.; Alrefaei, Y.N.; Mann, V.H.; Rinaldi, G.; Brindley, P.J. Functional analysis of vasa/PL10-like genes in the ovary of Schistosoma mansoni. Mol. Biochem. Parasitol. 2020, 236, 111259. [Google Scholar] [CrossRef] [PubMed]
- Rosner, A.; Moiseeva, E.; Rinkevich, Y.; Lapidot, Z.; Rinkevich, B. Vasa and the germ line lineage in a colonial urochordate. Dev. Biol. 2009, 331, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, R.; Sugio, M.; Kutsuna, J.; Tochinai, S.; Takahashi, Y. Early Segregation of Germ and Somatic Lineages during Gonadal Regeneration in the Annelid Enchytraeus japonensis. Curr. Biol. 2006, 16, 1012–1017. [Google Scholar] [CrossRef]
- Sugio, M.; Takeuchi, K.; Kutsuna, J.; Tadokoro, R.; Takahashi, Y.; Yoshida-noro, C.; Tochinai, S. Exploration of embryonic origins of germline stem cells and neoblasts in Enchytraeus japonensis (Oligochaeta, Annelida). Gene Expr. Patterns 2008, 8, 227–236. [Google Scholar] [CrossRef]
- Yoshida-noro, C.; Tochinai, S. Stem cell system in asexual and sexual reproduction of Enchytraeus japonensis (Oligochaeta, Annelida). Dev. Growth Differ. 2010, 52, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Giani, V.C.; Yamaguchi, E.; Boyle, M.J.; Seaver, E.C. Somatic and germline expression of piwi during development and regeneration in the marine polychaete annelid Capitella teleta. Evodevo 2011, 2, 10. [Google Scholar] [CrossRef]
- Oyama, A.; Shimizu, T. Transient occurrence of vasa-expressing cells in nongenital segments during embryonic development in the oligochaete annelid Tubifex tubifex. Dev. Genes Evol. 2007, 217, 675–690. [Google Scholar] [CrossRef]
- Álvarez-Campos, P.; Kenny, N.J.; Verdes, A.; Fernández, R.; Novo, M.; Giribet, G.; Riesgo, A. Delegating Sex: Differential Gene Expression in Stolonizing Syllids Uncovers the Hormonal Control of Reproduction. Genome Biol. Evol. 2019, 11, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Nikanorova, D.D.; Kupriashova, E.E.; Kostyuchenko, R.P. Regeneration in Annelids: Cell Sources, Tissue Remodeling, and Differential Gene Expression. Russ. J. Dev. Biol. 2020, 51, 148–161. [Google Scholar] [CrossRef]
- Kostyuchenko, R.P.; Kozin, V.V. Comparative Aspects of Annelid Regeneration: Towards Understanding the Mechanisms of Regeneration. Genes 2021, 12, 1148. [Google Scholar] [CrossRef]
- Kostyuchenko, R.P. Nanos Is Expressed in Somatic and Germline Tissue during Larval and Post-Larval Development of the Annelid Alitta virens. Genes 2022, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Kozin, V.V.; Kostyuchenko, R.P. Vasa, PL10, and Piwi gene expression during caudal regeneration of the polychaete annelid Alitta virens. Dev. Genes Evol. 2015, 225, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, B.; Ballarin, L.; Martinez, P.; Somorjai, I.; Ben-Hamo, O.; Borisenko, I.; Berezikov, E.; Ereskovsky, A.; Gazave, E.; Khnykin, D.; et al. A pan-metazoan concept for adult stem cells: The wobbling Penrose landscape. Biol. Rev. 2022, 97, 299–325. [Google Scholar] [CrossRef]
- Xu, C.-M.; Sun, S.-C. Expression of Piwi Genes during the Regeneration of Lineus sanguineus (Nemertea, Pilidiophora, Heteronemertea). Genes 2020, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Palakodeti, D.; Smielewska, M.; Lu, Y.-C.; Yeo, G.W.; Graveley, B.R. The PIWI Proteins SMEDWI-2 and SMEDWI-3 are Required for Stem Cell Function and piRNA Expression in Planarians. RNA 2008, 14, 1174–1186. [Google Scholar] [CrossRef]
- Rosner, A.; Paz, G.; Rinkevich, B. Divergent roles of the DEAD-box protein BS-PL10, the urochordate homologue of human DDX3 and DDX3Y proteins, in colony astogeny and ontogeny. Dev. Dyn. 2006, 235, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Bely, A.E. Decoupling of fission and regenerative capabilities in an asexual oligochaete. Hydrobiologia 1999, 406, 243–251. [Google Scholar] [CrossRef]
- Bely, A.E.; Wray, G.A. Evolution of regeneration and fission in annelids: Insights from engrailed- and orthodenticle-class gene expression. Development 2001, 128, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Kharin, A.V.; Zagainova, I.V.; Kostyuchenko, R.P. Formation of the paratomic fission zone in freshwater oligochaetes. Russ. J. Dev. Biol. 2006, 37, 354–365. [Google Scholar] [CrossRef]
- Zattara, E.E.; Bely, A.E. Phylogenetic distribution of regeneration and asexual reproduction in Annelida: Regeneration is ancestral and fission evolves in regenerative clades. Invertebr. Biol. 2016, 135, 400–414. [Google Scholar] [CrossRef]
- Chekanovskaya, O.V. Aquatic Oligochaeta of the USSR; Amerind Publishing Co.: New Delhi, India, 1981; 513p. [Google Scholar]
- Korn, H. Morphogenese der Tiere. Lieferung 5: H-I. Annelida (einschließlich Echiurida und Sipunculida); VEB Gustav Fischer Verlag: Jena, Germany, 1982; 599p. [Google Scholar]
- Özpolat, B.D.; Sloane, E.S.; Zattara, E.E.; Bely, A.E. Plasticity and regeneration of gonads in the annelid Pristina leidyi. Evol. Dev. 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Özpolat, B.D.; Bely, A.E. Gonad Establishment during Asexual Reproduction in the Annelid Pristina leidyi. Dev. Biol. 2015, 405, 123–136. [Google Scholar] [CrossRef]
- Zattara, E.E.; Bely, A.E. Evolution of a Novel Developmental Trajectory: Fission Is Distinct from Regeneration in the Annelid Pristina leidyi. Evol. Dev. 2011, 13, 80–95. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Kostyuchenko, R.P.; Amosov, A.V. Spatial colinear but broken temporal expression of duplicated ParaHox genes in asexually reproducing annelids, Nais communis and Pristina longiseta. Genes 2023, 14, 1501. [Google Scholar] [CrossRef]
- Kostyuchenko, R.P.; Kozin, V.V. Morphallaxis versus Epimorphosis? Cellular and Molecular Aspects of Regeneration and Asexual Reproduction in Annelids. Biol. Bull. 2020, 47, 237–246. [Google Scholar] [CrossRef]
- Schroeder, P.C.; Hermans, C.O. Annelida: Polychaeta. In Reproduction of Marine Invertebrates; Giese, A.C., Pearse, J.S., Eds.; Academic Press: New York, NY, USA, 1975; pp. 1–213. [Google Scholar]
- Bely, A.E.; Sikes, J.M. Latent regeneration abilities persist following recent evolutionary loss in asexual annelids. Proc. Natl. Acad. Sci. USA 2010, 107, 1464–1469. [Google Scholar] [CrossRef]
- Hughes, R.N. A Functional Biology of Clonal Animals; Chapman and Hall: New York, NY, USA, 1989; 290p. [Google Scholar]
- del Olmo, I.; Verdes, A.; Álvarez-Campos, P. Distinct patterns of gene expression during regeneration and asexual reproduction in the annelid Pristina leidyi. J. Exp. Zool. Part B Mol. Dev. Evol. 2022, 338, 405. [Google Scholar] [CrossRef]
- Michaelsen, W. Clitellata. In Handbuchder Zoologie; Kükenthal, W., Krumbach, T., Eds.; Bd. 2; De Gruyter: Berlin, Germany, 1928; pp. 1–112. [Google Scholar]
- Stolte, H.A. Über selektive Eibildung bei Stylaria lacustris L.(Blastocytenstudien). Z. Wiss. Zool. Abt. A. 1934, 145, 79–98. [Google Scholar]
- Gorgoń, S.; Wardas, A.; Krodkiewsk, M.; Świątek, P. Oogenesis in three species of Naidinae (Annelida, Clitellata) isextraovarian of the Stylaria type. Zoology 2017, 121, 111–124. [Google Scholar] [CrossRef]
- Rebscher, N.; Zelada-González, F.; Banisch, T.U.; Raible, F.; Arendt, D. Vasa unveils a common origin of germ cells and of somatic stem cells from the posterior growth zone in the polychaete Platynereis dumerilii. Dev. Biol. 2007, 306, 599–611. [Google Scholar] [CrossRef]
- Ponz-Segrelles, G.; Bleidorn, C.; Aguado, M.T. Expression of vasa, piwi, and nanos during gametogenesis in Typosyllis antoni (Annelida, Syllidae). Evol. Dev. 2018, 20, 132–145. [Google Scholar] [CrossRef]
- Planques, A.; Malem, J.; Parapar, J.; Vervoort, M.; Gazave, E. Morphological, Cellular and Molecular Characterization of Posterior Regeneration in the Marine Annelid Platynereis dumerilii. Dev. Biol. 2019, 445, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Campos, P.; García-Castro, H.; Emili, E.; Pérez-Posada, A.; Salamanca-Díaz, D.A.; Mason, V.; Metzger, B.; Bely, A.E.; Kenny, N.; Özpolat, B.D.; et al. Annelid adult cell type diversity and their pluripotent cellular origins. bioRxiv 2023, 537979. [Google Scholar] [CrossRef]
- Sugio, M.; Yoshida-noro, C.; Ozawa, K.; Tochinai, S. Stem cells in asexual reproduction of Enchytraeus japonensis (Oligochaeta, Annelid): Proliferation and migration of neoblasts. Dev. Growth Differ. 2012, 54, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Zattara, E.E.; Turlington, K.W.; Bely, A.E. Long-Term Time-Lapse Live Imaging Reveals Extensive Cell Migration during Annelid Regeneration. BMC Dev. Biol. 2016, 16, 6. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostyuchenko, R.P.; Smirnova, N.P. Vasa, Piwi, and Pl10 Expression during Sexual Maturation and Asexual Reproduction in the Annelid Pristina longiseta. J. Dev. Biol. 2023, 11, 34. https://doi.org/10.3390/jdb11030034
Kostyuchenko RP, Smirnova NP. Vasa, Piwi, and Pl10 Expression during Sexual Maturation and Asexual Reproduction in the Annelid Pristina longiseta. Journal of Developmental Biology. 2023; 11(3):34. https://doi.org/10.3390/jdb11030034
Chicago/Turabian StyleKostyuchenko, Roman P., and Natalia P. Smirnova. 2023. "Vasa, Piwi, and Pl10 Expression during Sexual Maturation and Asexual Reproduction in the Annelid Pristina longiseta" Journal of Developmental Biology 11, no. 3: 34. https://doi.org/10.3390/jdb11030034
APA StyleKostyuchenko, R. P., & Smirnova, N. P. (2023). Vasa, Piwi, and Pl10 Expression during Sexual Maturation and Asexual Reproduction in the Annelid Pristina longiseta. Journal of Developmental Biology, 11(3), 34. https://doi.org/10.3390/jdb11030034





