The Shape of the Jaw—Zebrafish Col11a1a Regulates Meckel’s Cartilage Morphogenesis and Mineralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Care and Transgenic Lines
2.2. Morpholino Injections
2.3. Cartilage and Bone Staining
2.4. Imaging
3. Results: Characterization of Jaw Development in Zebrafish Col11a1a Knockdown
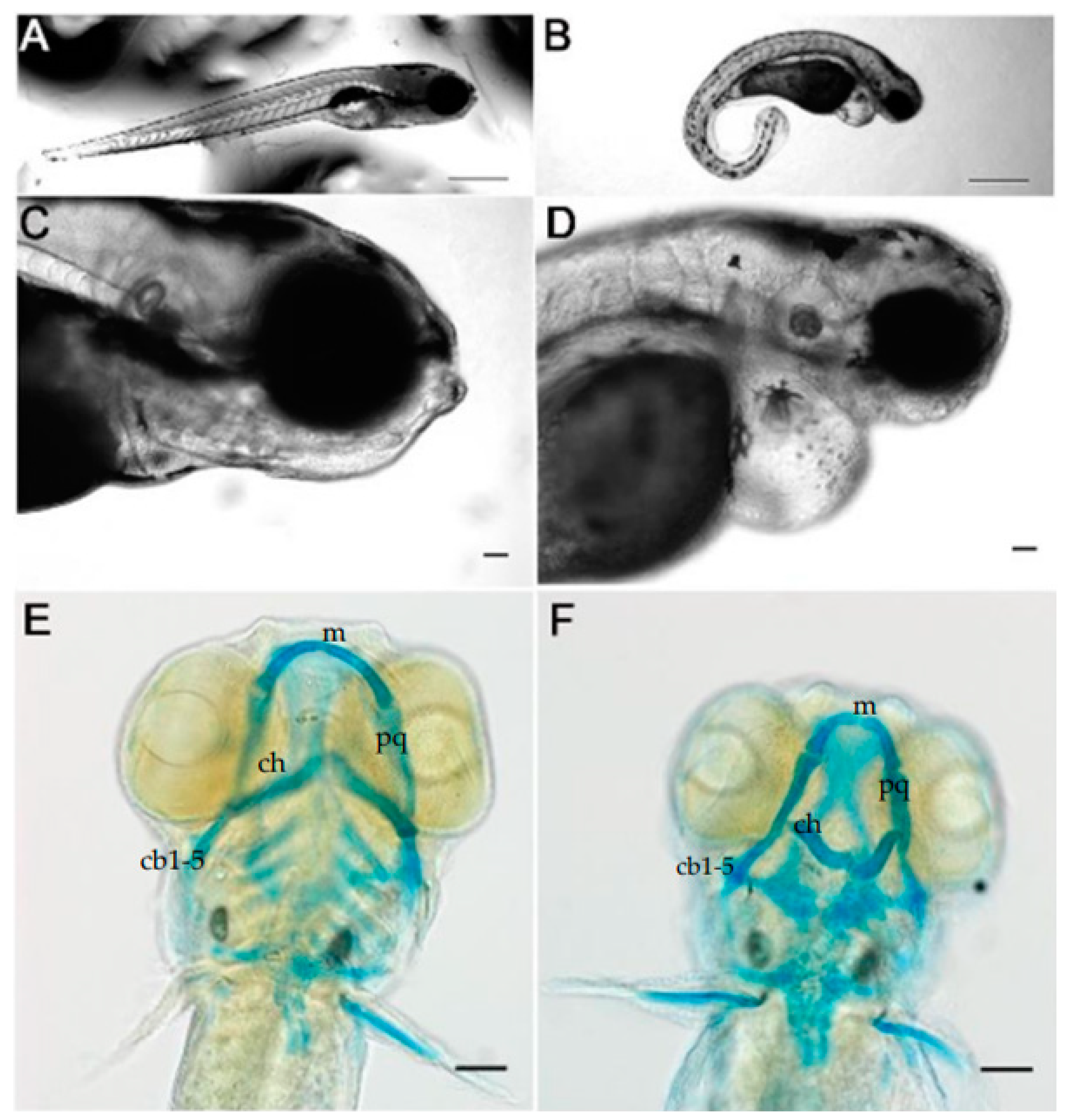
3.1. Knockdown of Col11a1a Leads to Skeletal Deformities in Zebrafish
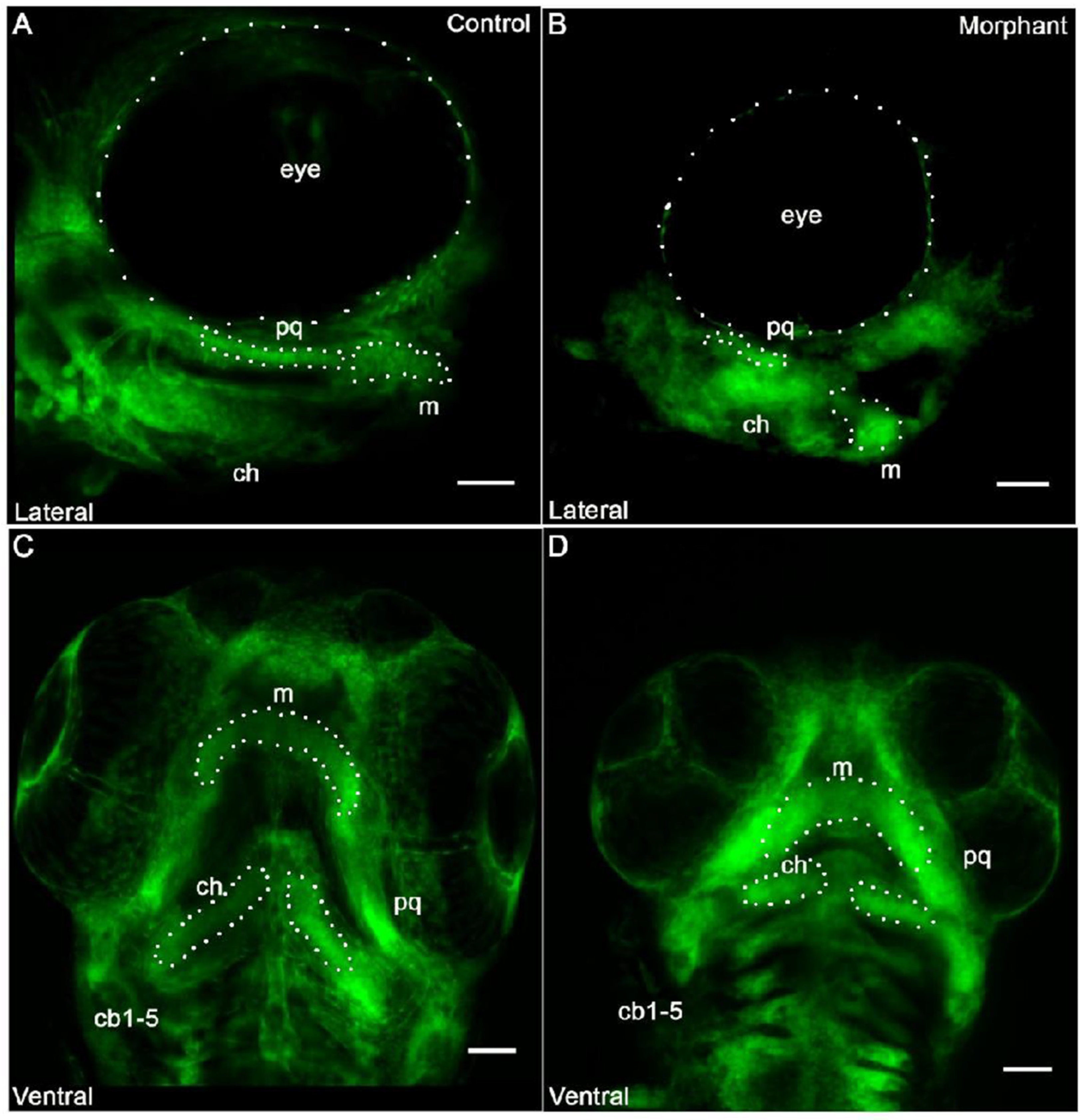
3.2. Cranial Neural Crest Derived Cells Form Segmented Pharyngeal Arches with Abnormal Shape
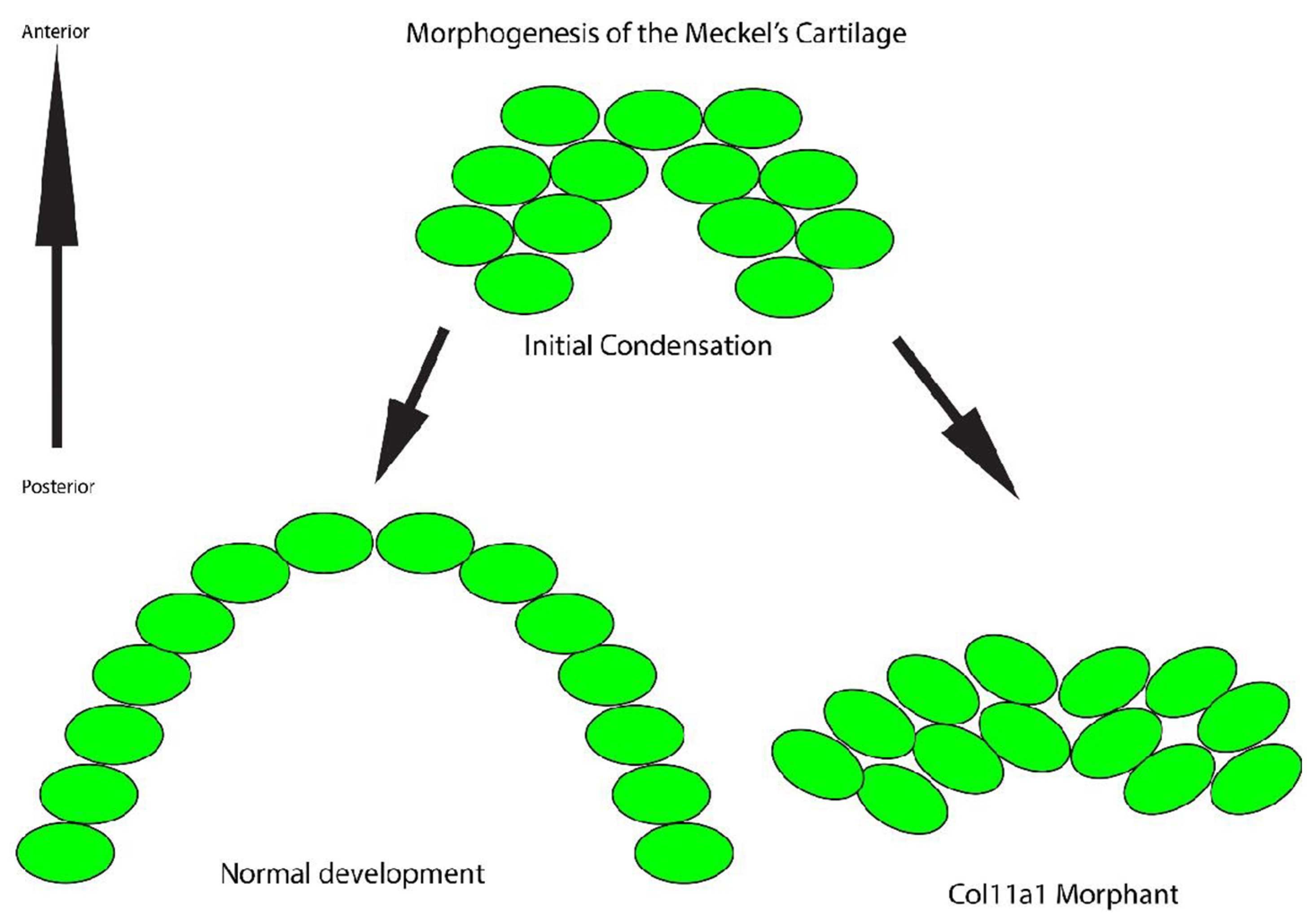
3.3. Cells in the Meckel’s Cartilage Failed to Converge and Extend in Col11a1a Morphants
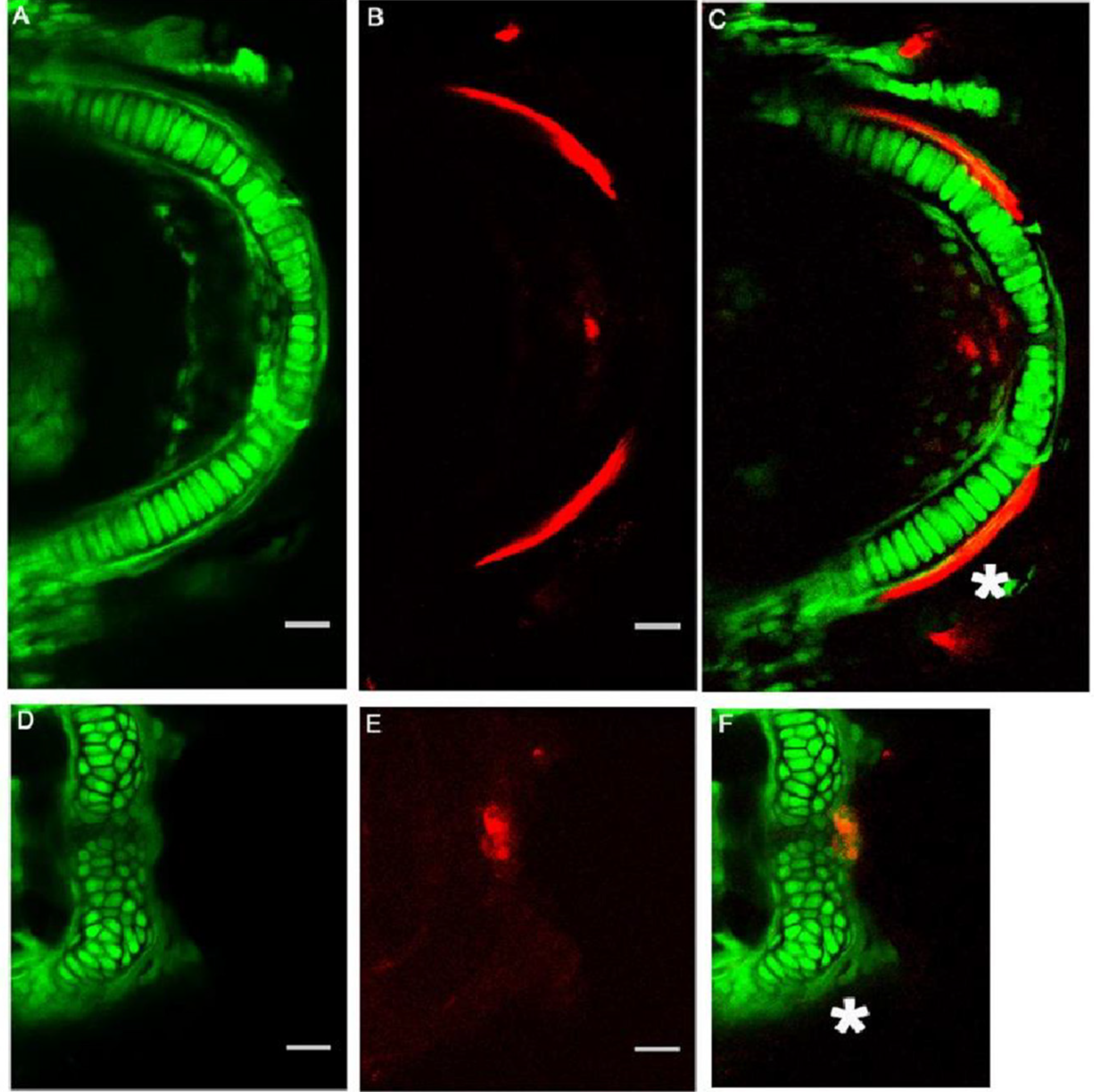
3.4. Mineralization of Meckel’s Cartilage in Col11a1a Morphants Compared to Controls
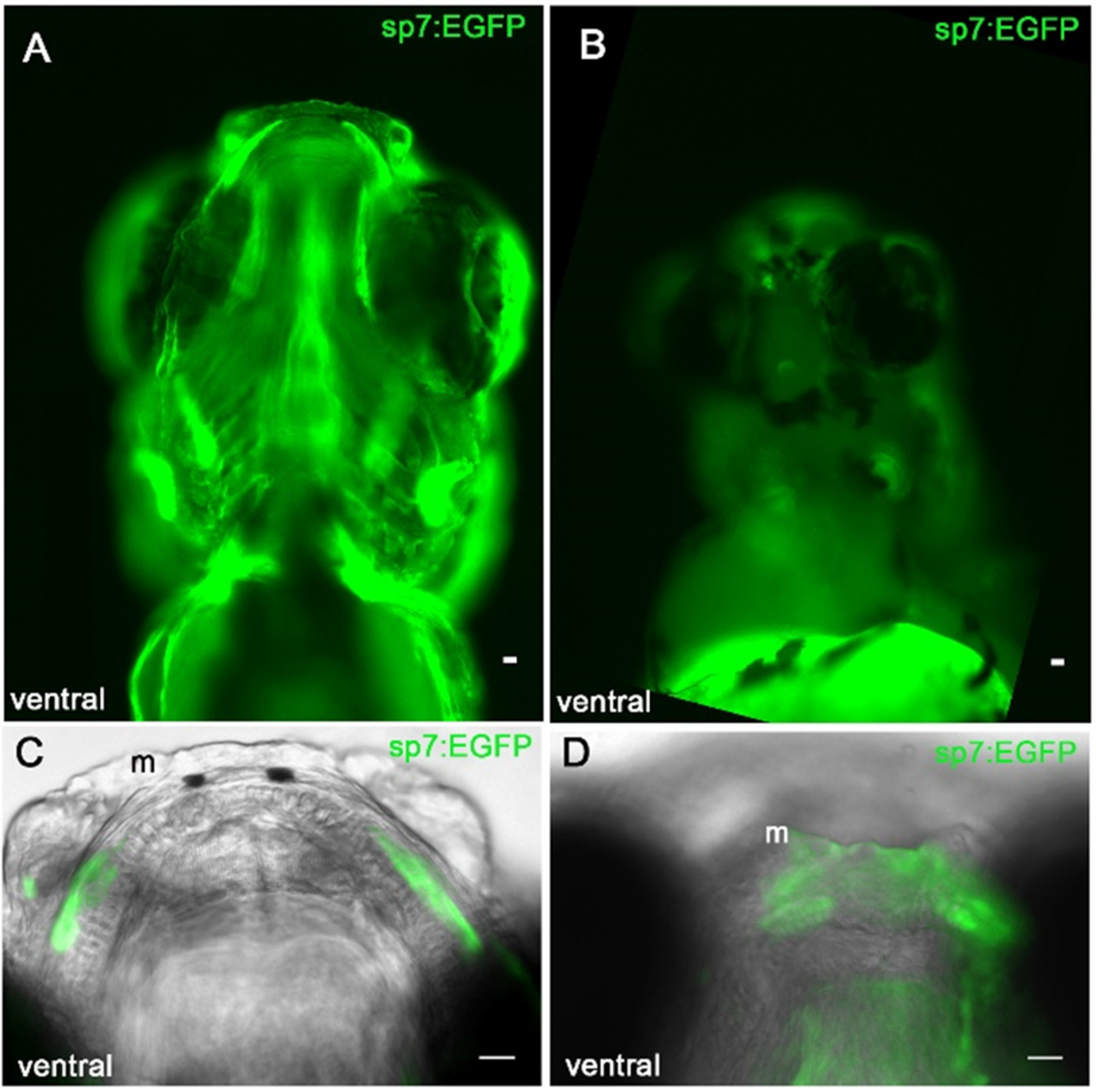
3.5. Sp7/Osterix Expression by Bone Forming Cells in the Morphant Meckel’s Cartilage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kimmel, C.; Miller, C.; Kruze, G.; Ullmann, B.; BreMiller, R.; Larison, K.; Snyder, H. The shaping of pharyngeal cartilages during early development of the zebrafish. Dev. Biol. 1998, 203, 245–263. [Google Scholar]
- Kague, E.; Gallagher, M.; Burke, S.; Parsons, M.; Franz-Odendaal, T.; Fisher, S. Skeletogenic Fate of Zebrafish Cranial and Trunk Neural Crest. PLoS ONE 2012, 7, e47394. [Google Scholar]
- Berendsen, A.; Olsen, B. Bone development. Bone 2015, 80, 14–18. [Google Scholar]
- Schilling, T.; Kimmel, C. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 1994, 120, 483–494. [Google Scholar]
- Mork, L.; Crump, G. Zebrafish Craniofacial Development: A Window into Early Patterning. Curr. Top. Dev. Biol. 2015, 115, 235–269. [Google Scholar]
- Eames, B.; DeLaurier, A.; Ullmann, B.; Huycke, T.; Nichols, J.; Dowd, J.; McFadden, M.; Sasaki, M.; Kimmel, C. FishFace: Interactive atlas of zebrafish craniofacial development at cellular resolution. BMC Dev. Biol. 2013, 13, 23. [Google Scholar]
- Carter, E.; Raggio, C. Genetic and orthopedic aspects of collagen disorders. Curr. Opin. Pediatr. 2009, 21, 46–54. [Google Scholar]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar]
- Kadler, K.; Baldock, C.; Bella, J.; Boot-Handford, R. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar]
- Poole, C.; Flint, M.; Beaumont, B. Chondrons in cartilage: Ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J. Orthop. Res. 1987, 5, 509–522. [Google Scholar]
- Eyre, D. Collagen of articular cartilage. Arthritis Res. 2002, 4, 30–35. [Google Scholar]
- Tompson, S.; Bacino, C.; Safina, N.; Bober, M.; Proud, V.; Funari, T.; Wangler, M.; Nevarez, L.; Ala-Kokko, L.; Wilcox, W.; et al. Fibrochondrogenesis results from mutations in the COL11A1 type XI collagen gene. Am. J. Hum. Genet. 2010, 87, 708–712. [Google Scholar]
- Vijzelaar, R.; Waller, S.; Errami, A.; Donaldson, A.; Lourenco, T.; Rodrigues, M.; McConnell, V.; Fincham, G.; Snead, M.; Richards, A. Deletions within COL11A1 in Type 2 stickler syndrome detected by multiplex ligation-dependent probe amplification (MLPA). BMC Med. Genet. 2013, 14, 48. [Google Scholar]
- Acke, F.; Malfait, F.; Vanakker, O.; Steyaert, W.; De Leeneer, K.; Mortier, G.; Dhooge, I.; De Paepe, A.; De Leenheer, E.; Coucke, P. Novel pathogenic COL11A1/COL11A2 variants in Stickler syndrome detected by targeted NGS and exome sequencing. Mol. Genet. Metab. 2014, 113, 230–235. [Google Scholar]
- Whitley, C.; Langer, L.; Ophoven, J.; Gilbert, E.; Gonzalez, C.; Mammel, M.; Coleman, M.; Rosemberg, S.; Rodriques, C.; Sibley, R.; et al. Fibrochondrogenesis: Lethal, autosomal recessive chondrodysplasia with distinctive cartilage histopathology. Am. J. Med. Genet. 1984, 19, 265–275. [Google Scholar]
- Akawi, N.; Al-Gazali, L.; Ali, B. Clinical and molecular analysis of UAE fibrochondrogenesis patients expands the phenotype and reveals two COL11A1 homozygous null mutations. Clin. Genet. 2012, 82, 147–156. [Google Scholar]
- Hufnagel, S.; Weaver, K.; Hufnagel, R.; Bader, P.; Schorry, E.; Hopkin, R. A novel dominant COL11A1 mutation resulting in a severe skeletal dysplasia. Am. J. Med. Genet. A 2014, 164A, 2607–2612. [Google Scholar]
- Warner, L.; Brown, R.; Yingst, S.; Oxford, J. Isoform-specific heparan sulfate binding within the amino-terminal noncollagenous domain of collagen alpha1(XI). J. Biol. Chem. 2006, 281, 39507–39516. [Google Scholar]
- McDougal, O.; Warner, L.; Mallory, C.; Oxford, J. Predicted structure and binding motifs of collagen α1(XI). GSTF Int. J. Bioinform. Biotechnol. 2011, 1, 43–48. [Google Scholar]
- Brown, R.; Mallory, C.; McDougal, O.; Oxford, J. Proteomic analysis of Col11a1-associated protein complexes. Proteomics 2011, 11, 4660–4676. [Google Scholar]
- Ryan, R.; Martin, B.; Mellor, L.; Jacob, R.; Tawara, K.; McDougal, O.; Oxford, J.; Jorcyk, C. Oncostatin M binds to extracellular matrix in a bioactive conformation: Implications for inflammation and metastasis. Cytokine 2015, 72, 71–85. [Google Scholar]
- Fernandes, R.; Weis, M.; Scott, M.; Seegmiller, R.; Eyre, D. Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse. Matrix Biol. 2007, 26, 597–603. [Google Scholar]
- Li, Y.; Lacerda, D.; Warman, M.; Beier, D.; Yoshioka, H.; Ninomiya, Y.; Oxford, J.; Morris, N.; Andrikopoulos, K.; Ramirez, F.; et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell 1995, 80, 423–430. [Google Scholar]
- Fang, M.; Adams, J.; McMahan, B.; Brown, R.; Oxford, J. The expression patterns of minor fibrillar collagens during development in zebrafish. Gene Expr. Patterns 2010, 10, 315–322. [Google Scholar]
- Hardy, M.J.; Reeck, J.C.; Fang, M.; Adams, J.S.; Oxford, J.T. Col11a1a Expression is required for zebrafish development. J. Dev. Biol. 2020, 8, 16. [Google Scholar] [CrossRef]
- Reeck, J.C.; Hardy, M.J.; Pu, X.; Keller-Peck, C.; Oxford, J.T. Authentication of a novel antibody to zebrafish collagen type XI alpha 1 chain (Col11a1a). BMC Res. Notes 2021, 14, 359. [Google Scholar] [CrossRef]
- DeLaurier, A.; Eames, B.; Blanco-Sánchez, B.; Peng, G.; He, X.; Swartz, M.; Ullmann, B.; Westerfield, M.; Kimmel, C. Zebrafish sp7:EGFP: A transgenic for studying otic vesicle formation, skeletogenesis, and bone regeneration. Genesis 2010, 48, 505–511. [Google Scholar]
- Lawson, N.; Weinstein, B. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio Rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Kimmel, C.B.; DeLaurier, A.; Ullmann, B.; Dowd, J.; McFadden, M. Modes of developmental outgrowth and shaping of a craniofacial bone in zebrafish. PLoS ONE 2010, 5, e9475. [Google Scholar] [CrossRef]
- Yonkers, M.; Oxford, J. Knockdown of Collagen 11a1 decreases zebrafish lens and optic cup diameter during early development. J. Investig. Ophthalmol. Vis. Sci. 2013, 54, 465. [Google Scholar]
- Piotrowski, T.; Schilling, T.; Brand, M.; Jiang, Y.; Heisenberg, C.; Beuchle, D.; Grandel, H.; van Eeden, F.; Furutani-Seiki, M.; Granato, M.; et al. Jaw and branchial arch mutants in zebrafish II: Anterior arches and cartilage differentiation. Development 1996, 123, 345–356. [Google Scholar]
- Schilling, T.; Piotrowski, T.; Grandel, H.; Brand, M.; Heisenberg, C.; Jiang, Y.; Beuchle, D.; Hammerschmidt, M.; Kane, D.; Mullins, M.; et al. Jaw and branchial arch mutants in zebrafish I: Branchial arches. Development 1996, 123, 329–344. [Google Scholar]
- Eames, B.; Amores, A.; Yan, Y.; Postlethwait, J.H. Evolution of the osteoblast: Skeletogenesis in gar and zebrafish. BMC Evol. Biol. 2012, 12, 27. [Google Scholar]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.; Behringer, R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar]
- Jobling, R.; D’Souza, R.; Baker, N.; Lara-Corrales, I.; Mendoza-Londono, R.; Dupuis, L.; Savarirayan, R.; Ala-Kokko, L.; Kannu, P. The Collagenopathies: Review of Clinical Phenotypes and Molecular Correlations. Curr. Rheumatol. Rep. 2014, 16, 394. [Google Scholar]
- Deng, H.; Huang, X.; Yuan, L. Molecular genetics of the COL2A1-related disorders. Mutat. Res. Mutat. Res. 2016, 768, 1–13. [Google Scholar]
- Kronenberg, H. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar]
- Meckel, J.F. Handbuch der Menschlichem Anatomie; Lehre und Geschichte des Foetud; Buchhandlung des Hallischen waisenhauses: Berlin, Germany, 1820; Volume IV. [Google Scholar]
- Xu, L.; Flahiff, C.M.; Waldman, B.A.; Wu, D.; Olsen, B.R.; Setton, L.A.; Li, Y. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho). Arthritis Rheum. 2003, 48, 2509–2518. [Google Scholar] [CrossRef]
- Wu, J.; Eyre, D. Structural analysis of cross-linking domains in cartilage type XI collagen. Insights on polymeric assembly. J. Biol. Chem. 1995, 270, 18865–18870. [Google Scholar]
- Baas, D.; Malbouyres, M.; Haftek-Terreau, Z.; Le Guellec, D.; Ruggiero, F. Craniofacial cartilage morphogenesis requires zebrafish col11a1 activity. Matrix Biol. 2009, 28, 490–502. [Google Scholar]
- Hafez, A.; Squires, R.; Pedracini, A.; Joshi, A.; Seegmiller, R.; Oxford, J. Col11a1 Regulates Bone Microarchitecture during Embryonic Development. J. Dev. Biol. 2015, 3, 158–176. [Google Scholar]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P.; Han, J.; Rowitch, D.; Soriano, P.; McMahon, A.; Sucov, H. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar]
- McKenzie, J. The first arch syndrome. Dev. Med. Child Neurol. 1966, 8, 55–66. [Google Scholar] [CrossRef]
- Nemoto, Y.; Chatani, M.; Inohaya, K.; Hiraki, Y.; Kudo, A. Expression of marker genes during otolith development in medaka. Gene Expr. Patterns 2008, 8, 92–95. [Google Scholar]
- Acke, F.; Dhooge, I.; Malfait, F.; De Leenheer, E. Hearing impairment in Stickler syndrome: A systematic review. Orphanet J. Rare Dis. 2012, 7, 84. [Google Scholar]
- Lazzaroni-Fossati, F. Fibrochondrogenesis. Arch. Fr. Pédiatr. 1978, 35, 1096–1104. [Google Scholar]
- Stevenson, D.; Vanzo, R.; Damjanovich, K.; Hanson, H.; Muntz, H.; Hoffman, R.; Bayrak-Toydemir, P. Mosaicism in Stickler syndrome. Eur. J. Med. Genet. 2012, 55, 418–422. [Google Scholar]
- Aszodi, A.; Hunziker, E.; Brakebusch, C.; Fässler, R. Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 2003, 17, 2465–2479. [Google Scholar]
- Lee, J.; Kim, G.; Kim, J. Androgen receptor is up-regulated by a BLT2-linked pathway to contribute to prostate cancer progression. Biochem. Biophys. Res. Commun. 2012, 420, 428–433. [Google Scholar]
- Wu, Y.; Chang, T.; Huang, Y.; Chen, C.; Chou, C. COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBPβ pathway and PDK1 stabilization. Oncotarget 2015, 6, 23748–23763. [Google Scholar]
- Rodriguez, R.; Seegmiller, R.; Stark, M.; Bridgewater, L.C. A type XI collagen mutation leads to increased degradation of type II collagen in articular cartilage. OsteoArthritis Cart 2004, 12, 314–320. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reeck, J.C.; Oxford, J.T. The Shape of the Jaw—Zebrafish Col11a1a Regulates Meckel’s Cartilage Morphogenesis and Mineralization. J. Dev. Biol. 2022, 10, 40. https://doi.org/10.3390/jdb10040040
Reeck JC, Oxford JT. The Shape of the Jaw—Zebrafish Col11a1a Regulates Meckel’s Cartilage Morphogenesis and Mineralization. Journal of Developmental Biology. 2022; 10(4):40. https://doi.org/10.3390/jdb10040040
Chicago/Turabian StyleReeck, Jonathon C., and Julia Thom Oxford. 2022. "The Shape of the Jaw—Zebrafish Col11a1a Regulates Meckel’s Cartilage Morphogenesis and Mineralization" Journal of Developmental Biology 10, no. 4: 40. https://doi.org/10.3390/jdb10040040
APA StyleReeck, J. C., & Oxford, J. T. (2022). The Shape of the Jaw—Zebrafish Col11a1a Regulates Meckel’s Cartilage Morphogenesis and Mineralization. Journal of Developmental Biology, 10(4), 40. https://doi.org/10.3390/jdb10040040







