Impermeable and Compliant: SIBS as a Promising Encapsulant for Ionically Electroactive Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Fabrication
2.2. Encapsulation Process
2.3. Material Characterization
2.4. Permeability Measurement
2.5. Actuation Test
3. Results and Discussions
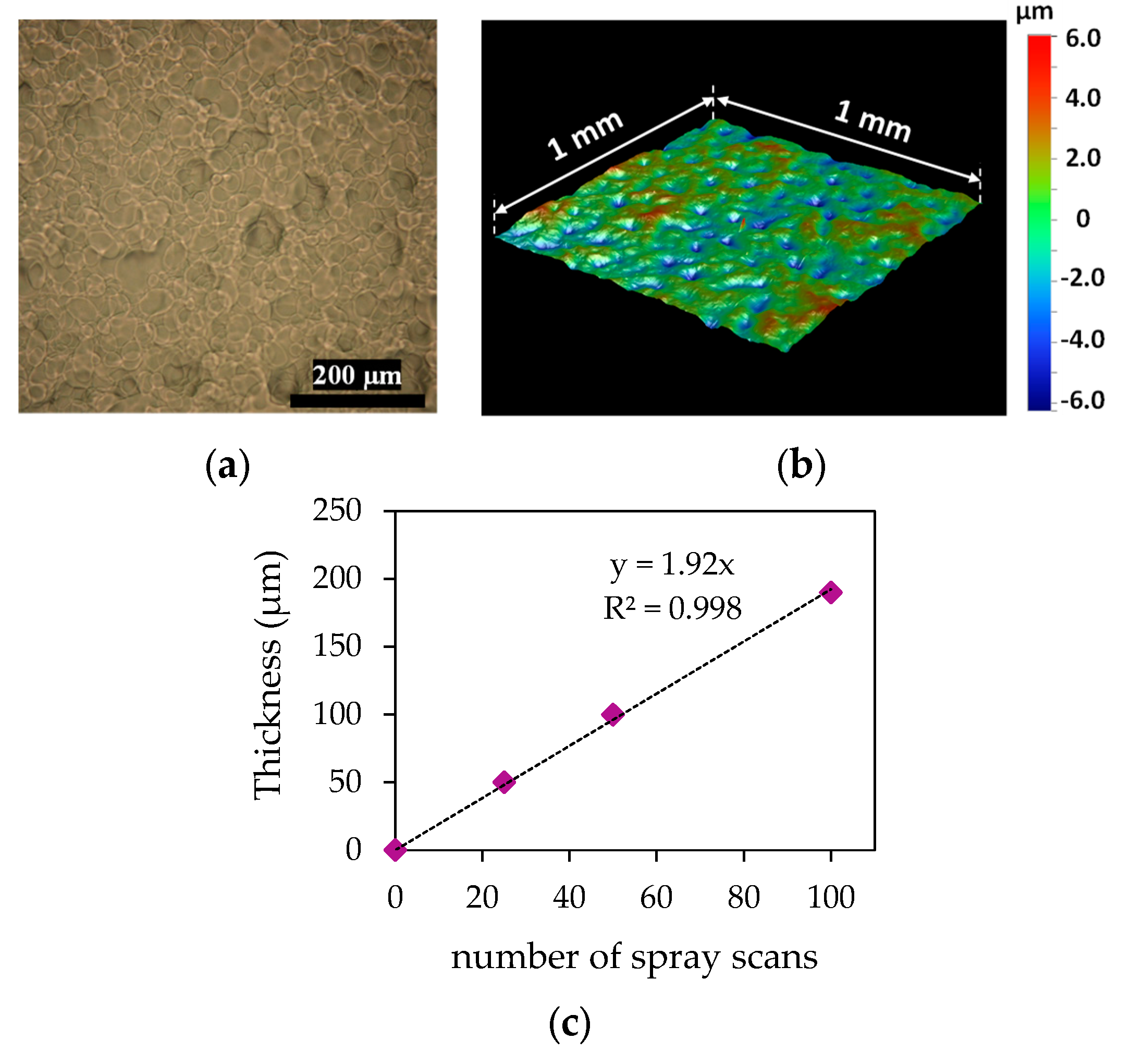
3.1. SIBSTAR Film Characterization
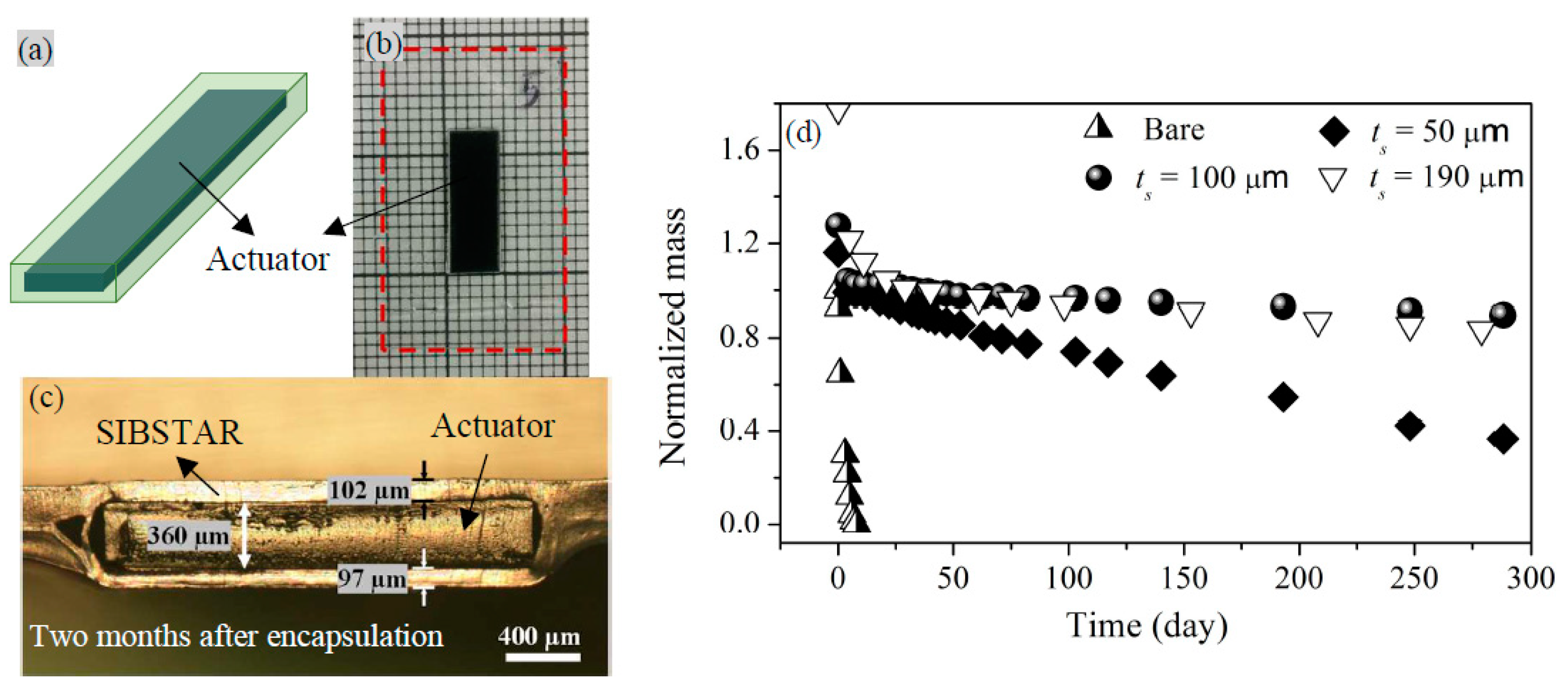
3.2. Characterization of Permeant Mass Transfer in SIBSTAR Sprayed Films
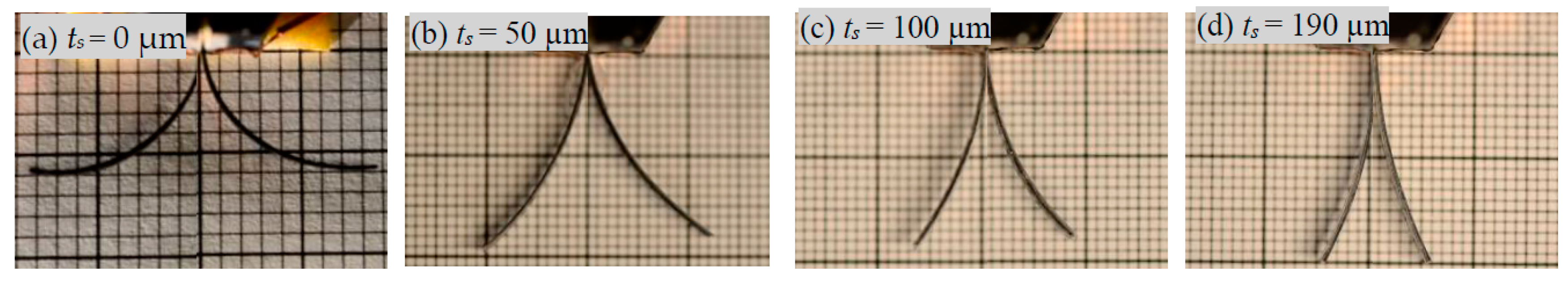
3.3. Effect of Encapsulation on the Extent to Which the Trilayer Can Bend
3.4. Effect of Encapsulation on the Cycling Response of Tri-Layer Actuators in Flowing Air
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
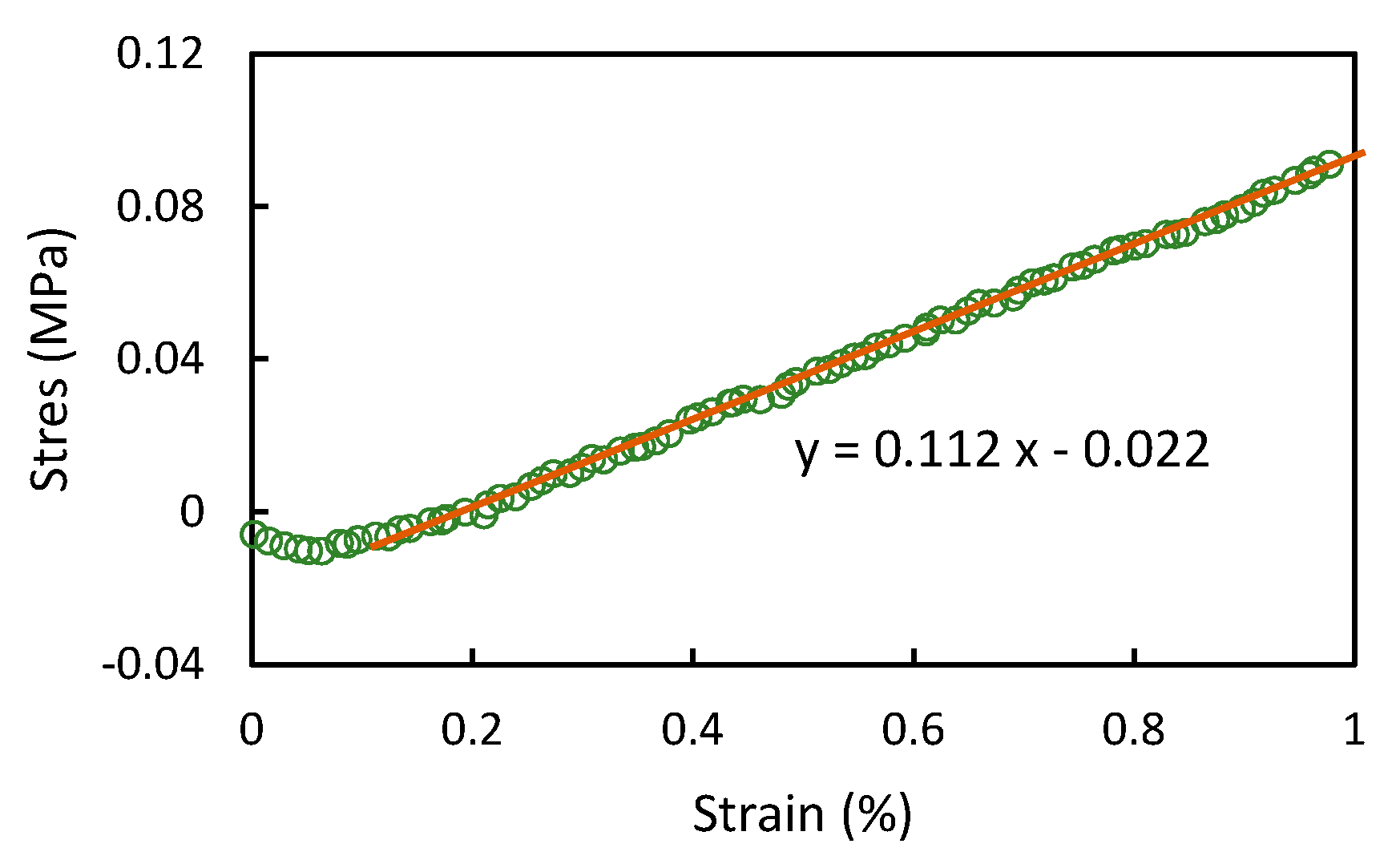
Appendix C

References
- Naficy, S.; Stoboi, N.; Whitten, P.G.; Spinks, G.M.; Wallace, G.G. Evaluation of encapsulating coatings on the performance of polypyrrole actuators. Smart Mater. Struct. 2013, 22, 075005. [Google Scholar] [CrossRef]
- Takalloo, S.E.; Fannir, A.; Nguyen, G.T.M.; Plesse, C.; Vidal, F.; Madden, J.D.W. Evaluating performance of wet unencapsulated PEDOT trilayer actuators operating in air and water. Multifunct. Mater. 2019, 2, 014003. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, I.T.; Lee, H.-Y.; Kim, Y.H. Performance improvement of an ionic polymer–metal composite actuator by parylene thin film coating. Smart Mater. Struct. 2006, 15, 1540–1546. [Google Scholar] [CrossRef]
- Lei, H.; Li, W.; Tan, X. Encapsulation of ionic polymer-metal composite (IPMC) sensors with thick parylene: Fabrication process and characterization results. Sens. Actuators A 2014, 217, 1–12. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Dobashi, Y.; Preston, C.; Wyss, J.K.M.; Mirabbasi, S.; Madden, J.D.W. Bend, stretch, and touch: Locating a finger on an actively deformed transparent sensor array. Sci. Adv. 2017, 3, e1602200. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Schougaard, S.B.; Goodenough, J.B. Conducting-Polymer/Iron-Redox- Couple Composite Cathodes for Lithium Secondary Batteries. Adv. Mater. 2007, 19, 848–851. [Google Scholar] [CrossRef]
- Mirvakili, S.M.; Hunter, I.W. Vertically Aligned Niobium Nanowire Arrays for Fast-Charging Micro-Supercapacitors. Adv. Mater. 2017, 29, 1700671. [Google Scholar] [CrossRef] [PubMed]
- Somani, P.; Mandale, A.B.; Radhakrishnan, S. Study and development of conducting polymer-based electrochromic display devices. Acta Mater. 2000, 48, 2859–2871. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Jaakson, P.; Aabloo, A.; Tamm, T. Encapsulation of Ionic Electroactive Polymers: Reducing the Interaction with Environment; Bar-Cohen, Y., Vidal, F., Eds.; International Society for Optics and Photonics: Las Vegas, NV, USA, 2016. [Google Scholar]
- Shoa, T.; Madden, J.D.; Fekri, N.; Munce, N.R.; Yang, V.X.D. Conducting polymer based active catheter for minimally invasive interventions inside arteries. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, USA, 20–24 August 2008; pp. 2063–2066. [Google Scholar]
- Smela, E. Conjugated Polymer Actuators for Biomedical Applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Ebrahimi Takalloo, S.; Seifi, H.; Madden, J.D.W. Design of Ultra-Thin High Frequency Trilayer Conducting Polymer Micro-Actuators for Tactile Feedback Interfaces; Bar-Cohen, Y., Ed.; International Society for Optics and Photonics: Portland, OR, USA, 2017. [Google Scholar]
- Fukushima, T.; Asaka, K.; Kosaka, A.; Aida, T. Fully Plastic Actuator through Layer-by-Layer Casting with Ionic-Liquid-Based Bucky Gel. Angew. Chem. Int. Ed. 2005, 44, 2410–2413. [Google Scholar] [CrossRef] [PubMed]
- Woishnis, W. Permeability and other Film Properties of Plastics and Elastomers; Plastics Design Library: Norwich, NY, USA, 1995; ISBN 978-1-884207-14-3. [Google Scholar]
- Crompton, T.R. Mechanical Properties of Polymers. In Physical Testing of Plastics; Smithers Rapra: Shrewsbury, UK, 2012. [Google Scholar]
- Noda, K.; Kimura, K.; Fukuda, R.; Tawada, M. Performance Characterization of Styrene-Isobutylene Block Copolymer; Thermoplastic Elastomers: Munich, Germany, 2006. [Google Scholar]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.-M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef] [PubMed]
- TAXUS™ Liberté™ Paclitaxel-Eluting Coronary Stent System. Available online: http://www.bostonscientific.com/en-US/products/stents--coronary/taxus-liberte-coronary-stent-system.html (accessed on 23 May 2019).
- Kamath, K.R.; Barry, J.J.; Miller, K.M. The TaxusTM drug-eluting stent: A new paradigm in controlled drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K.; Weber, B.A.; Kwon, Y.; et al. The development of a micro-shunt made from poly(styrene- block -isobutylene- block -styrene) to treat glaucoma: SIBS-BASED INNFOCUS MICROSHUNT®. J. Biomed. Mater. Res. Part B 2017, 105, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Gallocher, S.L.; Aguirre, A.F.; Kasyanov, V.; Pinchuk, L.; Schoephoerster, R.T. A novel polymer for potential use in a trileaflet heart valve. J. Biomed. Mater. Res. Part B 2006, 79B, 325–334. [Google Scholar] [CrossRef] [PubMed]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Ebrahimi Takalloo, S. Actualizing Fast Conducting Polymer Actuators: Design Optimization, Fabrication, and Encapsulation. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2019. [Google Scholar]
- Girotto, C.; Rand, B.P.; Genoe, J.; Heremans, P. Exploring spray coating as a deposition technique for the fabrication of solution-processed solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 454–458. [Google Scholar] [CrossRef]
- Perevosnik, K.A. Investigation pf the Mechanical and Thermal Properties of poly(styrene-block-isobutylene-block-styrene) (SIBS) and Its Blends with thymine-functionalized Polystyrene. Ph.D. Thesis, University of Akron, Akron, OH, USA, 2008. [Google Scholar]
- Daynes, H.A. The Process of Diffusion through a Rubber Membrane. Proc. R. Soc. Lond. Ser. A 1920, 97, 286–307. [Google Scholar] [CrossRef]
- Chen, Z.; To, J.W.F.; Wang, C.; Lu, Z.; Liu, N.; Chortos, A.; Pan, L.; Wei, F.; Cui, Y.; Bao, Z. A Three-Dimensionally Interconnected Carbon Nanotube–Conducting Polymer Hydrogel Network for High-Performance Flexible Battery Electrodes. Adv. Energy Mater. 2014, 4, 1400207. [Google Scholar] [CrossRef]


| Material’s Name | Abbreviation or Trade Name | Young’s Modulus | Strain at Yield or Fracture Strain | WVTRn | |
|---|---|---|---|---|---|
| (MPa) | (%) | (g mm m−2 day−1) | (MPa g mm m−2 day−1) | ||
| poly(styrene-b-isobutylene-b-styrene) | SIBSTAR 103T 1 | 11.2 2 | 700 | 0.24 | 2.7 |
| poly(styrene-b-ethylene-butylene-b-styrene) | SEBS, Kraton G27011 | 20 | 800 | 47 | 940 |
| Polyurethane | PU 1 | 55 | 700 | 2.2 | 120 |
| Low-density Polyethylene | LDPE | 250 | 19 | 0.5 | 125 |
| Polyvinylidene Chloride | PVDC, Saran 560 | 470 | 5 | 0.02 | 9.4 |
| High-density Polyethylene | HDPE | 1250 | 15 | 0.12 | 150 |
| Polyethylene terephthalate | PET | 2300 | 3.5 | 0.39 | 900 |
| Polyimide | PI, Upilex R | 2500 | 4 | 0.56 | 1400 |
| Poly(p-xylylene)- type C | Parylene C | 2800 | 2 | 0.08 | 225 |
| Polyvinyl chloride | PVC | 3000 | 3.5 | 1.18 | 3540 |
| Liquid Crystal | LC, Vectra A950 | 10000 | 2 | 0.05 | 500 |
| Polyepoxides | Epoxy | 80000 | 1.3 | 0.8 | 64,000 |
| Sample | Encapsulating Layer’s Thickness | Tri-Layer’s | Initial PC Mass | PC Mass Decay Rate | Time to Lose 20% of Stored PC | Operation Lifetime | |||
|---|---|---|---|---|---|---|---|---|---|
| Surface Area | Side Area | Total Area | |||||||
| (µm) | (mm2) | (mg) | (µg/day) | (µg m m−2 day−1) | (days) | (days) | |||
| S1 | 0 | 144.4 | 12.5 | 156.9 | 13.7 | - | - | 0.5 | 4 |
| S2 | 150.3 | 13 | 163.3 | 14.2 | - | - | 0.5 | 4 | |
| S3 | 161.8 | 13.8 | 175.6 | 15.6 | - | - | 0.5 | 4 | |
| S4 | 50 | 94.5 | 10.2 | 104.7 | 8.8 | 19 | 9.1 | 65 | 390 * |
| S5 | 96 | 10.2 | 106.2 | 8.9 | 20.3 | 9.6 | 65 | 370 * | |
| S6 | 96 | 10.2 | 106.4 | 9 | 50.5 | 23.9 | 35 | 300 | |
| S7 | 100 | 96 | 11.5 | 107.5 | 8.8 | 16.2 | 15.6 | 110 | 460 * |
| S8 | 120 | 12.2 | 132.2 | 11 | 4 | 3.1 | 550 * | 2350 * | |
| S9 | 100 | 11.7 | 111.7 | 8.9 | 4.6 | 4.4 | 390 * | 1650 * | |
| S10 | 190 | 126 | 13.3 | 139.3 | 10.5 | 6.4 | 9.9 | 330 * | 1400 * |
| S11 | 112 | 13 | 125 | 10.45 | 19.1 | 29.6 | 80 | 460 * | |
| S12 | 165 | 14.8 | 179.8 | 14.25 | 17.4 | 20.3 | 180 | 700 * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi Takalloo, S.; Fannir, A.; Nguyen, G.T.M.; Plesse, C.; Vidal, F.; Madden, J.D.W. Impermeable and Compliant: SIBS as a Promising Encapsulant for Ionically Electroactive Devices. Robotics 2019, 8, 60. https://doi.org/10.3390/robotics8030060
Ebrahimi Takalloo S, Fannir A, Nguyen GTM, Plesse C, Vidal F, Madden JDW. Impermeable and Compliant: SIBS as a Promising Encapsulant for Ionically Electroactive Devices. Robotics. 2019; 8(3):60. https://doi.org/10.3390/robotics8030060
Chicago/Turabian StyleEbrahimi Takalloo, Saeedeh, Adelyne Fannir, Giao T. M. Nguyen, Cedric Plesse, Frederic Vidal, and John D. W. Madden. 2019. "Impermeable and Compliant: SIBS as a Promising Encapsulant for Ionically Electroactive Devices" Robotics 8, no. 3: 60. https://doi.org/10.3390/robotics8030060
APA StyleEbrahimi Takalloo, S., Fannir, A., Nguyen, G. T. M., Plesse, C., Vidal, F., & Madden, J. D. W. (2019). Impermeable and Compliant: SIBS as a Promising Encapsulant for Ionically Electroactive Devices. Robotics, 8(3), 60. https://doi.org/10.3390/robotics8030060





