Coordination of Cilia Movements in Multi-Ciliated Cells
Abstract
1. Introduction
2. Roles of Core PCP Proteins in Coordinating Cilia Orientation
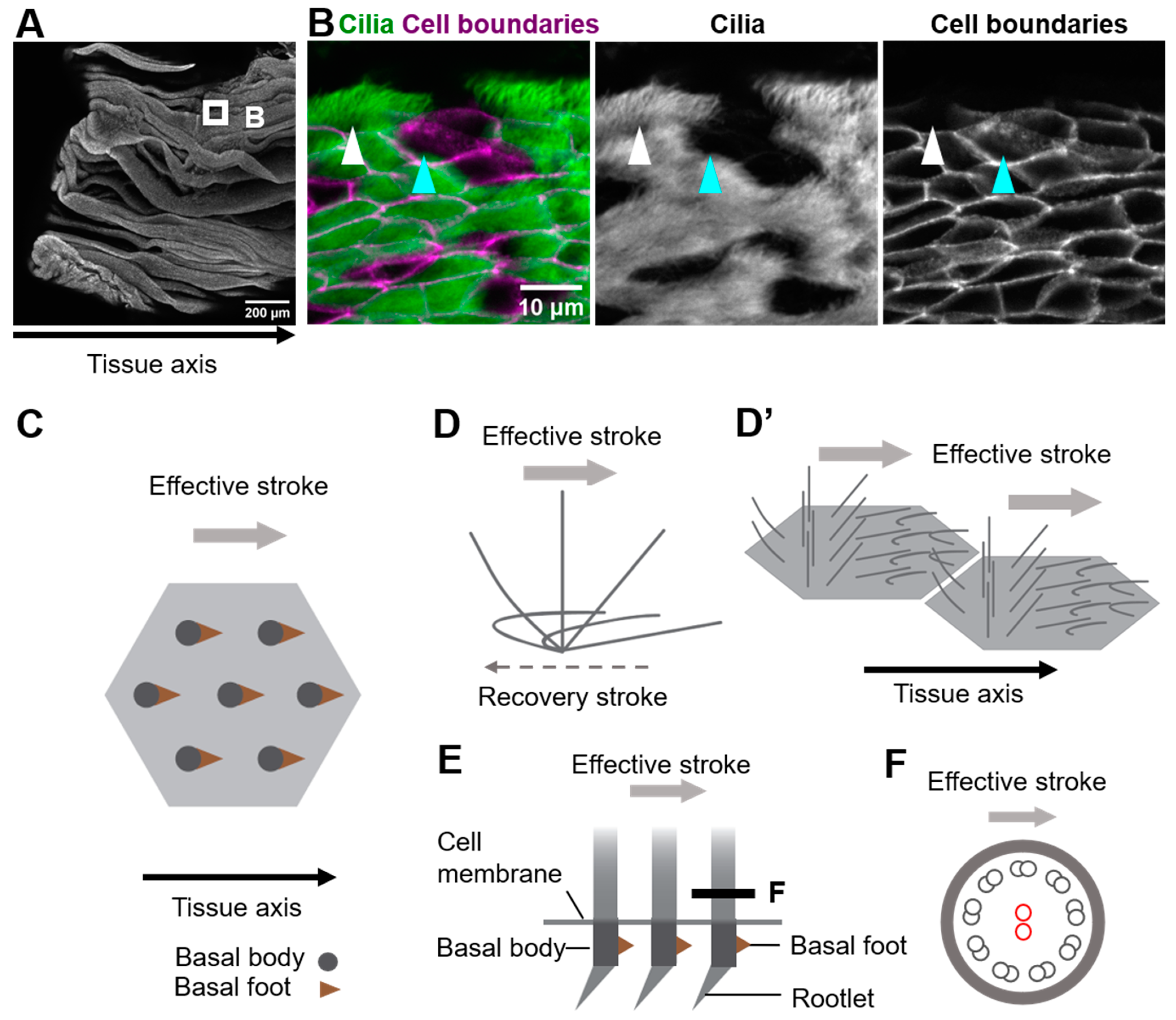
2.1. Multicellular and Tissue-Level Coordination of Cilia Orientation
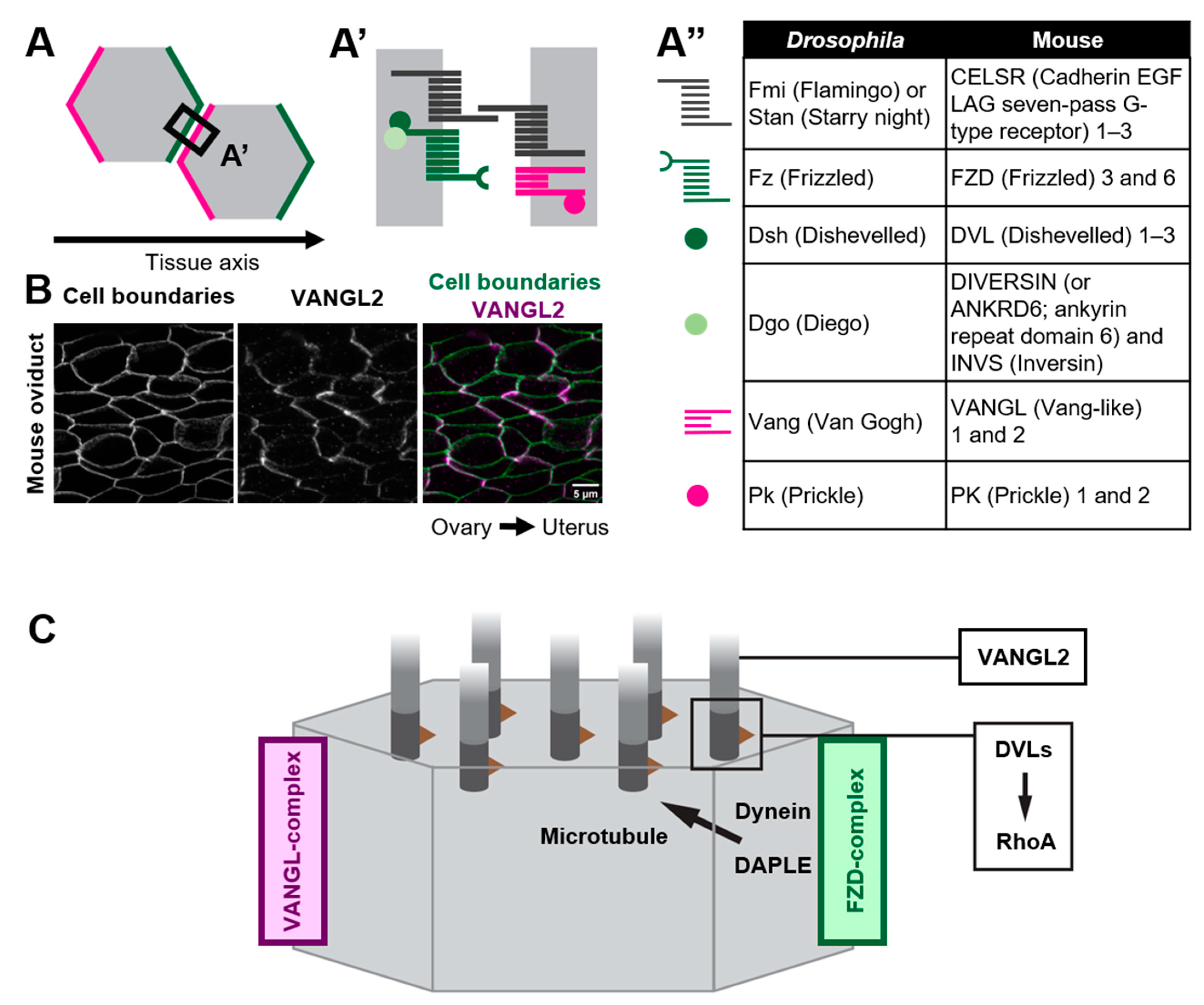
2.2. How Do Core PCP Proteins Orient Cilia?
2.3. Variable Roles of Members of Core PCP Proteins in Multi-Ciliated Cells
3. Fluid Flow Orients Cilia
4. Metachronal Wave: A Temporal Coordination of Cilia Movements
5. Roles of Cytoskeletons in Coordinating Cilia Movements
5.1. Microtubules
5.2. Actin Filaments
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, E.R.; Wallingford, J.B. Multiciliated Cells. Curr. Biol. 2014, 24, R973–R982. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Kodjabachian, L. Biology of Multiciliated Cells. Curr. Opin. Genet. Dev. 2019, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.F.; Leroux, M.R. Genes and Molecular Pathways Underpinning Ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Meunier, A.; Azimzadeh, J. Multiciliated Cells in Animals. Cold Spring Harb. Perspect. Biol. 2016, 8, a028233. [Google Scholar] [CrossRef]
- Spassky, N.; Meunier, A. The Development and Functions of Multiciliated Epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436. [Google Scholar] [CrossRef]
- Li, S.; Winuthayanon, W. Oviduct: Roles in Fertilization and Early Embryo Development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef]
- Shi, D.; Komatsu, K.; Uemura, T.; Fujimori, T. Analysis of Ciliary Beat Frequency and Ovum Transport Ability in the Mouse Oviduct. Genes Cells 2011, 16, 282–290. [Google Scholar] [CrossRef]
- Tilley, A.E.; Walters, M.S.; Shaykhiev, R.; Crystal, R.G. Cilia Dysfunction in Lung Disease. Annu. Rev. Physiol. 2015, 77, 379–406. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Ji, W.; Tang, Z.; Chen, Y.; Wang, C.; Tan, C.; Liao, J.; Tong, L.; Xiao, G. Ependymal Cilia: Physiology and Role in Hydrocephalus. Front. Mol. Neurosci. 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Satir, P.; Christensen, S.T. Structure and Function of Mammalian Cilia. Histochem. Cell Biol. 2008, 129, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.F.; Blacque, O.E.; Leroux, M.R. The Base of the Cilium: Roles for Transition Fibres and the Transition Zone in Ciliary Formation, Maintenance and Compartmentalization. EMBO Rep. 2012, 13, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Clare, D.K.; Magescas, J.; Piolot, T.; Dumoux, M.; Vesque, C.; Pichard, E.; Dang, T.; Duvauchelle, B.; Poirier, F.; Delacour, D. Basal Foot MTOC Organizes Pillar MTs Required for Coordination of Beating Cilia. Nat. Commun. 2014, 5, 4888. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Reiter, J.F. A Primer on the Mouse Basal Body. Cilia 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Klotz, C.; Bordes, N.; Laine, M.C.; Sandoz, D.; Bornens, M. A Protein of 175,000 Daltons Associated with Striated Rootlets in Ciliated Epithelia, as Revealed by a Monoclonal Antibody. Cell Motil. Cytoskelet. 1986, 6, 56–67. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Yue, G.; Adamian, M.; Bulgakov, O.; Li, T. Rootletin, a Novel Coiled-Coil Protein, Is a Structural Component of the Ciliary Rootlet. J. Cell Biol. 2002, 159, 431–440. [Google Scholar] [CrossRef]
- Hagiwara, H.; Harada, S.; Maeda, S.; Aoki, T.; Ohwada, N.; Takata, K. Ultrastructural and Immunohistochemical Study of the Basal Apparatus of Solitary Cilia in the Human Oviduct Epithelium. J. Anat. 2002, 200, 89–96. [Google Scholar] [CrossRef]
- Butler, M.T.; Wallingford, J.B. Planar Cell Polarity in Development and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 375–388. [Google Scholar] [CrossRef]
- Axelrod, J.D. Planar Cell Polarity Signaling in the Development of Left–Right Asymmetry. Curr. Opin. Cell Biol. 2020, 62, 61–69. [Google Scholar] [CrossRef]
- Shi, D.; Komatsu, K.; Hirao, M.; Toyooka, Y.; Koyama, H.; Tissir, F. Celsr1 Is Required for the Generation of Polarity at Multiple Levels of the Mouse Oviduct. Development 2014, 3, 4558–4568. [Google Scholar] [CrossRef]
- Adler, P.N. The Frizzled/Stan Pathway and Planar Cell Polarity in the Drosophila Wing. Curr. Top. Dev. Biol. 2012, 101, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Humphries, A.C.; Mlodzik, M. From Instruction to Output: Wnt/PCP Signaling in Development and Cancer. Curr. Opin. Cell Biol. 2018, 51, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Strutt, H.; Strutt, D. How Do the Fat-Dachsous and Core Planar Polarity Pathways Act Together and Independently to Coordinate Polarized Cell Behaviours? Open Biol. 2021, 11, 200356. [Google Scholar] [CrossRef] [PubMed]
- Aw, W.Y.; Devenport, D. Planar Cell Polarity: Global Inputs Establishing Cellular Asymmetry. Curr. Opin. Cell Biol. 2017, 44, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.A.; Casal, J. Planar Cell Polarity: Two Genetic Systems Use One Mechanism to Read Gradients. Development 2018, 145, dev168229. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mlodzik, M. Wnt-Frizzled/Planar Cell Polarity Signaling: Cellular Orientation by Facing the Wind (Wnt). Annu. Rev. Cell Dev. Biol. 2015, 31, 623–646. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.; Jülicher, F. Cell Flow and Tissue Polarity Patterns. Curr. Opin. Genet. Dev. 2011, 21, 747–752. [Google Scholar] [CrossRef]
- Usui, T.; Shima, Y.; Shimada, Y.; Hirano, S.; Burgess, R.W.; Schwarz, T.L.; Takeichi, M.; Uemura, T. Flamingo, a Seven-Pass Transmembrane Cadherin, Regulates Planar Cell Polarity under the Control of Frizzled. Cell 1999, 98, 585–595. [Google Scholar] [CrossRef]
- Wallingford, J.B. Planar Cell Polarity and the Developmental Control of Cell Behavior in Vertebrate Embryos. Annu. Rev. Cell Dev. Biol. 2012, 28, 627–653. [Google Scholar] [CrossRef]
- Hale, R.; Strutt, D. Conservation of Planar Polarity Pathway Function Across the Animal Kingdom. Annu. Rev. Genet. 2015, 49, 529–551. [Google Scholar] [CrossRef]
- Strutt, D.I. Asymmetric Localization of Frizzled and the Establishment of Cell Polarity in the Drosophila Wing. Mol. Cell 2001, 7, 367–375. [Google Scholar] [CrossRef]
- Axelrod, J.D. Unipolar Membrane Association of Dishevelled Mediates Frizzled Planar Cell Polarity Signaling. Genes Dev. 2001, 15, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Feiguin, F.; Hannus, M.; Mlodzik, M.; Eaton, S. The Ankyrin Repeat Protein Diego Mediates Frizzled-Dependent Planar Polarization. Dev. Cell 2001, 1, 93–101. [Google Scholar] [CrossRef]
- Tree, D.R.P.; Shulman, J.M.; Rousset, R.; Scott, M.P.; Gubb, D.; Axelrod, J.D. Prickle Mediates Feedback Amplification to Generate Asymmetric Planar Cell Polarity Signaling. Cell 2002, 109, 371–381. [Google Scholar] [CrossRef]
- Bastock, R.; Strutt, H.; Strutt, D. Strabismus Is Asymmetrically Localised and Binds to Prickle and Dishevelled during Drosophila Planar Polarity Patterning. Development 2003, 130, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Tissir, F.; Qu, Y.; Montcouquiol, M.; Zhou, L.; Komatsu, K.; Shi, D.; Fujimori, T.; Labeau, J.; Tyteca, D.; Courtoy, P.; et al. Lack of Cadherins Celsr2 and Celsr3 Impairs Ependymal Ciliogenesis, Leading to Fatal Hydrocephalus. Nat. Neurosci. 2010, 13, 700–707. [Google Scholar] [CrossRef]
- Guirao, B.; Meunier, A.; Mortaud, S.; Aguilar, A.; Corsi, J.M.; Strehl, L.; Hirota, Y.; Desoeuvre, A.; Boutin, C.; Han, Y.G.; et al. Coupling between Hydrodynamic Forces and Planar Cell Polarity Orients Mammalian Motile Cilia. Nat. Cell Biol. 2010, 12, 341–350. [Google Scholar] [CrossRef]
- Vladar, E.K.; Bayly, R.D.; Sangoram, A.M.; Scott, M.P.; Axelrod, J.D. Microtubules Enable the Planar Cell Polarity of Airway Cilia. Curr. Biol. 2012, 22, 2203–2212. [Google Scholar] [CrossRef]
- Usami, F.M.; Arata, M.; Shi, D.; Oka, S.; Higuchi, Y.; Tissir, F.; Takeichi, M.; Fujimori, T. Intercellular and Intracellular Cilia Orientation Is Coordinated by CELSR1 and CAMSAP3 in Oviduct Multi-Ciliated Cells. J. Cell Sci. 2021, 134, jcs257006. [Google Scholar] [CrossRef]
- Boutin, C.; Goffinet, A.M.; Tissir, F. Celsr1-3 Cadherins in PCP and Brain Development. Curr. Top. Dev. Biol. 2012, 101, 161–183. [Google Scholar] [CrossRef]
- Boutin, C.; Labedan, P.; Dimidschstein, J.; Richard, F.; Cremer, H.; André, P.; Yang, Y.; Montcouquiol, M.; Goffinet, A.M.; Tissir, F. A Dual Role for Planar Cell Polarity Genes in Ciliated Cells. Proc. Natl. Acad. Sci. USA 2014, 111, E3129–E3138. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Usami, F.; Komatsu, K.; Oka, S.; Abe, T.; Uemura, T.; Fujimori, T. Dynamics of Planar Cell Polarity Protein Vangl2 in the Mouse Oviduct Epithelium. Mech. Dev. 2016, 141, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.; Stubbs, J.L.; Huisman, F.; Taborek, P.; Yu, C.; Kintner, C. The PCP Pathway Instructs the Planar Orientation of Ciliated Cells in the Xenopus Larval Skin. Curr. Biol. 2009, 19, 924–929. [Google Scholar] [CrossRef]
- Chu, C.-W.; Sokol, S.Y. Wnt Proteins Can Direct Planar Cell Polarity in Vertebrate Ectoderm. Elife 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Gao, B.; Song, H.; Bishop, K.; Elliot, G.; Garrett, L.; English, M.A.; Andre, P.; Robinson, J.; Sood, R.; Minami, Y.; et al. Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Dev. Cell 2011, 20, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Koca, Y.; Collu, G.M.; Mlodzik, M. Wnt-Frizzled Planar Cell Polarity Signaling in the Regulation of Cell Motility. Curr. Top. Dev. Biol. 2022, 150, 255–297. [Google Scholar] [CrossRef]
- Fulford, A.D.; McNeill, H. Fat/Dachsous Family Cadherins in Cell and Tissue Organisation. Curr. Opin. Cell Biol. 2020, 62, 96–103. [Google Scholar] [CrossRef]
- Ma, D.; Yang, C.; Mcneill, H.; Simon, M.A. Fidelity in Planar Cell Polarity Signalling. Nature 2003, 6, 543–547. [Google Scholar] [CrossRef]
- Ambegaonkar, A.A.; Irvine, K.D. Coordination of Planar Cell Polarity Pathways through Spiny-Legs. eLife 2015, 4, e09946. [Google Scholar] [CrossRef]
- Arata, M.; Sugimura, K.; Uemura, T. Difference in Dachsous Levels between Migrating Cells Coordinates the Direction of Collective Cell Migration. Dev. Cell 2017, 42, 479–497.e10. [Google Scholar] [CrossRef]
- Aigouy, B.; Farhadifar, R.; Staple, D.B.; Sagner, A.; Röper, J.C.; Jülicher, F.; Eaton, S. Cell Flow Reorients the Axis of Planar Polarity in the Wing Epithelium of Drosophila. Cell 2010, 142, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Aw, W.Y.; Heck, B.W.; Joyce, B.; Devenport, D. Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Curr. Biol. 2016, 26, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Mii, Y.; Charras, G.; Michiue, T. Alignment of the Cell Long Axis by Unidirectional Tension Acts Cooperatively with Wnt Signalling to Establish Planar Cell Polarity. Development 2022, 149, 3–8. [Google Scholar] [CrossRef]
- Chien, Y.H.; Keller, R.; Kintner, C.; Shook, D.R. Mechanical Strain Determines the Axis of Planar Polarity in Ciliated Epithelia. Curr. Biol. 2015, 25, 2774–2784. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.; Jacobs, R.; Li, J.; Chien, S.; Kintner, C. A Positive Feedback Mechanism Governs the Polarity and Motion of Motile Cilia. Nature 2007, 447, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Takagishi, M.; Esaki, N.; Takahashi, K.; Takahashi, M. Cytoplasmic Dynein Functions in Planar Polarization of Basal Bodies within Ciliated Cells. iScience 2020, 23, 101213. [Google Scholar] [CrossRef]
- Namba, T.; Ishihara, S. Cytoskeleton Polarity Is Essential in Determining Orientational Order in Basal Bodies of Multi-Ciliated Cells. PLoS Comput. Biol. 2020, 16, 1–18. [Google Scholar] [CrossRef]
- Nakayama, S.; Yano, T.; Namba, T.; Konishi, S.; Takagishi, M.; Herawati, E.; Nishida, T.; Imoto, Y.; Ishihara, S.; Takahashi, M.; et al. Planar Cell Polarity Induces Local Microtubule Bundling for Coordinated Ciliary Beating. J. Cell Biol. 2021, 220, e202010034. [Google Scholar] [CrossRef]
- Takagishi, M.; Sawada, M.; Ohata, S.; Asai, N.; Enomoto, A.; Takahashi, K.; Weng, L.; Ushida, K.; Ara, H.; Matsui, S.; et al. Daple Coordinates Planar Polarized Microtubule Dynamics in Ependymal Cells and Contributes to Hydrocephalus. Cell Rep. 2017, 20, 960–972. [Google Scholar] [CrossRef]
- Butler, M.T.; Wallingford, J.B. Control of Vertebrate Core Planar Cell Polarity Protein Localization and Dynamics by Prickle 2. Development 2015, 142, 3429–3439. [Google Scholar] [CrossRef]
- Park, T.J.; Mitchell, B.J.; Abitua, P.B.; Kintner, C.; Wallingford, J.B. Dishevelled Controls Apical Docking and Planar Polarization of Basal Bodies in Ciliated Epithelial Cells. Nat. Genet. 2008, 40, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Meunier, A.; Huang, S.; Shimozawa, T.; Yamada, O.; Kida, Y.S.; Inoue, M.; Ito, T.; Kato, H.; Sakaguchi, M.; et al. Planar Polarity of Multiciliated Ependymal Cells Involves the Anterior Migration of Basal Bodies Regulated by Non-Muscle Myosin II. Development 2010, 137, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Ohata, S.; Nakatani, J.; Herranz-Pérez, V.; Cheng, J.G.; Belinson, H.; Inubushi, T.; Snider, W.D.; García-Verdugo, J.M.; Wynshaw-Boris, A.; Álvarez-Buylla, A. Loss of Dishevelleds Disrupts Planar Polarity in Ependymal Motile Cilia and Results in Hydrocephalus. Neuron 2014, 83, 558–571. [Google Scholar] [CrossRef]
- Shnitsar, I.; Bashkurov, M.; Masson, G.R.; Ogunjimi, A.A.; Mosessian, S.; Cabeza, E.A.; Hirsch, C.L.; Trcka, D.; Gish, G.; Jiao, J.; et al. PTEN Regulates Cilia through Dishevelled. Nat. Commun. 2015, 6, 8388. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, E.; Gsell, S.; Nommick, A.; Jomard, C.; Gras, D.; Chanez, P.; D’Ortona, U.; Kodjabachian, L.; Favier, J.; Viallat, A. Active Mucus–Cilia Hydrodynamic Coupling Drives Self-Organization of Human Bronchial Epithelium. Nat. Phys. 2020, 16, 1158–1164. [Google Scholar] [CrossRef]
- Pellicciotta, N.; Hamilton, E.; Kotar, J.; Faucourt, M.; Delgehyr, N.; Spassky, N.; Cicuta, P. Entrainment of Mammalian Motile Cilia in the Brain with Hydrodynamic Forces. Proc. Natl. Acad. Sci. USA 2020, 117, 8315–8325. [Google Scholar] [CrossRef]
- Pellicciotta, N.; Das, D.; Kotar, J.; Faucourt, M.; Spassky, N.; Lauga, E.; Cicuta, P. Cilia Density and Flow Velocity Affect Alignment of Motile Cilia from Brain Cells. J. Exp. Biol. 2020, 223, jeb229310. [Google Scholar] [CrossRef]
- Faubel, R.; Westendorf, C.; Bodenschatz, E.; Eichele, G. Cilia-Based Flow Network in the Brain Ventricles. Science 2016, 353, 176–178. [Google Scholar] [CrossRef]
- Hino, T.; Yanagimachi, R. Active Peristaltic Movements and Fluid Production of the Mouse Oviduct: Their Roles in Fluid and Sperm Transport and Fertilization. Biol. Reprod. 2019, 101, 40–49. [Google Scholar] [CrossRef]
- Knight-Jones, E.W. Relations between Metachronism and the Direction of Ciliary Beat in Metazoa. J. Cell Sci. 1954, 3, 503–521. [Google Scholar] [CrossRef]
- Elgeti, J.; Winkler, R.G.; Gompper, G. Physics of Microswimmers—Single Particle Motion and Collective Behavior: A Review. Rep. Prog. Phys. 2015, 78, 056601. [Google Scholar] [CrossRef] [PubMed]
- Elgeti, J.; Gompper, G. Emergence of Metachronal Waves in Cilia Arrays. Proc. Natl. Acad. Sci. USA 2013, 110, 4470–4475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Claydon, R.; Polin, M.; Brumley, D.R. Transitions in Synchronization States of Model Cilia through Basal-Connection Coupling. J. R. Soc. Interface 2018, 15, 20180450. [Google Scholar] [CrossRef]
- Wan, K.Y.; Goldstein, R.E. Coordinated Beating of Algal Flagella Is Mediated by Basal Coupling. Proc. Natl. Acad. Sci. USA 2016, 113, E2784–E2793. [Google Scholar] [CrossRef] [PubMed]
- Narematsu, N.; Quek, R.; Chiam, K.H.; Iwadate, Y. Ciliary Metachronal Wave Propagation on the Compliant Surface of Paramecium Cells. Cytoskeleton 2015, 72, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.E.; Hwang, P.; Huisman, F.; Taborek, P.; Yu, C.C.; Mitchell, B.J. Actin and Microtubules Drive Differential Aspects of Planar Cell Polarity in Multiciliated Cells. J. Cell Biol. 2011, 195, 19–26. [Google Scholar] [CrossRef]
- Burn, A.; Schneiter, M.; Ryser, M.; Gehr, P.; Rička, J.; Frenz, M. A Quantitative Interspecies Comparison of the Respiratory Mucociliary Clearance Mechanism. Eur. Biophys. J. 2022, 51, 51–65. [Google Scholar] [CrossRef]
- Sandoz, D.; Chailley, B.; Boisvieux-Ulrich, E.; Lemullois, M.; Laine, M.C.; Bautista-Harris, G. Organization and Functions of Cytoskeleton in Metazoan Ciliated Cells. Biol. Cell 1988, 63, 183–193. [Google Scholar] [CrossRef]
- Antoniades, I.; Stylianou, P.; Skourides, P.A. Making the Connection: Ciliary Adhesion Complexes Anchor Basal Bodies to the Actin Cytoskeleton. Dev. Cell 2014, 28, 70–80. [Google Scholar] [CrossRef]
- Chevalier, B.; Adamiok, A.; Mercey, O.; Revinski, D.R.; Zaragosi, L.E.; Pasini, A.; Kodjabachian, L.; Barbry, P.; Marcet, B. MiR-34/449 Control Apical Actin Network Formation during Multiciliogenesis through Small GTPase Pathways. Nat. Commun. 2015, 6, 8386. [Google Scholar] [CrossRef]
- Herawati, E.; Taniguchi, D.; Kanoh, H.; Tateishi, K.; Ishihara, S.; Tsukita, S. Multiciliated Cell Basal Bodies Align in Stereotypical Patterns Coordinated by the Apical Cytoskeleton. J. Cell Biol. 2016, 214, 571–586. [Google Scholar] [CrossRef]
- Tateishi, K.; Nishida, T.; Inoue, K.; Tsukita, S. Three-Dimensional Organization of Layered Apical Cytoskeletal Networks Associated with Mouse Airway Tissue Development. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.; Avolio, J.; Satir, P. The Cytoskeleton of the Apical Border of the Lateral Cells of Freshwater Mussel Gill: Structural Integration of Microtubule and Actin Filament-Based Organelles. J. Cell Sci. 1984, 68, 1–33. [Google Scholar] [CrossRef]
- Lemullois, M.; Marty, M.C. Immunocytochemical Study of the Formation of Striated Rootlets during Ciliogenesis in Quail Oviduct. J. Cell Sci. 1990, 95, 423–432. [Google Scholar] [CrossRef]
- Kunimoto, K.; Yamazaki, Y.; Nishida, T.; Shinohara, K.; Ishikawa, H.; Hasegawa, T.; Okanoue, T.; Hamada, H.; Noda, T.; Tamura, A.; et al. Coordinated Ciliary Beating Requires Odf2-Mediated Polarization of Basal Bodies via Basal Feet. Cell 2012, 148, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Kano, A.; Aoki, T.; Ohwada, N.; Takata, K. Localization of γ-Tubulin to the Basal Foot Associated with the Basal Body Extending a Cilium. Histochem. J. 2000, 32, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Matsukawa-Usami, F.; Fujimori, T.; Kimura, T.; Ide, T.; Yamamoto, T.; Shibata, T.; Onoue, K.; Okayama, S.; Yonemura, S.; et al. Tracheal Motile Cilia in Mice Require CAMSAP3 for the Formation of Central Microtubule Pair and Coordinated Beating. Mol. Biol. Cell 2021, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.M.; Takahashi, S.; Brotslaw, E.J.; Ahmad, A.; Ferrer, E.; Procissi, D.; Richter, C.P.; Cheatham, M.A.; Mitchell, B.J.; Zheng, J. CAMSAP3 Facilitates Basal Body Polarity and the Formation of the Central Pair of Microtubules in Motile Cilia. Proc. Natl. Acad. Sci. USA 2020, 117, 13571–13579. [Google Scholar] [CrossRef]
- Kimura, T.; Saito, H.; Kawasaki, M.; Takeichi, M. CAMSAP3 Is Required for MTORC1-Dependent Ependymal Cell Growth and Lateral Ventricle Shaping in Mouse Brains. Development 2021, 148, dev195073. [Google Scholar] [CrossRef]
- Basquin, C.; Ershov, D.; Gaudin, N.; Vu, H.T.K.; Louis, B.; Papon, J.F.; Orfila, A.M.; Mansour, S.; Rink, J.C.; Azimzadeh, J. Emergence of a Bilaterally Symmetric Pattern from Chiral Components in the Planarian Epidermis. Dev. Cell 2019, 51, 516–525.e5. [Google Scholar] [CrossRef]
- Hoffman, H.K.; Prekeris, R. Roles of the Actin Cytoskeleton in Ciliogenesis. J. Cell Sci. 2022, 135, jcs259030. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, T.; Wiegel, J.; Bergen, M.D.; Helmstädter, M.; Epting, D.; Paolini, A.; Çiçek, Ö.; Radziwill, G.; Engel, C.; Brox, T.; et al. Microridge-like Structures Anchor Motile Cilia. Nat. Commun. 2022, 13, 2056. [Google Scholar] [CrossRef]
- Mahuzier, A.; Shihavuddin, A.; Fournier, C.; Lansade, P.; Faucourt, M.; Menezes, N.; Meunier, A.; Garfa-traoré, M.; Carlier, M.; Voituriez, R.; et al. Ependymal Cilia Beating Induces an Actin Network to Protect Centrioles against Shear Stress. Nat. Commun. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Dema, A.; van Haren, J. Lights, Cytoskeleton, Action: Optogenetic Control of Cell Dynamics. Curr. Opin. Cell Biol. 2020, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alabi, S.B.; Crews, C.M. Major Advances in Targeted Protein Degradation: PROTACs, LYTACs, and MADTACs. J. Biol. Chem. 2021, 296, 100647. [Google Scholar] [CrossRef]
- Brette, R. Integrative Neuroscience of Paramecium, a “Swimming Neuron”. eNeuro 2021, 8, ENEURO.0018-21.2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arata, M.; Usami, F.M.; Fujimori, T. Coordination of Cilia Movements in Multi-Ciliated Cells. J. Dev. Biol. 2022, 10, 47. https://doi.org/10.3390/jdb10040047
Arata M, Usami FM, Fujimori T. Coordination of Cilia Movements in Multi-Ciliated Cells. Journal of Developmental Biology. 2022; 10(4):47. https://doi.org/10.3390/jdb10040047
Chicago/Turabian StyleArata, Masaki, Fumiko Matsukawa Usami, and Toshihiko Fujimori. 2022. "Coordination of Cilia Movements in Multi-Ciliated Cells" Journal of Developmental Biology 10, no. 4: 47. https://doi.org/10.3390/jdb10040047
APA StyleArata, M., Usami, F. M., & Fujimori, T. (2022). Coordination of Cilia Movements in Multi-Ciliated Cells. Journal of Developmental Biology, 10(4), 47. https://doi.org/10.3390/jdb10040047






