Transcription of HOX Genes Is Significantly Increased during Neuronal Differentiation of iPSCs Derived from Patients with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Human iPSC-Derived NPCs
2.2. Targeted Differentiation of NPCs into TDNs
2.3. Immunofluorescence Staining
2.4. Bioinformatic Analysis
2.5. Real-Time PCR Analysis (qPCR)
2.6. Statistical Analysis
3. Results
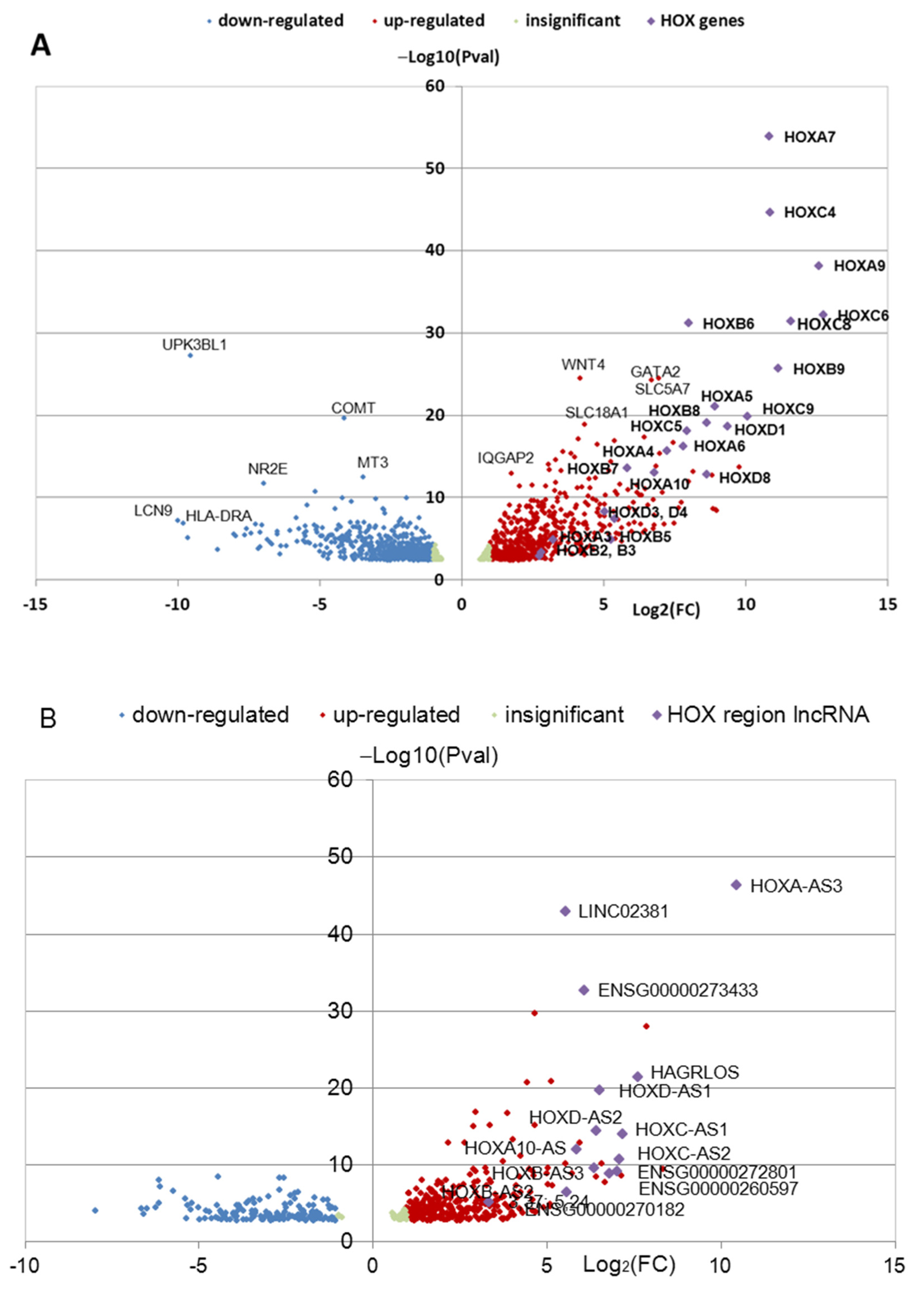
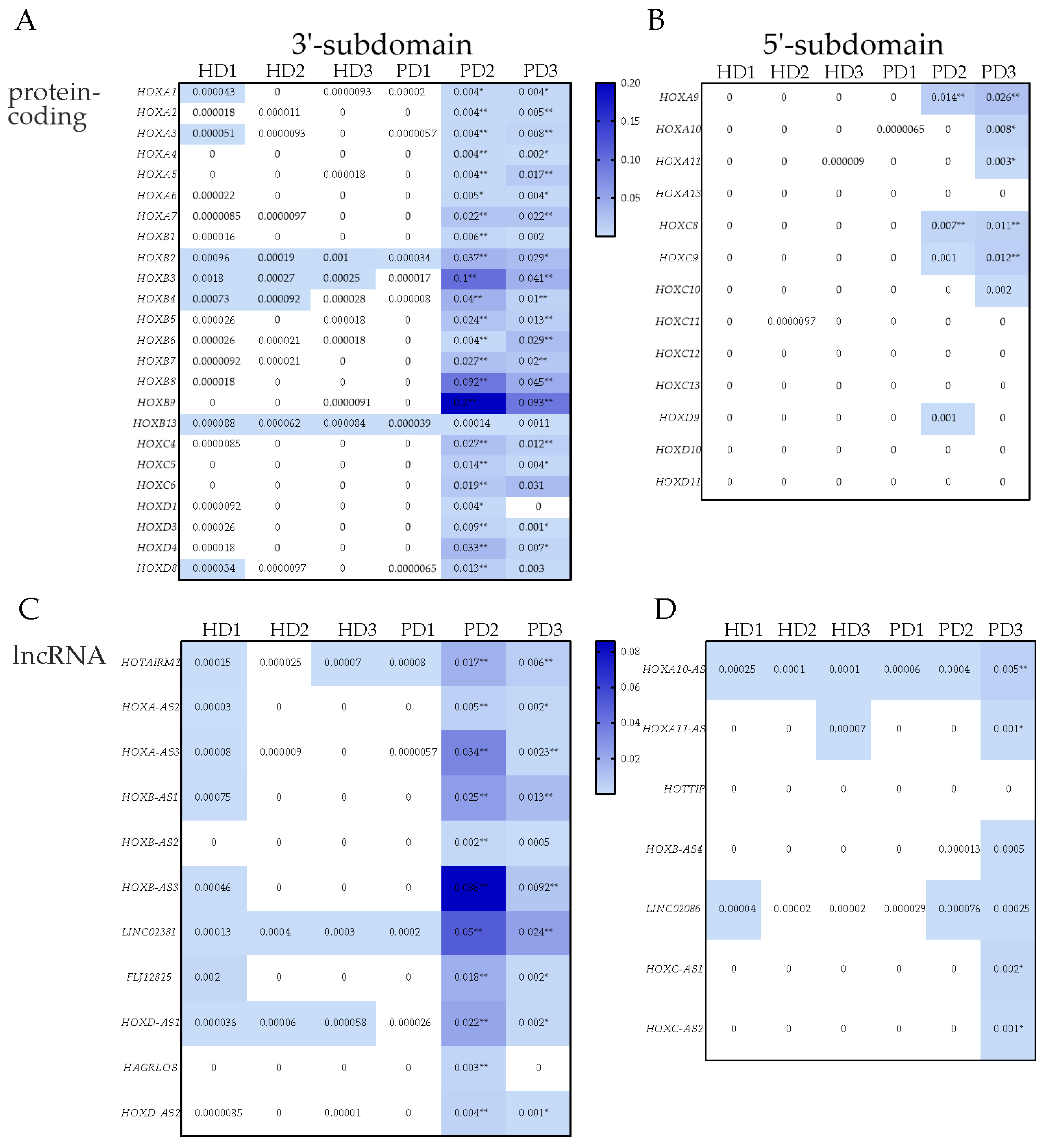
3.1. Identification of Differentially Expressed HOX Cluster Genes in NPCs and TDNs from PD Patients
3.2. Transcription of HOX Gene Clusters in NPCs Derived from PD Patients and HD
3.3. Transcription of HOX Gene Clusters in TDNs Derived from PD Patients and HD
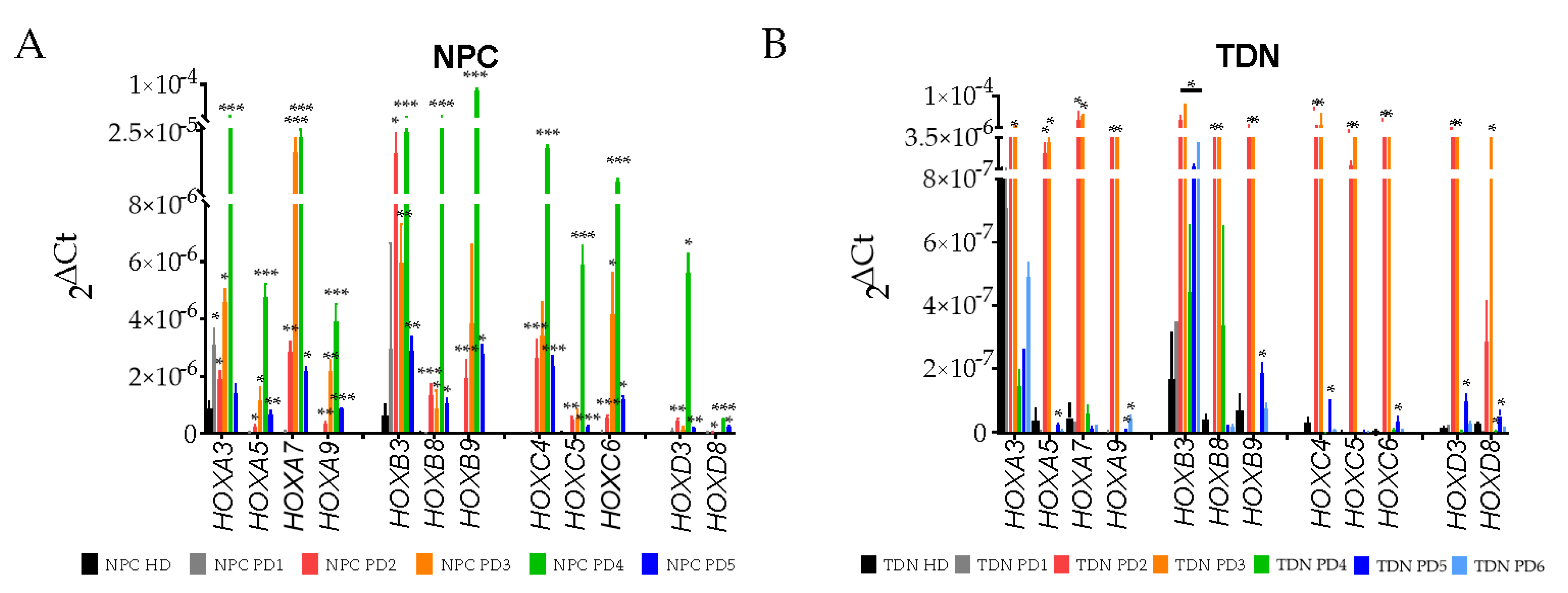
3.4. Real-Time PCR Analysis of HOX Cluster Gene Expression
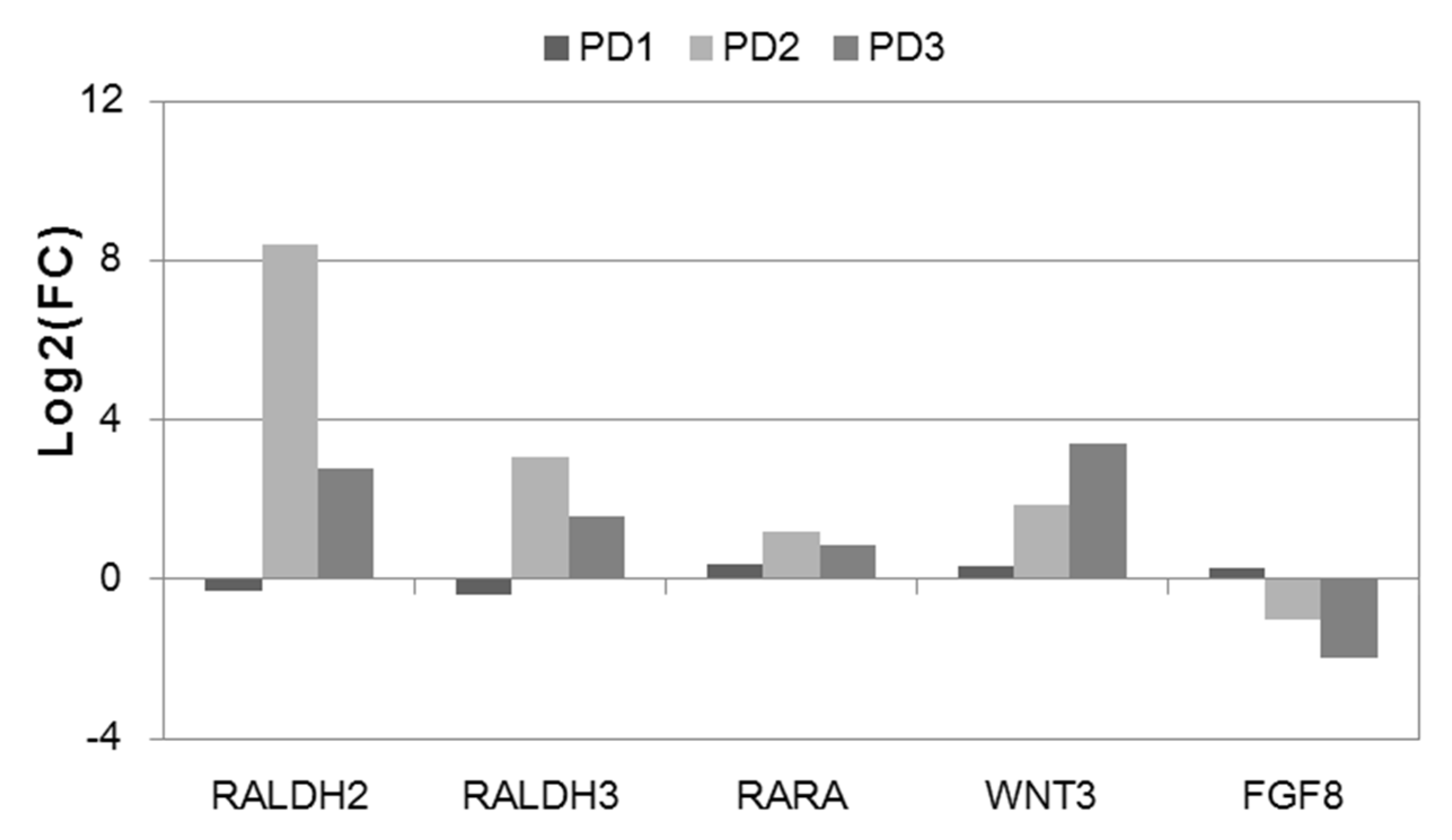
3.5. Differential Expression of the Activators of the HOX Genes in NPC and TDN from PD Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Lau, L.M.L.; Breteler, M.M.B. Epidemiology of Parkinson’s Disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Day, J.O.; Mullin, S. The Genetics of Parkinson’s Disease and Implications for Clinical Practice. Genes 2021, 12, 1006. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Birla, H.; Singh, S.S.; Zahra, W.; Dilnashin, H.; Singh, R.; Keshri, P.K.; Singh, S.P. Epigenetic Modulation in Parkinson’s Disease and Potential Treatment Therapies. Neurochem. Res. 2021, 46, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, T.; Corbillé, A.-G.; Leclair-Visonneau, L.; de Lataillade, A.G.; Lionnet, A.; Preterre, C.; Damier, P.; Derkinderen, P. (2021) Pathophysiology of Parkinson’s disease: Mitochondria, alpha-synuclein and much more. Rev. Neurol. 2021, 177, 2–271. [Google Scholar] [CrossRef] [PubMed]
- Novosadova, E.V.; Grivennikov, I.A. Induced pluripotent stem cells: From derivation to application in biochemical and biomedical research. Biochemistry 2014, 79, 1425–1441. [Google Scholar] [CrossRef]
- Novosadova, E.V.; Nekrasov, E.D.; Chestkov, I.V.; Surdina, A.V.; Vasina, E.M.; Bogomazova, A.N.; Manuilova, E.S.; Arsenyeva, E.L.; Simonova, V.V.; Konovalova, E.V.; et al. A platform for studying molecular and cellular mechanisms of Parkinson s disease based on human induced pluripotent stem cells. Sovrem. Tehnol. V Med. 2016, 8, 157–166. [Google Scholar] [CrossRef]
- Okano, H.; Yamanaka, S. iPS cell technologies: Significance and applications to CNS regeneration and disease. Mol. Brain 2014, 7, 22. [Google Scholar] [CrossRef]
- Torrent, R.; De Angelis Rigotti, F.; Dell’Era, P.; Memo, M.; Raya, A.; Consiglio, A. Using iPS Cells toward the Understanding of Parkinson’s Disease. J. Clin. Med. 2015, 4, 548–566. [Google Scholar] [CrossRef]
- Antonov, S.A.; Novosadova, E.V. Current State-of-the-Art and Unresolved Problems in Using Human Induced Pluripotent Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease Drug Development. Int. J. Mol. Sci. 2021, 22, 3381. [Google Scholar] [CrossRef]
- Grivennikov, I.A.; Tarantul, V.Z. Genome Editing Technology for the Study and Correction of Neurodegenerative Diseases. Neurochem. J. 2021, 38, 339–352. [Google Scholar] [CrossRef]
- Avazzadeh, S.; Baena, J.M.; Keighron, C.; Feller-Sanchez, Y.; Quinlan, L.R. Modelling Parkinson’s Disease: iPSCs towards Better Understanding of Human Pathology. Brain Sci. 2021, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Novosadova, E.; Anufrieva, K.; Kazantseva, E.; Arsenyeva, E.; Fedoseyeva, V.; Stepanenko, E.; Poberezhniy, D.; Illarioshkin, S.; Novosadova, L.; Gerasimova, T.; et al. Transcriptome datasets of neural progenitors and neurons differentiated from induced pluripotent stem cells of healthy donors and Parkinson’s disease patients with mutations in the PARK2 gene. Data Brief 2022, 41, 107958. [Google Scholar] [CrossRef]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Liau, E.S.; Lee, Y.H.; Huang, Y.Z.; Liu, Z.; Willems, A.; Garside, V.; McGlinn, E.; Chen, J.A.; Hong, T. MicroRNA governs bistable cell differentiation and lineage segregation via a noncanonical feedback. Mol. Syst. Biol. 2021, 17, e9945. [Google Scholar] [CrossRef]
- Chen, T.H.; Chen, J.A. Multifaceted roles of microRNAs: From motor neuron generation in embryos to degeneration in spinal muscular atrophy. ELife 2019, 8, e50848. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Guttman, M.; Huarte, M.; Rinn, J.L. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 1667–1672. [Google Scholar] [CrossRef]
- Tsai, M.C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef]
- de Kumar, B.; Parrish, M.E.; Slaughter, B.D. Analysis of dynamic changes in retinoid-induced transcription and epigenetic profiles of murine HOX clusters in ES cells. Genome. Res. 2015, 25, 1229–1243. [Google Scholar] [CrossRef]
- Montavon, T.; Soshnikova, N. HOX gene regulation and timing in embryogenesis. Semin. Cell Dev. Biol. 2014, 34, 76–84. [Google Scholar] [CrossRef]
- Gonçalves, C.S.; Le Boiteux, E.; Arnaud, P.; Costa, B.M. HOX gene cluster (de)regulation in brain: From neurodevelopment to malignant glial tumours. Cell Mol. Life Sci. 2020, 77, 3797–3821. [Google Scholar] [CrossRef]
- Hutlet, B.; Theys, N.; Coste, C.; Ahn, M.T.; Doshishti-Agolli, K.; Lizen, B.; Gofflot, F. Systematic expression analysis of Hox genes at adulthood reveals novel patterns in the central nervous system. Brain Struct. Funct. 2016, 221, 1223–1243. [Google Scholar] [CrossRef] [PubMed]
- Gofflot, F.; Lizen, B. Emerging roles for HOX proteins in synaptogenesis. Int. J. Dev. Biol. 2018, 62, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Quinonez, S.C.; Innis, J.W. Human HOX gene disorders. Mol. Genet. Metab. 2014, 111, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.A.B.; Araújo, M.; Pereira, T.; Mouta, J.; Freitas, R. HOX genes function in Breast Cancer development. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188358. [Google Scholar] [CrossRef]
- Casaca, A.; Hauswirth, G.M.; Bildsoe, H.; Mallo, M.; McGlinn, E. Regulatory landscape of the HOX transcriptome. Int. J. Dev. Biol. 2018, 62, 693–704. [Google Scholar] [CrossRef]
- Chopra, V.S.; Mishra, R.K. “Mir” acles in HOX gene regulation. Bioassays 2006, 28, 445–448. [Google Scholar] [CrossRef]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; Van Ryo, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome. Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Novosadova, E.V.; Arsenyeva, E.L.; Manuilova, E.S.; Khaspekov, L.G.; Bobrov, M.Y.; Bezuglov, V.V.; Illarioshkin, S.N.; Grivennikov, I.A. Neuroprotective properties of endocannabinoids N-arachidonoyl dopamine and N-docosahexaenoyl dopamine examined in neuronal precursors derived from human pluripotent stem cells. Biochemistry 2017, 82, 1367–1372. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2020, 26, 139–140. [Google Scholar] [CrossRef]
- WEB-Based GEne SeT AnaLysis Toolkit. Available online: https://www.Web.Gestalt.org (accessed on 20 September 2021).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Novosadova, E.V.; Nenasheva, V.V.; Makarova, I.V.; Dolotov, O.V.; Inozemtseva, L.S.; Arsenyeva, E.L.; Chernyshenko, S.V.; Sultanov, R.I.; Illarioshkin, S.N.; Grivennikov, I.A.; et al. Parkinson’s Disease-Associated Changes in the Expression of Neurotrophic Factors and their Receptors upon Neuronal Differentiation of Human Induced Pluripotent Stem Cells. J. Mol. Neurosci. 2020, 70, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Shahhoseini, M.; Taghizadeh, Z.; Hatami, M.; Baharvand, H. Retinoic acid dependent histone 3 demethylation of the clustered HOX genes during neural differentiation of human embryonic stem cells. Biochemistry and Cell Biol. 2013, 91, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bel-Vialar, S.; Itasaki, N.; Krumlauf, R. Initiating HOX gene expression: In the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HOXB genes in two distinct groups. Development 2002, 129, 5103–5115. [Google Scholar] [CrossRef] [PubMed]
- Oosterveen, T.; van Vliet, P.; Deschamps, J.; Meijlink, F. The direct context of a hox retinoic acid response element is crucial for its activity. J. Bio. Chem. 2003, 278, 24103–24107. [Google Scholar] [CrossRef]
- Niederreither, K.; Subbarayan, V.; Dollé, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999, 21, 444–448. [Google Scholar] [CrossRef]
- Sockanathan, S.; Jessell, T.M. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell 1998, 94, 503–514. [Google Scholar] [CrossRef]
- Liu, J.P.; Laufer, E.; Jessell, T.M. Assigning the positional identity of spinal motor neurons: Rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron 2001, 32, 997–1012. [Google Scholar] [CrossRef]
- Mao, X.M.; Li, H.; Zhang, X.Y.; Zhou, P.; Fu, Q.R.; Chen, Q.E.; Shen, J.X.; Liu, Y.; Chen, Q.X.; Shen, D.Y. Retinoic Acid Receptor α Knockdown Suppresses the Tumorigenicity of Esophageal Carcinoma via Wnt/β-catenin Pathway. Dig. Dis. Sci. 2018, 63, 3348–3358. [Google Scholar] [CrossRef]
- Vlasov, I.N.; Alieva, A.K.; Novosadova, E.V.; Arsenyeva, E.L.; Rosinskaya, A.V.; Partevian, S.A.; Grivennikov, I.A.; Shadrina, M.I. Transcriptome Analisis of induced Pluripotent Stem Cells and Neuronal Progenitor Cells, Derived from Discordant Monozygotic Twins with Parkinson’s Disease. Cells 2021, 10, 3478. [Google Scholar] [CrossRef]
- Hoss, A.G.; Kartha, V.K.; Dong, X.; Latourelle, J.C.; Dumitriu, A.; Hadzi, T.C.; Macdonald, M.E.; Gusella, J.F.; Akbarian, S.; Chen, J.F.; et al. MicroRNAs located in the HOX gene clusters are implicated in huntington’s disease pathogenesis. PLoS Genet. 2014, 10, e1004188. [Google Scholar] [CrossRef]
- Li, L.; Zhuang, Y.; Zhao, X.; Li, X. Long non-coding RNA in neuronal development and neurological disorders. Front. Genet. 2018, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Sessa, L.; Breiling, A.; Lavorgna, G.; Silvestri, L.; Casari, G.; Orlando, V. Noncoding RNA synthesis and loss of Polycomb group repression accompanies the colinear activation of the human HOXA cluster. RNA 2007, 13, 223–239. [Google Scholar] [CrossRef]
- Ahn, Y.; Mullan, H.E.; Krumlauf, R. Long-range regulation by shared retinoic acid response elements modulates dynamic expression of posterior HOXB genes in CNS development. Dev. Biol. 2014, 388, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Liu, J. Long Non-coding RNAs in Parkinson’s Disease. Neurochem. Res. 2021, 46, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Kmita, M. Polycomb Repressive Complexes in Hox Gene Regulation: Silencing and Beyond: The functional Dynamics of Polycomb Repressive Complexes in Hox Gene Regulation. BioEssays 2020, 42, e1900249. [Google Scholar] [CrossRef]
- Wang, X.Q.; Dostie, J. Reciprocal regulation of chromatin state and architecture by HOTAIRM1 contributes to temporal collinear HOXA gene activation. Nucleic Acids Res. 2017, 45, 1091–1104. [Google Scholar] [CrossRef]
- Saldaña-Meyer, R.; Rodriguez-Hernaez, J.; Escobar, T.; Nishana, M.; Jácome-López, K.; Nora, E.P.; Bruneau, B.G.; Tsirigos, A.; Furlan-Magaril, M.; Skok, J.; et al. RNA Interactions Are Essential for CTCF-Mediated Genome Organization. Mol. Cell 2019, 76, 412–422. [Google Scholar] [CrossRef]
- Wicks, K.; Knight, J.C. Transcriptional repression and DNA looping associated with a novel regulatory element in the final exon of the lymphotoxin-β gene. Genes Immun. 2021, 12, 126–135. [Google Scholar] [CrossRef]
- Lobanenkov, V.V.; Nicolas, R.H.; Adler, V.V.; Paterson, H.; Klenova, E.M.; Polotskaja, A.V.; Goodwin, G.H. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5’-flanking sequence of the chicken c-myc gene. Oncogene 1990, 5, 1743–1753. [Google Scholar]
- Rawal, N.; Corti, O.; Sacchetti, P.; Ardilla-Osorio, H.; Sehat, B.; Brice, A.; Arenas, E. Parkin protects dopaminergic neurons from excessive Wnt/beta-catenin signaling. Biochem. Biophys. Res. Commun. 2009, 388, 473–478. [Google Scholar] [CrossRef]
- Berwick, D.C.; Harvey, K. The regulation and deregulation of Wnt signaling by PARK genes in health and disease. J. Mol. Cell Biol. 2014, 6, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Neijts, R.; Amin, S.; van Rooijen, C.; Tan, S.; Creyghton, M.P.; de Laat, W.; Deschamps, J. Polarized regulatory landscape and Wnt responsiveness underlie HOX activation in embryos. Genes. Dev. 2016, 30, 1937–1942. [Google Scholar] [CrossRef]
- Shen, W.F.; Krishnan, K.; Lawrence, H.J.; Largman, C. The HOX homeodomain proteins block CBP histone acetyltransferase activity. Mol. Cell Biol. 2001, 21, 7509–7522. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Zhang, L.; Smith, D.; Hayakawa, Y.; McCaffery, P. A critical period for retinoic acid teratogenesis and loss of neurophilic migration of pontine nuclei neurons. Mech. Dev. 2003, 120, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Fujinuma, M.; Hirano, S.; Hayakawa, Y.; Clagett-Dame, M.; Zhang, J.; McCaffery, P. Retinoic acid influences the development of the inferior olivary nucleus in the rodent. Dev. Biol. 2005, 280, 421–433. [Google Scholar] [CrossRef]
- Conlon, R.A.; Rossant, J. Exogenous retinoic acid rapidly induces anterior ectopic expression of murine Hox-2 genes in vivo. Development 1992, 116, 357–368. [Google Scholar] [CrossRef]
- Feng, W.; Li, Y.; Kratsios, P. Emerging Roles for Hox Proteins in the Last Steps of Neuronal Development in Worms, Flies, and Mice. Front. Neurosci. 2022, 15, 801791. [Google Scholar] [CrossRef]


| Designation | Description of PD Patients and HD | Genotype | NPC Cell Line Name | TDN Cell Line Name | Abbreviation Name of Cell Lines |
|---|---|---|---|---|---|
| IPSRG2L [12] | Healthy male, 60 years | normal | NP RG2L | TDN RG2L | HD1 |
| IPSHD1.1S [12] | Healthy female, 18 years | normal | NP HD 1.1S | TDN HD 1.1S | HD2 |
| IPSFD3.9L [12] | Healthy female, 26 years | normal | NP RFD 3.9 L | TDN RFD 3.9 L | HD3 |
| PARK2-PDL1 [12] | Male with PD, the disease onset—38 years, biopsy—40 years | (del 202-203 AG: IVS1 + 1G/A) PARK2 | NP PDL1.5L | TDN PLD1.5L | PD1 |
| PSPDPS8 [12] | Female with PD, the disease onset—30 years, biopsy—41 years | EX8 del PARK2 | NP PDS13 | TDN PDS13 | PD2 |
| IPSPDPS2d [12] | Male with PD, the disease onset—38 years, biopsy—40 years | het EX2 del PARK2 | NP PDS14 | TDN PDS14 | PD3 |
| IPSPDL2.15L [32] | Male with PD the disease onset—50 years, biopsy—63 years | G2019S PARK8 | NP PDL2.15L | TDN PDL2.15L | PD4 |
| IPSPDG1.1S [32] | Male with PD the disease onset—44 years, biopsy—60 years | N370S GBA | NP PDG1.1S | TDN PDG1.1S | PD5 |
| IPSPDL1.6L [32] | Male with PD, the disease onset—47 years, biopsy—58 years | G2019S PARK8 N370S GBA | - | TDN PDL1.6L | PD6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedoseyeva, V.B.; Novosadova, E.V.; Nenasheva, V.V.; Novosadova, L.V.; Grivennikov, I.A.; Tarantul, V.Z. Transcription of HOX Genes Is Significantly Increased during Neuronal Differentiation of iPSCs Derived from Patients with Parkinson’s Disease. J. Dev. Biol. 2023, 11, 23. https://doi.org/10.3390/jdb11020023
Fedoseyeva VB, Novosadova EV, Nenasheva VV, Novosadova LV, Grivennikov IA, Tarantul VZ. Transcription of HOX Genes Is Significantly Increased during Neuronal Differentiation of iPSCs Derived from Patients with Parkinson’s Disease. Journal of Developmental Biology. 2023; 11(2):23. https://doi.org/10.3390/jdb11020023
Chicago/Turabian StyleFedoseyeva, Viya B., Ekaterina V. Novosadova, Valentina V. Nenasheva, Lyudmila V. Novosadova, Igor A. Grivennikov, and Vyacheslav Z. Tarantul. 2023. "Transcription of HOX Genes Is Significantly Increased during Neuronal Differentiation of iPSCs Derived from Patients with Parkinson’s Disease" Journal of Developmental Biology 11, no. 2: 23. https://doi.org/10.3390/jdb11020023
APA StyleFedoseyeva, V. B., Novosadova, E. V., Nenasheva, V. V., Novosadova, L. V., Grivennikov, I. A., & Tarantul, V. Z. (2023). Transcription of HOX Genes Is Significantly Increased during Neuronal Differentiation of iPSCs Derived from Patients with Parkinson’s Disease. Journal of Developmental Biology, 11(2), 23. https://doi.org/10.3390/jdb11020023







