Organophosphate Insecticide Toxicity in Neural Development, Cognition, Behaviour and Degeneration: Insights from Zebrafish
Abstract
1. The Need for Insecticides
2. Insecticides—The Move to Organophosphates (OPs)
3. OPs—Mode of Action In Vivo
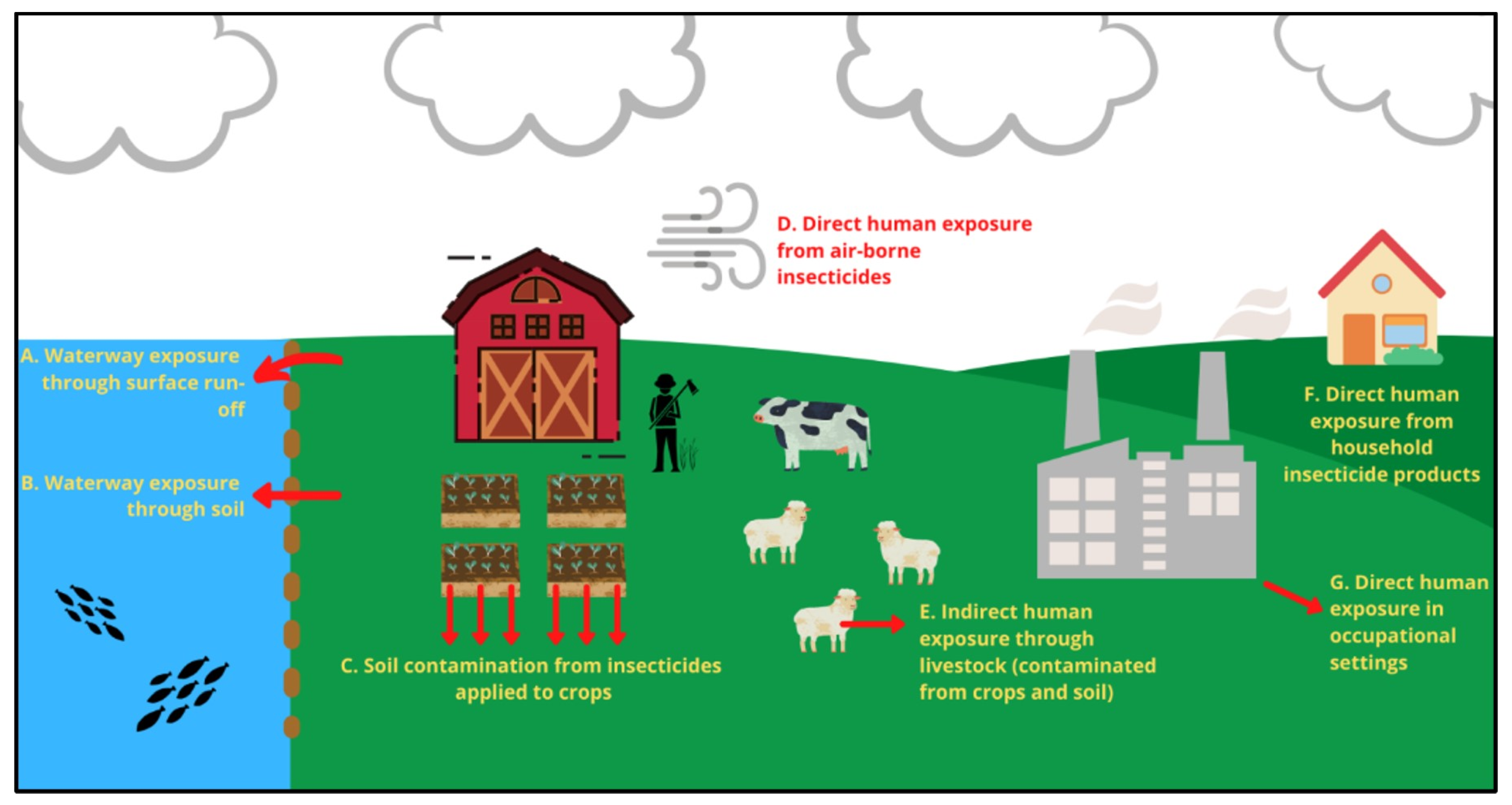
4. OPs—Occupational, Household and Waterway Exposure
5. Major Findings: The Zebrafish as Model for Testing Organophosphate (OP) Insecticides
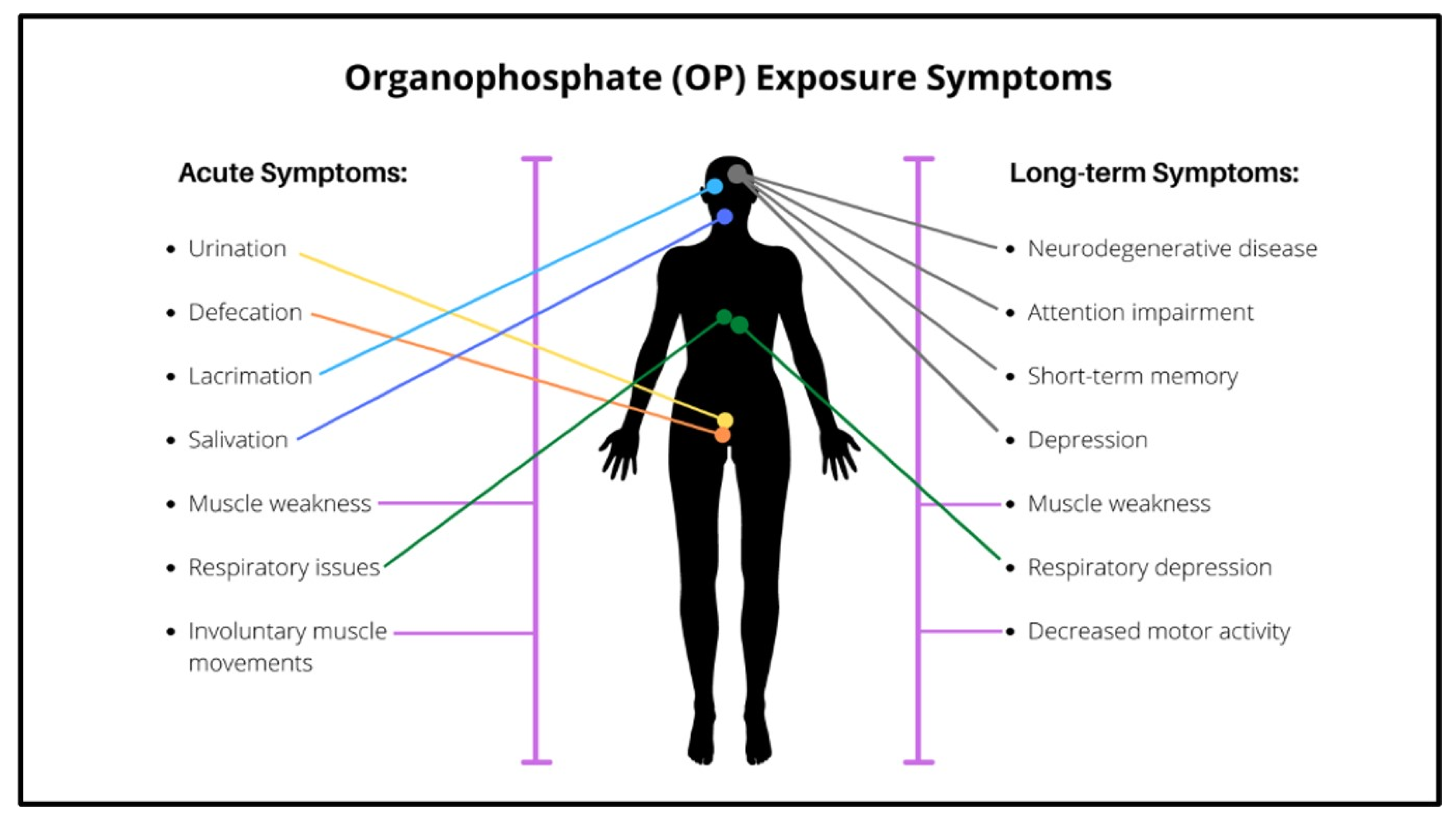
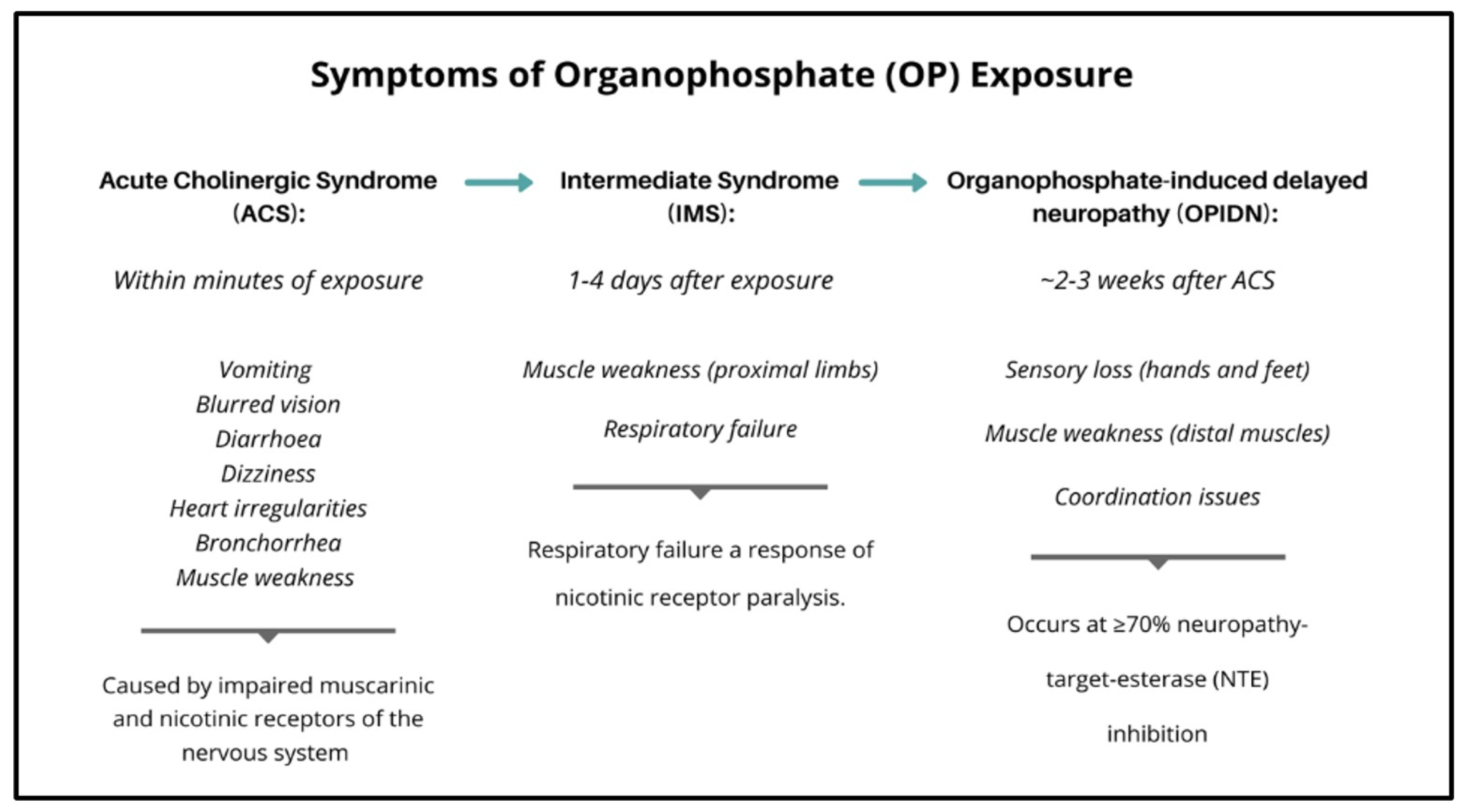
6. Organophosphate Toxicity—Acute Cholinergic Syndrome (ACS)
7. Organophosphate Toxicity—Intermediate Syndrome (IMS)
8. Organophosphate Toxicity—Organophosphate-Induced Delayed Neuropathy (OPIDN)
9. Organophosphate Toxicity—Effects on Embryogenesis
10. Organophosphate Toxicity—Effects on Neurodevelopment and Early Behaviour
11. Organophosphate Toxicity—Effects in Adulthood and Neurodegenerative Diseases
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pamanji, R.; Bethu, M.S.; Yashwanth, B.; Leelavathi, S.; Venkateswara Rao, J. Developmental toxic effects of monocrotophos, an organophosphorous pesticide, on zebrafish (Danio rerio) embryos. Environ. Sci. Pollut Res. Int. 2015, 22, 7744–7753. [Google Scholar] [CrossRef] [PubMed]
- Velki, M.; Di Paolo, C.; Nelles, J.; Seiler, T.B.; Hollert, H. Diuron and diazinon alter the behavior of zebrafish embryos and larvae in the absence of acute toxicity. Chemosphere 2017, 180, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.M.; Shen, J.; Hu, R.F. Productivity effect and overuse of pesticide in crop production in China. J. Integr. Agr. 2015, 14, 1903–1910. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Yu, X.Y.; Wang, D.L.; Yan, H.J.; Liu, X.J. Acute toxicity to zebrafish of two organophosphates and four pyrethroids and their binary mixtures. Pest Manag. Sci. 2010, 66, 84–89. [Google Scholar] [CrossRef]
- Naughton, S.X.; Terry, A.V., Jr. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018, 408, 101–112. [Google Scholar] [CrossRef]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- Barton, P.S.; Evans, M.J. Insect biodiversity meets ecosystem function: Differential effects of habitat and insects on carrion decomposition. Ecol. Entomol. 2017, 42, 364–374. [Google Scholar] [CrossRef]
- Scudder, G.G.E. The Importance of Insects. In Insect Biodiversity: Science and Society, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1. [Google Scholar]
- APVMA. Agricultural and veterinary chemicals. In Authority; The Australian Pesticides and Veterinary Medicines Authority: Kingston, ACT, Australia, 2014. [Google Scholar]
- Perry, J.; Cotton, J.; Rahman, M.A.; Brumby, S.A. Organophosphate exposure and the chronic effects on farmers: A narrative review. Rural Remote Health 2020, 20, 4508. [Google Scholar] [CrossRef]
- Bonner, M.R.; Freeman, L.E.; Hoppin, J.A.; Koutros, S.; Sandler, D.P.; Lynch, C.F.; Hines, C.J.; Thomas, K.; Blair, A.; Alavanja, M.C. Occupational exposure to pesticides and the incidence of lung cancer in the agricultural health study. Environ. Health Perspect. 2017, 125, 544–551. [Google Scholar] [CrossRef]
- Kaushik, P.; Kaushik, G. An assessment of structure and toxicity correlation in organochlorine pesticides. J. Hazard Mater. 2007, 143, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, J.R.; Soderlund, D.M. Pyrethroid insecticides and DDT modify alkaloid-dependent sodium channel activation and its enhancement by sea anemone toxin. Mol. Pharmacol. 1988, 33, 543–550. [Google Scholar]
- Karami-Mohajeri, S.; Abdollahi, M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: A systematic review. Hum. Exp. Toxicol. 2011, 30, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M. Environmental toxins and health--the health impact of pesticides. Aust. Fam. Physician 2007, 36, 1002–1004. [Google Scholar] [PubMed]
- Cao, F.; Souders, C.L., 2nd; Li, P.; Pang, S.; Qiu, L.; Martyniuk, C.J. Biological impacts of organophosphates chlorpyrifos and diazinon on development, mitochondrial bioenergetics, and locomotor activity in zebrafish (Danio rerio). Neurotoxicol. Teratol. 2018, 70, 18–27. [Google Scholar] [CrossRef]
- Modra, H.; Vrskova, D.; Macova, S.; Kohoutkova, J.; Hajslova, J.; Haluzova, I.; Svobodova, Z. Comparison of diazinon toxicity to embryos of Xenopus laevis and Danio rerio; degradation of diazinon in water. Bull. Environ. Contam. Toxicol. 2011, 86, 601–604. [Google Scholar] [CrossRef]
- Pope, C.N.; Chakraborti, T.K. Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures. Toxicology 1992, 73, 35–43. [Google Scholar] [CrossRef]
- Marrs, T.C. Organophosphate poisoning. Pharmacol. Ther. 1993, 58, 51–66. [Google Scholar] [CrossRef]
- Costa, L.G. Current issues in organophosphate toxicology. Clin. Chim. Acta 2006, 366, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Abass, K.S. Experimental measurements for the effect of dilution procedure in blood esterases as animals biomarker for exposure to OP compounds. Biomed. Res. Int. 2014, 2014, 451982. [Google Scholar] [CrossRef]
- Wessels, D.; Barr, D.B.; Mendola, P. Use of biomarkers to indicate exposure of children to organophosphate pesticides: Implications for a longitudinal study of children’s environmental health. Environ. Health Perspect. 2003, 111, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Lambert, W.E.; Lasarev, M.; Muniz, J.; Scherer, J.; Rothlein, J.; Santana, J.; McCauley, L. Variation in organophosphate pesticide metabolites in urine of children living in agricultural communities. Environ. Health Perspect. 2005, 113, 504–508. [Google Scholar] [CrossRef]
- Munoz-Quezada, M.T.; Lucero, B.; Bradman, A.; Baumert, B.; Iglesias, V.; Munoz, M.P.; Concha, C. Reliability and factorial validity of a questionnaire to assess organophosphate pesticide exposure to agricultural workers in Maule, Chile. Int. J. Environ. Health Res. 2019, 29, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Reiss, R.; Chang, E.T.; Richardson, R.J.; Goodman, M. A review of epidemiologic studies of low-level exposures to organophosphorus insecticides in non-occupational populations. Crit. Rev. Toxicol. 2015, 45, 531–641. [Google Scholar] [CrossRef]
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.H. Effects of low-dose chlorpyrifos on neurobehavior and potential mechanisms: A review of studies in rodents, zebrafish, and Caenorhabditis elegans. Birth Defects Res. 2020, 112, 445–479. [Google Scholar] [CrossRef]
- Daubas, P.; Devillers-Thiery, A.; Geoffroy, B.; Martinez, S.; Bessis, A.; Changeux, J.P. Differential expression of the neuronal acetylcholine receptor alpha 2 subunit gene during chick brain development. Neuron 1990, 5, 49–60. [Google Scholar] [CrossRef]
- Obis, T.; Besalduch, N.; Hurtado, E.; Nadal, L.; Santafe, M.M.; Garcia, N.; Tomas, M.; Priego, M.; Lanuza, M.A.; Tomas, J. The novel protein kinase C epsilon isoform at the adult neuromuscular synapse: Location, regulation by synaptic activity-dependent muscle contraction through TrkB signaling and coupling to ACh release. Mol. Brain 2015, 8, 8. [Google Scholar] [CrossRef]
- Obis, T.; Hurtado, E.; Nadal, L.; Tomas, M.; Priego, M.; Simon, A.; Garcia, N.; Santafe, M.M.; Lanuza, M.A.; Tomas, J. The novel protein kinase C epsilon isoform modulates acetylcholine release in the rat neuromuscular junction. Mol. Brain 2015, 8, 80. [Google Scholar] [CrossRef]
- Akbarsha, M.A.; Sivasamy, P. Male reproductive toxicity of phosphamidon: Histopathological changes in epididymis. Indian J. Exp. Biol. 1998, 36, 34–38. [Google Scholar] [PubMed]
- Hamm, J.T.; Wilson, B.W.; Hinton, D.E. Organophosphate-induced acetylcholinesterase inhibition and embryonic retinal cell necrosis in vivo in the teleost (Oryzias latipes). Neurotoxicology 1998, 19, 853–869. [Google Scholar] [PubMed]
- Barranger, A.; Benabdelmouna, A.; Degremont, L.; Burgeot, T.; Akcha, F. Parental exposure to environmental concentrations of diuron leads to aneuploidy in embryos of the Pacific oyster, as evidenced by fluorescent in situ hybridization. Aquat Toxicol. 2015, 159, 36–43. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, J.; Xu, B.; Chen, Z. Potential of chlorpyrifos and cypermethrin forming DNA adducts. Mutat Res. 2006, 604, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Autrup, H.; Daneshvar, B.; Dragsted, L.O.; Gamborg, M.; Hansen, M.; Loft, S.; Okkels, H.; Nielsen, F.; Nielsen, P.S.; Raffn, E.; et al. Biomarkers for exposure to ambient air pollution--comparison of carcinogen-DNA adduct levels with other exposure markers and markers for oxidative stress. Environ. Health Perspect. 1999, 107, 233–238. [Google Scholar]
- Moyano, P.; Del Pino, J.; Anadon, M.J.; Diaz, M.J.; Gomez, G.; Frejo, M.T. Toxicogenomic profile of apoptotic and necrotic SN56 basal forebrain cholinergic neuronal loss after acute and long-term chlorpyrifos exposure. Neurotoxicol. Teratol. 2017, 59, 68–73. [Google Scholar] [CrossRef]
- Mohammadzadeh, L.; Hosseinzadeh, H.; Abnous, K.; Razavi, B.M. Neuroprotective potential of crocin against malathion-induced motor deficit and neurochemical alterations in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 4904–4914. [Google Scholar] [CrossRef]
- Eftekhari, A.; Ahmadian, E.; Azami, A.; Johari-Ahar, M.; Eghbal, M.A. Protective effects of coenzyme Q10 nanoparticles on dichlorvos-induced hepatotoxicity and mitochondrial/lysosomal injury. Environ. Toxicol. 2018, 33, 167–177. [Google Scholar] [CrossRef]
- Pimentel, D.; Levitan, L. Pesticides—Amounts applied and amounts reaching pests. BioScience 1986, 36, 86–91. [Google Scholar] [CrossRef]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Galloway, T.; Handy, R. Immunotoxicity of organophosphorous pesticides. Ecotoxicology 2003, 12, 345–363. [Google Scholar] [CrossRef]
- Ghazala; Mahboob, S.; Ahmad, L.; Sultana, S.; Alghanim, K.; Al-Misned, F.; Ahmad, Z. Fish cholinesterases as biomarkers of sublethal effects of organophosphorus and carbamates in tissues of Labeo rohita. J. Biochem. Mol. Toxicol. 2014, 28, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Jaga, K.; Dharmani, C. Sources of exposure to and public health implications of organophosphate pesticides. Rev. Panam. Salud Publica 2003, 14, 171–185. [Google Scholar] [CrossRef]
- Oates, L.; Cohen, M.; Braun, L.; Schembri, A.; Taskova, R. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ. Res. 2014, 132, 105–111. [Google Scholar] [CrossRef]
- Fenske, R.A.; Kedan, G.; Lu, C.; Fisker-Andersen, J.A.; Curl, C.L. Assessment of organophosphorous pesticide exposures in the diets of preschool children in Washington State. J. Expo. Anal. Environ. Epidemiol. 2002, 12, 21–28. [Google Scholar] [CrossRef]
- Holme, F.; Thompson, B.; Holte, S.; Vigoren, E.M.; Espinoza, N.; Ulrich, A.; Griffith, W.; Faustman, E.M. The role of diet in children’s exposure to organophosphate pesticides. Environ. Res. 2016, 147, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kamanyire, R.; Karalliedde, L. Organophosphate toxicity and occupational exposure. Occup. Med. 2004, 54, 69–75. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Peng, T.; Fu, Z. The toxicity of chlorpyrifos on the early life stage of zebrafish: A survey on the endpoints at development, locomotor behavior, oxidative stress and immunotoxicity. Fish Shellfish. Immunol. 2015, 43, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Zanella, R.; Prestes, O.D.; Meinhart, A.D.; Da Silva, A.S.; Baldisserotto, B. Behavioral impairment and neurotoxic responses of silver catfish Rhamdia quelen exposed to organophosphate pesticide trichlorfon: Protective effects of diet containing rutin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 239, 108871. [Google Scholar] [CrossRef]
- Trujillo-Gonzalez, A.; Becker, J.A.; Vaughan, D.B.; Hutson, K.S. Monogenean parasites infect ornamental fish imported to Australia. Parasitol. Res. 2018, 117, 995–1011. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.J.; Munn, M.D. Organophosphate and carbamate insecticides in agricultural waters and cholinesterase (ChE) inhibition in common carp (Cyprinus carpio). Arch. Environ. Contam. Toxicol. 1998, 35, 391–396. [Google Scholar] [CrossRef]
- Gilliom, R.J.; Barbash, J.E.; Kolpin, D.W.; Larson, S.J. Peer reviewed: Testing water quality for pesticide pollution. Environ. Sci. Technol. 1999, 33, 164A–169A. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.P.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, M.; Zhou, J.; Jin, Y. Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 216, 19–28. [Google Scholar] [CrossRef]
- Shen, W.; Lou, B.; Xu, C.; Yang, G.; Yu, R.; Wang, X.; Li, X.; Wang, Q.; Wang, Y. Lethal toxicity and gene expression changes in embryonic zebrafish upon exposure to individual and mixture of malathion, chlorpyrifos and lambda-cyhalothrin. Chemosphere 2020, 239, 124802. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Chrysanthis, E.; Yacisin, K.; Linney, E. Chlorpyrifos exposure of developing zebrafish: Effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol. Teratol. 2003, 25, 51–57. [Google Scholar] [CrossRef]
- Levin, E.D.; Swain, H.A.; Donerly, S.; Linney, E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol. Teratol 2004, 26, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.W.; Paradise, C.J.; Lom, B. The pesticide malathion reduces survival and growth in developing zebrafish. Environ. Toxicol. Chem. 2005, 24, 1745–1750. [Google Scholar] [CrossRef]
- Senger, M.R.; Rico, E.P.; de Bem Arizi, M.; Rosemberg, D.B.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Carbofuran and malathion inhibit nucleotide hydrolysis in zebrafish (Danio rerio) brain membranes. Toxicology 2005, 212, 107–115. [Google Scholar] [CrossRef]
- Osterauer, R.; Kohler, H.R. Temperature-dependent effects of the pesticides thiacloprid and diazinon on the embryonic development of zebrafish (Danio rerio). Aquat. Toxicol. 2008, 86, 485–494. [Google Scholar] [CrossRef]
- Kienle, C.; Kohler, H.R.; Gerhardt, A. Behavioural and developmental toxicity of chlorpyrifos and nickel chloride to zebrafish (Danio rerio) embryos and larvae. EcoToxicol. Environ. Saf. 2009, 72, 1740–1747. [Google Scholar] [CrossRef]
- Jacobson, K.A. Introduction to adenosine receptors as therapeutic targets. Handb. Exp. Pharmacol. 2009, 193, 1–24. [Google Scholar]
- Eddins, D.; Cerutti, D.; Williams, P.; Linney, E.; Levin, E.D. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol. Teratol. 2010, 32, 99–108. [Google Scholar] [CrossRef]
- Jacobson, S.M.; Birkholz, D.A.; McNamara, M.L.; Bharate, S.B.; George, K.M. Subacute developmental exposure of zebrafish to the organophosphate pesticide metabolite, chlorpyrifos-oxon, results in defects in Rohon-Beard sensory neuron development. Aquat. Toxicol. 2010, 100, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sisman, T. Dichlorvos-induced developmental toxicity in zebrafish. Toxicol. Ind. Health 2010, 26, 567–573. [Google Scholar] [CrossRef]
- Tilton, F.A.; Bammler, T.K.; Gallagher, E.P. Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately, or as mixtures. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lauridsen, H.; Buels, K.; Chi, L.H.; La Du, J.; Bruun, D.A.; Olson, J.R.; Tanguay, R.L.; Lein, P.J. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol. Sci. 2011, 121, 146–159. [Google Scholar] [CrossRef]
- Yen, J.; Donerly, S.; Levin, E.D.; Linney, E.A. Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotoxicol. Teratol. 2011, 33, 735–741. [Google Scholar] [CrossRef]
- Richendrfer, H.; Pelkowski, S.D.; Colwill, R.M.; Creton, R. Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae. Neurotoxicol. Teratol. 2012, 34, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, L.; Yang, K.; Tian, H.; Wang, W.; Ru, S. Monocrotophos pesticide modulates the expression of sexual differentiation genes and causes phenotypic feminization in zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 33–40. [Google Scholar] [CrossRef]
- Watson, F.L.; Schmidt, H.; Turman, Z.K.; Hole, N.; Garcia, H.; Gregg, J.; Tilghman, J.; Fradinger, E.A. Organophosphate pesticides induce morphological abnormalities and decrease locomotor activity and heart rate in Danio rerio and Xenopus laevis. Environ. Toxicol. Chem. 2014, 33, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fuentes, G.; Rubio-Escalante, F.J.; Norena-Barroso, E.; Escalante-Herrera, K.S.; Schlenk, D. Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following Chlorpyrifos exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 172–173, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Bui-Nguyen, T.M.; Baer, C.E.; Lewis, J.A.; Yang, D.; Lein, P.J.; Jackson, D.A. Dichlorvos exposure results in large scale disruption of energy metabolism in the liver of the zebrafish, Danio rerio. BMC Genomics 2015, 16, 853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhong, Y.; Tian, H.; Wang, W.; Ru, S. Impairment of the cortisol stress response mediated by the hypothalamus-pituitary-interrenal (HPI) axis in zebrafish (Danio rerio) exposed to monocrotophos pesticide. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 176–177, 10–16. [Google Scholar] [CrossRef]
- Gomez-Canela, C.; Prats, E.; Pina, B.; Tauler, R. Assessment of chlorpyrifos toxic effects in zebrafish (Danio rerio) metabolism. Environ. Pollut. 2017, 220 Pt B, 1231–1243. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, L.; Yu, Y.; Yang, G.; Xu, Z.; Wang, Q.; Cai, L. Single and joint toxic effects of five selected pesticides on the early life stages of zebrafish (Denio rerio). Chemosphere 2017, 170, 61–67. [Google Scholar] [CrossRef]
- Shukla, S.; Jhamtani, R.C.; Dahiya, M.S.; Agarwal, R. Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol. Rep. 2017, 4, 240–244. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.; Qian, Y.; Chen, C.; Jiao, B.; Cai, L.; Wang, Q. Joint acute and endocrine disruptive toxicities of malathion, cypermethrin and prochloraz to embryo-larval zebrafish, Danio rerio. Chemosphere 2017, 166, 63–71. [Google Scholar] [CrossRef]
- D’Costa, A.H.; Shyama, S.K.; Praveen Kumar, M.K.; Fernandes, T.M. Induction of DNA damage in the peripheral blood of zebrafish (Danio rerio) by an agricultural organophosphate pesticide, monocrotophos. Int. Aquat. Res. 2018, 10, 243–251. [Google Scholar] [CrossRef]
- Korkmaz, V.; Gungordu, A.; Ozmen, M. Comparative evaluation of toxicological effects and recovery patterns in zebrafish (Danio rerio) after exposure to phosalone-based and cypermethrin-based pesticides. EcoToxicol. Environ. Saf. 2018, 160, 265–272. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Shen, M.; Shen, J.; Zhang, X.; Jin, Y. Chlorpyrifos exposure induces lipid metabolism disorder at the physiological and transcriptomic levels in larval zebrafish. Acta Biochim. Biophys. Sin. 2019, 51, 890–899. [Google Scholar] [CrossRef]
- Schmitt, C.; McManus, M.; Kumar, N.; Awoyemi, O.; Crago, J. Comparative analyses of the neurobehavioral, molecular, and enzymatic effects of organophosphates on embryo-larval zebrafish (Danio rerio). Neurotoxicol. Teratol. 2019, 73, 67–75. [Google Scholar] [CrossRef]
- Altenhofen, S.; Nabinger, D.D.; Bitencourt, P.E.R.; Bonan, C.D. Dichlorvos alters morphology and behavior in zebrafish (Danio rerio) larvae. Environ. Pollut. 2019, 245, 1117–1123. [Google Scholar] [CrossRef]
- Shahjahan, M.; Rahman, M.S.; Islam, S.M.M.; Uddin, M.H.; Al-Emran, M. Increase in water temperature increases acute toxicity of sumithion causing nuclear and cellular abnormalities in peripheral erythrocytes of zebrafish Danio rerio. Environ. Sci. Pollut. Res. Int. 2019, 26, 36903–36912. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, A.B.; Glazer, L.; Dean, C.; Wells, C.N.; Odamah, K.A.; Slotkin, T.A.; Seidler, F.J.; Levin, E.D. Adult exposure to insecticides causes persistent behavioral and neurochemical alterations in zebrafish. Neurotoxicol. Teratol. 2020, 78, 106853. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, A.B.; Holloway, Z.; Dean, C.; Koburov, R.; Slotkin, T.A.; Seidler, F.J.; Levin, E.D. Neurobehavioral anomalies in zebrafish after sequential exposures to DDT and chlorpyrifos in adulthood: Do multiple exposures interact? Neurotoxicol. Teratol. 2021, 87, 106985. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Islam, S.M.M.; Haque, A.; Shahjahan, M. Toxicity of the organophosphate insecticide sumithion to embryo and larvae of zebrafish. Toxicol. Rep. 2020, 7, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Boyda, J.; Hawkey, A.B.; Holloway, Z.R.; Trevisan, R.; Di Giulio, R.T.; Levin, E.D. The organophosphate insecticide diazinon and aging: Neurobehavioral and mitochondrial effects in zebrafish exposed as embryos or during aging. Neurotoxicol. Teratol. 2021, 87, 107011. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, O.; Horyn, O.; Khatib, I.; Falfushynska, H. Multibiomarker assessment in zebrafish Danio rerio after the effects of malathion and chlorpyrifos. Toxicol. Environ. Health Sci. 2021, 13, 165–174. [Google Scholar] [CrossRef]
- Falfushynska, H.; Khatib, I.; Kasianchuk, N.; Lushchak, O.; Horyn, O.; Sokolova, I.M. Toxic effects and mechanisms of common pesticides (Roundup and chlorpyrifos) and their mixtures in a zebrafish model (Danio rerio). Sci. Total Environ. 2022, 833, 155236. [Google Scholar] [CrossRef]
- Povey, A.C. Gene-environmental interactions and organophosphate toxicity. Toxicology 2010, 278, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Deng, J.F. Intermediate syndrome following organophosphate insecticide poisoning. J. Chin. Med. Assoc. 2007, 70, 467–472. [Google Scholar] [CrossRef]
- Vacca, V.M. Organophosphate poisoning. Nursing 2012, 42, 72. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, A.; Muco, E.; Pierre, L. Organophosphates; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Peter, J.V.; Sudarsan, T.I.; Moran, J.L. Clinical features of organophosphate poisoning: A review of different classification systems and approaches. Indian J. Crit. Care Med. 2014, 18, 735–745. [Google Scholar] [CrossRef]
- Abdollahi, M.; Karami-Mohajeri, S. A comprehensive review on experimental and clinical findings in intermediate syndrome caused by organophosphate poisoning. Toxicol. Appl. Pharmacol. 2012, 258, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Karalliedde, L.; Baker, D.; Marrs, T.C. Organophosphate-induced intermediate syndrome: Aetiology and relationships with myopathy. Toxicol. Rev. 2006, 25, 1–14. [Google Scholar] [CrossRef]
- Giyanwani, P.R.; Zubair, U.; Salam, O.; Zubair, Z. Respiratory failure following organophosphate poisoning: A literature review. Cureus 2017, 9, e1651. [Google Scholar] [CrossRef]
- Casida, J.E. Organophosphorus xenobiotic toxicology. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 309–327. [Google Scholar] [CrossRef]
- Lotti, M.; Becker, C.E.; Aminoff, M.J. Organophosphate polyneuropathy: Pathogenesis and prevention. Neurology 1984, 34, 658–662. [Google Scholar] [CrossRef]
- Lotti, M.; Moretto, A. Organophosphate-induced delayed polyneuropathy. Toxicol. Rev. 2005, 24, 37–49. [Google Scholar] [CrossRef]
- Flynn, K.C. The cytoskeleton and neurite initiation. Bioarchitecture 2013, 3, 86–109. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K. Organophosphates and delayed neuropathy--is NTE alive and well? Toxicol. Appl. Pharmacol. 1990, 102, 385–399. [Google Scholar] [CrossRef]
- Richardson, R.J.; Fink, J.K.; Glynn, P.; Hufnagel, R.B.; Makhaeva, G.F.; Wijeyesakere, S.J. Neuropathy target esterase (NTE/PNPLA6) and organophosphorus compound-induced delayed neurotoxicity (OPIDN). Adv. Neurotoxicol. 2020, 4, 1–78. [Google Scholar]
- Peraica, M.; Capodicasa, E.; Moretto, A.; Lotti, M. Organophosphate polyneuropathy in chicks. Biochem. Pharmacol. 1993, 45, 131–135. [Google Scholar] [CrossRef]
- Rauh, V.A.; Garfinkel, R.; Perera, F.P.; Andrews, H.F.; Hoepner, L.; Barr, D.B.; Whitehead, R.; Tang, D.; Whyatt, R.W. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 2006, 118, e1845–e1859. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.P.; Jha, R.R.; Ahmad, H.; Patel, D.K.; Ravi Ram, K. Xenobiotic mediated diabetogenesis: Developmental exposure to dichlorvos or atrazine leads to type 1 or type 2 diabetes in Drosophila. Free Radic. Biol. Med. 2019, 141, 461–474. [Google Scholar] [CrossRef]
- Furlong, C.E.; Holland, N.; Richter, R.J.; Bradman, A.; Ho, A.; Eskenazi, B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharm. Genom. 2006, 16, 183–190. [Google Scholar] [CrossRef]
- Holland, N.; Furlong, C.; Bastaki, M.; Richter, R.; Bradman, A.; Huen, K.; Beckman, K.; Eskenazi, B. Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns. Environ. Health Perspect. 2006, 114, 985–991. [Google Scholar] [CrossRef]
- Sastry, B.V. Human placental cholinergic system. Biochem. Pharmacol. 1997, 53, 1577–1586. [Google Scholar] [CrossRef]
- Eskenazi, B.; Harley, K.; Bradman, A.; Weltzien, E.; Jewell, N.P.; Barr, D.B.; Furlong, C.E.; Holland, N.T. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ. Health Perspect. 2004, 112, 1116–1124. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Camann, D.; Perera, F.P.; Rauh, V.A.; Tang, D.; Kinney, P.L.; Garfinkel, R.; Andrews, H.; Hoepner, L.; Barr, D.B. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol. Appl. Pharmacol. 2005, 206, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.M.; Berkowitz, G.S.; Barr, D.B.; Teitelbaum, S.L.; Siskind, J.; Meisel, S.J.; Wetmur, J.G.; Wolff, M.S. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. J. Epidemiol. 2007, 165, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Bellinger, D.C.; Wright, R.O.; Weisskopf, M.G. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 2010, 125, e1270–e1277. [Google Scholar] [CrossRef]
- Saunders, N.R.; Liddelow, S.A.; Dziegielewska, K.M. Barrier mechanisms in the developing brain. Front. Pharmacol. 2012, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Bradman, A.; Barr, D.B.; Claus Henn, B.G.; Drumheller, T.; Curry, C.; Eskenazi, B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: A validation study. Environ. Health Perspect. 2003, 111, 1779–1782. [Google Scholar] [CrossRef]
- Sultatos, L.G. Mammalian toxicology of organophosphorus pesticides. J. Toxicol. Environ. Health 1994, 43, 271–289. [Google Scholar] [CrossRef]
- Suwannakul, B.; Sapbamrer, R.; Wiwattanadittakul, N.; Hongsibsong, S. Organophosphate pesticide exposures in early and late pregnancy influence different aspects of infant developmental performance. Toxics 2021, 9, 99. [Google Scholar] [CrossRef]
- Qiao, D.; Seidler, F.J.; Slotkin, T.A. Developmental neurotoxicity of chlorpyrifos modeled in vitro: Comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ. Health Perspect. 2001, 109, 909–913. [Google Scholar] [CrossRef]
- Kamel, F.; Hoppin, J.A. Association of pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect. 2004, 112, 950–958. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Chevrier, J.; Harley, K.G.; Kogut, K.; Vedar, M.; Calderon, N.; Trujillo, C.; Johnson, C.; Bradman, A.; Barr, D.B.; et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect. 2011, 119, 1189–1195. [Google Scholar] [CrossRef]
- Guillette, E.A.; Meza, M.M.; Aquilar, M.G.; Soto, A.D.; Garcia, I.E. An anthropological approach to the evaluation of preschool children exposed to pesticides in Mexico. Environ. Health Perspect. 1998, 106, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Santed, F.; Colomina, M.T.; Herrero Hernandez, E. Organophosphate pesticide exposure and neurodegeneration. Cortex 2016, 74, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.L. Acetylcholine, aging, and Alzheimer’s disease. Pharmacol. Biochem. Behav. 1997, 56, 687–696. [Google Scholar] [CrossRef]
- Obeso, J.A. Modeling clinical features of neurodegeneration. Nat. Med. 2010, 16, 1372. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Khuder, S.A.; Schaub, E.A.; Shrivastava, S. A meta-analysis of Parkinson’s disease and exposure to pesticides. Neurotoxicology 2000, 21, 435–440. [Google Scholar]
- Hancock, D.B.; Martin, E.R.; Mayhew, G.M.; Stajich, J.M.; Jewett, R.; Stacy, M.A.; Scott, B.L.; Vance, J.M.; Scott, W.K. Pesticide exposure and risk of Parkinson’s disease: A family-based case-control study. BMC Neurol. 2008, 8, 6. [Google Scholar] [CrossRef]
- Seidler, A.; Hellenbrand, W.; Robra, B.P.; Vieregge, P.; Nischan, P.; Joerg, J.; Oertel, W.H.; Ulm, G.; Schneider, E. Possible environmental, occupational, and other etiologic factors for Parkinson’s disease: A case-control study in Germany. Neurology 1996, 46, 1275–1284. [Google Scholar] [CrossRef]
- Manthripragada, A.D.; Costello, S.; Cockburn, M.G.; Bronstein, J.M.; Ritz, B. Paraoxonase 1, agricultural organophosphate exposure, and Parkinson disease. Epidemiology 2010, 21, 87–94. [Google Scholar] [CrossRef]
- Freire, C.; Koifman, S. Pesticide exposure and Parkinson’s disease: Epidemiological evidence of association. Neurotoxicology 2012, 33, 947–971. [Google Scholar] [CrossRef]
- Wang, A.; Cockburn, M.; Ly, T.T.; Bronstein, J.M.; Ritz, B. The association between ambient exposure to organophosphates and Parkinson’s disease risk. Occup. Environ. Med. 2014, 71, 275–281. [Google Scholar] [CrossRef]
- Diouf, J. FAO’s Director-General on How to Feed the World in 2050. Popul. Dev. Rev. 2009, 35, 837–839. [Google Scholar]

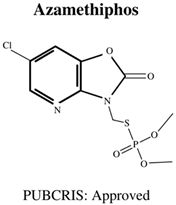 | Molecular Formula: C9H10CIN2O5PS General Uses of OP: Used in Aquatic Farming (Atlantic Salmon) to Control Parasites. |  | Molecular Formula: C10H12N3O3PS2 General Uses of OP: Used on Orchard Fruits and Nut Crops to Control Moths. |
 | Molecular Formula: C9H11Cl3NO3PS General Uses of OP: Used broadly (crops/animals/buildings) to control roundworms, mosquitos and termites. | 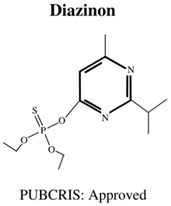 | Molecular Formula: C12H21N2O3PS General Uses of OP: Used on crops (fruits/vegetables/nuts/field crops) to control ants, fleas and cockroaches. |
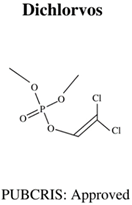 | Molecular Formula: C4H7Cl2O4P General Uses of OP: Used broadly (household/ agriculture) to control flies, caterpillars, thrips and mites. |  | Molecular Formula: C9H12NO5PS General Uses of OP: Used broadly (public health/agriculture) to control beetles, grubs, locusts, flies, mosquitos, etc. |
 | Molecular Formula: C10H19O6PS2 General Uses of OP: Used broadly (landscaping/public health/agriculture) to control mosquitos, fleas and ants. |  | Molecular Formula: C8H10NO5PS General Uses of OP: Used in open fields (cotton, soybean, vegetable) to control boll weevils, etc. |
 | Molecular Formula: C10H14NO5PS General Uses of OP: No longer used (banned largely worldwide) due to its high toxicity. | 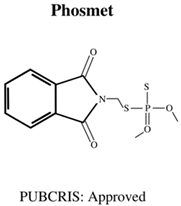 | Molecular Formula: C11H12NO4PS2 General Uses of OP: Used broadly (plants/animals) to control moths, mites, flies and aphids. |
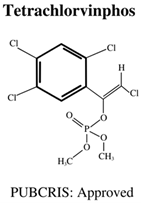 | Molecular Formula: C10H9Cl4O4P General Uses of OP: Used on animals (cattle, hogs, goats, chickens, and horses) to control flies and mites. | ||
| Organophosphate(s) | Dosage(s) * | Gene(s) Involved ** | Exposure Period | Observations | Reference |
|---|---|---|---|---|---|
| Chlorpyrifos (CPF) | CPF: 10 & 100 ng/mL | - | 0–5 dpf |
| [58] |
| Chlorpyrifos (CPF) | CPF: 100 ng/mL | - | 0–5 dpf |
| [59] |
| Malathion (MAL) | MAL: 2.5 & 3 mg/L | - | 3 hpf–5 dpf |
| [60] |
| Malathion (MAL) | MAL: 0.25, 0.5, 1, 3 & 5 mM | - | Adult (sexually mature) |
| [61] |
| Diazinon (DZN) | DZN: 2000 & 3000 μg/L | - | 8 hpf–96 hpf |
| [62] |
| Chlorpyrifos (CPF) | CPF: 0.25, 0.5, 0.75 & 1 mg/L | - | Acute: 5 dpf for 2 h Sub-chronic: ≤1 hpf–11dpf |
| [63] |
| Chlorpyrifos (CPF) | CPF: 300, 1500 & 3000 nM | Rohon-Beard Development/Axonogenesis: agrin↓, cntn2↓, ntf3↓, sema3d↓ | 3 hpf–27 hpf/51 hpf/72 hpf/4 dpf |
| [64] |
| Chlorpyrifos (CPF) | CPF: 0.29 μM | - | 0–5 dpf |
| [65] |
| Chlorpyrifos-oxon (CPF metabolite) | CPF: 300 nM | 0.1 μg/L, 3 μg/L | 3 hpf–75 hpf |
| [66] |
| Dichlorvos (DCV) | DCV: 20.81, 25 & 66.78 mg/L | - | 0 hpf–96 hpf |
| [67] |
| Dichlorvos (DCV) Phoxim (PHO) | DCV: N/A PHO: 0.469, 0.513, 0.700 & 1.28 mg/L | - | Adult (sexually mature) |
| [4] |
| Chlorpyrifos (CPF) | CPF: 0.6 μM | - | 1 ypf for 24 h |
| [68] |
| Chlorpyrifos (CPF) | CPF: 0.01, 0.1 & 1 μM | - | 6 hpf–24/48/72 hpf |
| [69] |
| Chlorpyrifos (CPF) Diazinon (DZN) Parathion (PA) | CPF: 0.3, 3 & 30 μM DZN: 10 & 30 μM PA: 10 & 30 μM | - | 6 hpf–5 dpf |
| [70] |
| Chlorpyrifos (CPF) | CPF: 0.01 & 0.1 μM | - | 0–7 dpf |
| [71] |
| Monocrotophos (MCP) | MCP: 0.001 & 0.100 mg/L | Sexual Differentiation: cyp19a1a↑, cyp19a1b↑, foxl2↑, dmrt1↓, B-actin, ef1-a | 72 hpfV–16 dpf |
| [72] |
| Chlorpyrifos (CPF) Dichlorvos (DCV) Diazinon (DZN) | CPF: 1, 10, 100 & 1000 μM DCV: 100 & 1000 μM DZN: 100 & 1000 μM | - | 1 hpf–5 dpf |
| [73] |
| Chlorpyrifos (CPF) | CPF: 30, 100 & 300 μg/L | Gfap, Mbp↓, Elavl3↑, Ngn1↑, Nestin↑, Shha↑ | 0–5 dpf |
| [50] |
| Chlorpyrifos (CPF) | CPF: 200 & 400 μg/L | - | 2 hpfV–72 hpf |
| [74] |
| Dichlorvos (DCV) | DCV: 6, 19, & 32 mg/L | Oxidative Stress: Nrf2 (many other associated genes within the Nrf2 pathway also examined) | 6–12 mpf |
| [75] |
| Monocrotophos (MCP) | MCP: 10, 20, 30, 40, 50 & 60 mg/L | - | 4 hpf–96 hpf |
| [1] |
| Monocrotophos (MCP) | MCP: 100 μg/L | HPI Axis: Crf, Gr↓, POMC↓, P45011β, 11B-HSD2, StAR, 20B-HSD2↑, MC2R↓, TAT, PEPCK | Adult (sexually mature)—21 d exposure |
| [76] |
| Chlorpyrifos (CPF) | CPF: 2 & 5 μM | - | Adult (sexually mature) |
| [77] |
| Chlorpyrifos (CPF) Phoxim (PHO) | CPF: 0.28- 13.03 mg/L PHO: 0.89–26.48 mg/L | - | Embryo (1 hpf), larvae (72 hpf) and juvenile (1 mpf)—96 h exposure |
| [78] |
| Diazinon (DZN) | DZN: 6.5 mg/L | - | 6 hpf–5 dpf |
| [2] |
| Dichlorvos (DCV) | DCV: 15 mg/L | - | Adult (sexually mature) 4–5 m—24 h exposure |
| [79] |
| Malathion (MAL) | MAL: 250, 500 & 1000 μg/L | HPG Axis: vtg1, vtg2, era↑, erB1, erB2, cyp19a1a, cyp19a1b↑ | 6 dpf–10 dpf |
| [80] |
| Chlorpyrifos (CPF) Diazinon (DZN) | CPF: 1, 10, & 25 μM DZN: 10 & 100 μM | - | 6 hpf–102 hpf |
| [18] |
| Monocrotophos (MCP) | MCP: 0.125, 0.625 & 1.25 uL/L | 24–72 hpf |
| [81] | |
| Phosalone (PSL) | PSL: 86–505 μg/L | - | 8 wpf–96 h exposure |
| [82] |
| Chlorpyrifos (CPF) | CPF: 30, 100 & 300 μg/L | Oxidative stress: Mn-Sod↑/↓, Cu/Zn-Sod↓, Gpx↓, Cat↓, Ucp2↓, bc12, Cox1↓ Glycolysis/Lipid: Gk↓, HK1, Pk↓, Pepckc↓, Aco↓, CPt1↓, Ppar-A↓, Acc1↓, Srebp 1a↓, Ppar-y↓, Fas↓, Fabp6, Apo↓, Dgat↓, LDLR↓, HMGCR, Fabp5 | Adult (sexually mature) |
| [56] |
| Chlorpyrifos (CPF) | CPF: 30, 100 & 300 μg/L | Cardiovascular: Mef2c↓, Bmp4↓, VEGFR-2, JunB↑, Tbx2 Lipid: Ppar-a, Ppar-y↓, Srebp 1a, Acc1, Fas↓, Cpt1↓, Aco, Apo↓, Fabp5, Fabp6↓, Dgat↓, LDLR | 2 hpf–7 dpf |
| [83] |
| Diazinon (DZN) Dichlorvos (DCV) Malathion (MAL) Parathion (PA) | DZN: 0.1 & 100 μg/L DCV: N/A MAL: 100 μg/L PA: 0.1 μg/L | Cholinergic: AChE↑/↓ Neurodegeneration: c-Fos, lingo-1b↑, grin-1b↓ | 5 hpf–5 dpf |
| [84] |
| Dichlorvos (DCV) | DCV: 1, 5 & 10 mg/L | - | 1 hpf–7 dpf |
| [85] |
| Sumithion (SMT) | SMT: 1 mg/L | - | Adult (sexually mature)—96h exposure |
| [86] |
| Chlorpyrifos (CPF) | CPF: 1 & 3 μM | - | Adult (sexually mature) 6–8 m—2/5 w exposure |
| [87,88] |
| Chlorpyrifos (CPF) Malathion (MAL) | CPF: 0.019, 0.077, 0.31, 0.41, 1.01, 1.53 & 6.15 mg/L MAL: 0.039, 0.16, 0.62, 2.90, 8.04, 8.54 & 12.45 mg/L | Oxidative Stress: Cat, CuSod, MnSod Immunity: Cxcl↓, IL↑, Tnf↑/↓ Apoptosis: Cas8↑/↓, Cas9, P53, Bax Endocrine: TRa, TRb↓, ERa, Tsh↓, Crh, cyp19a↑ | 1 hpf–96 hpf |
| [57] |
| Sumithion (SMT) | SMT: 0.1, 0.2, 0.4, 0.8 & 1.6 mg/L | - | Embryo/larvae |
| [89] |
| Chlorpyrifos (CPF) | CPF: 1μM | - | Adult (sexually mature)—5 w exposure |
| (Hawkey, 2021) |
| Diazinon (DZN) | DZN: 0.4, 1.25 & 4.0 μM | 5–120 hpf |
| [90] | |
| Malathion (MAL) Chlorpyrifos (CPF) | MAL: 5, 50 ug/L CPF: 0.1 & 3 ug/L | 0–14 dpf |
| [91] | |
| Chlorpyrifos (CPF) | Caspase 3↓, Bcl-2↓, | Adult—8–12 months old 14 day exposure |
| [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neylon, J.; Fuller, J.N.; van der Poel, C.; Church, J.E.; Dworkin, S. Organophosphate Insecticide Toxicity in Neural Development, Cognition, Behaviour and Degeneration: Insights from Zebrafish. J. Dev. Biol. 2022, 10, 49. https://doi.org/10.3390/jdb10040049
Neylon J, Fuller JN, van der Poel C, Church JE, Dworkin S. Organophosphate Insecticide Toxicity in Neural Development, Cognition, Behaviour and Degeneration: Insights from Zebrafish. Journal of Developmental Biology. 2022; 10(4):49. https://doi.org/10.3390/jdb10040049
Chicago/Turabian StyleNeylon, Jeremy, Jarrad N. Fuller, Chris van der Poel, Jarrod E. Church, and Sebastian Dworkin. 2022. "Organophosphate Insecticide Toxicity in Neural Development, Cognition, Behaviour and Degeneration: Insights from Zebrafish" Journal of Developmental Biology 10, no. 4: 49. https://doi.org/10.3390/jdb10040049
APA StyleNeylon, J., Fuller, J. N., van der Poel, C., Church, J. E., & Dworkin, S. (2022). Organophosphate Insecticide Toxicity in Neural Development, Cognition, Behaviour and Degeneration: Insights from Zebrafish. Journal of Developmental Biology, 10(4), 49. https://doi.org/10.3390/jdb10040049






