Abstract
Atrial fibrillation, the most prevalent cardiac arrhythmia, is treated by catheter ablation to isolate electrical triggers. Clinical trials on robotic catheter systems hold promise for improving the safety and efficacy of the procedure. However, expense and proprietary designs hinder accessibility to such systems. This paper details an open-source, modular, three-degree-of-freedom robotic platform for teleoperating commercial ablation catheters through joystick navigation. We also demonstrate a catheter-agnostic handle interface permitting customization with commercial catheters. Collaborating clinicians performed benchtop targeting trials, comparing manual and robotic catheter navigation performance. The robot reduced task duration by 1.59 s across participants and five trials. Validation through mean motion jerk analysis revealed 35.2% smoother robotic navigation for experts (≥10 years experience) compared to the intermediate group. Yet, both groups achieved smoother robot motion relative to the manual approach, with the experts and intermediates exhibiting 42.2% and 13.6% improvements, respectively. These results highlight the potential of this system for enhancing catheter-based procedures. The source code and designs of CardioXplorer have been made publicly available to lower boundaries and drive innovations that enhance procedure efficacy beyond human capabilities.
1. Introduction
Atrial fibrillation (AF) is caused by irregular electrical signals that induce an irregular cardiac rhythm. Yet, the pathophysiology of AF is complex, and the underlying mechanisms of advanced/persistent AF remain incompletely understood. Consequently, it is associated with considerable patient morbidity and mortality, disabling symptoms, and stroke risk [1]. Catheter ablation is now considered a first-line treatment for paroxysmal AF. However, it faces challenges in consistency and efficacy, contributing to significant re-occurrence rates of up to 50% [1]. The rising incidence of 30 million people diagnosed with AF worldwide, coupled with the need for repeat catheterization, has set off a healthcare problem of epidemic proportions and significant economic costs [1,2,3]. The alarming prevalence of this condition calls for imminent collaborative efforts toward the clinical translation of effective solutions.
Robotic catheter ablation systems hold promise for enhancing precision, catheter stability, and patient outcomes and reducing total radiation exposure to the patient and operator [4]. An extensive review of the successes and challenges of clinical trials for these systems is detailed in [5]. These commercial systems, including Niobe® (Stereotaxis Inc., St. Louis, MO, USA) [6] and Sensei® X (Hansen Medical, Inc., Mountain View, CA, USA) [7], require access to proprietary systems costing over USD 100,000, limiting their widespread adoption due to expense and lack of versatility [5,8]. As such, the goal of Amigo™ RCS (Catheter Precision, Inc., Mount Olive, NJ, USA) was to address these challenges and provide a more practical and lower-profile remote catheter-manipulation solution [9]. However, these remain closed systems of mechanical designs and algorithms, limiting community contributions due to constrained accessibility. As a result, systems continue to feature non-automated teleoperation [4,5]. Automated navigation systems have only been explored in prototype catheters in research [10], yet have remained constrained to benchtop environments for over a decade and overlook clinical workflow challenges. Compounding this issue is the insight gained from [11], which illustrates that even well-integrated systems face such challenges, contributing to adverse patient outcomes.
Prior works have explored catheter modifications, such as soft artificial actuators [12]. Moreover, four main types of transmission systems for actuator-driven catheters have been investigated: cable-driven actuators [13], shape memory alloys (SMA) [14], soft fluidic actuators (SFAs) [15], and magnetic actuators [16]. However, their high-cost, closed-source solutions restrict transparency as well as research and clinical adoption [8]. Actuating commercial tools through robotic manipulation can preserve clinical workflows while enabling sensing and control refinements, similar to the approach taken by the Amigo system [9] and leveraged in related work [17,18].
Open-source projects, such as the open-source hardware platform for soft robotics (Soft Robotics Toolkit, Paradox Robotics, New York City, NY, USA) [19], the Open Framework Architecture (SOFA Framework) [20], and SoftRobot [21], collectively foster collaboration across groups to solve obstacles restricting clinical translation [22].
We hypothesize that an open-source modular robot actuating existing clinical catheters would provide enhanced motion smoothness and accuracy to meet clinical needs. Proposed in this paper is CardioXplorer, which validates this through motion jerk analysis, phantom studies, and comprehensive clinician feedback. Thus, innovation can be centered on sensing, navigation, and safety.
The contributions of this article are as follows:
- The entire mechanical and software architecture is fully open-source (CC0-1.0 license) to reduce the focus on mechanical design and encourage community-driven advancement of the platform to address higher-level challenges, especially clinical translation [23].
- A modularized architecture, where the isolated actuator modules for each degree of freedom (DoF) facilitate seamless reconfiguration for similar tasks beyond the initial targeted application of AF ablation; this versatility can boost adoption across domains and integration of the solution with existing software. It overcomes the limitations set by systems designed for specific procedures and those constrained to single-catheter models.
- A catheter-agnostic design to accommodate a variety of commercial catheters, promoting interoperability and accessibility—this is a highlighted limitation of most vascular robots [24]. While the CathROB system is an open platform, it is only compatible with commercially available standard electrophysiology catheters and is limited by its lack of customization and adaptability with various endoscopic catheters for remote navigation outside the scope of standard arrhythmias [18]. Therefore, our goal was to address the shortcomings in prior works that suffer from poor compatibility with interventional devices, either relying on modified research catheters or being limited to specific commercial models [24].
- We demonstrate the system’s capabilities through extensive multi-user clinical evaluation, emphasizing a human-centered design approach to account for clinical needs, practices, and the importance of its integration with the catheterization lab and usability assessments from clinical experts. We capture the system’s performance through benchtop phantom experiments and direct assessment by experienced clinicians with 0–25 years of experience. In conjunction with this article, we have released the presented work in [23].
2. System Description
CardioXplorer is designed for teleoperated catheter control within the left atrium (LA) via a joystick interface. The system provides three DoFs: axial insertion/retraction, catheter handle rotation, and catheter tip. The robot platform is divided into three main parts: a peripheral unit, a controller unit, and an operator workstation.
Section 2.1 introduces the peripheral unit, including the robot manipulator and its mechanical design. Section 2.2 reviews the overarching software architecture tying the motion control (velocity open-loop) and data pipelines. Finally, Section 3 describes the real-time visual feedback presented to the operator.
2.1. Peripheral Unit: Robot
The modular mechanical design (Figure 1) comprises three key modules: a linear module to advance/retract the catheter, a rotation module for axial and knob rotation, and a catheter module housing the handle. The purpose of modularity is to create a set of modules capable of accommodating a variety of common catheters to facilitate reusability.
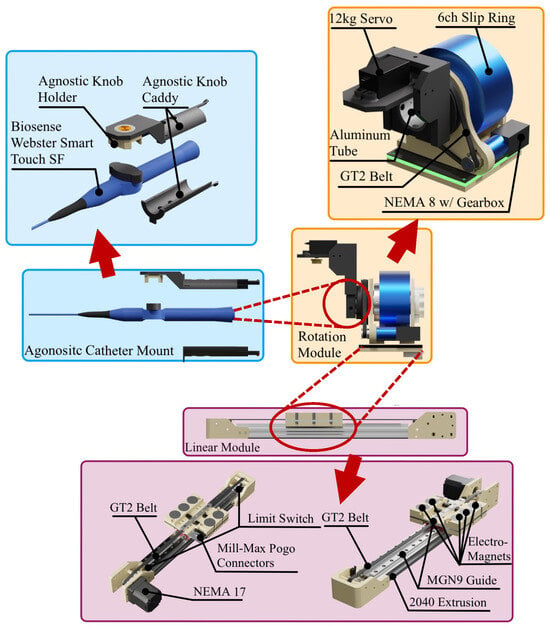
Figure 1.
Schematic of the modular robot design, composed of three modules: linear module, rotation module, and agnostic catheter mount module.
The robot architecture (Figure 2) was designed using computer-aided design (CAD) software (Fusion 360 (2.0.18961), Autodesk, San Francisco, CA, USA). Its components were then fabricated via fused deposition modeling (FDM) (Prusa i3 MK3S, Prusa Research, Prague, Czech Republic) and stereolithography (SLA) (Form 3+, Formlabs, Somerville, MA, USA) using Tough 2000 Resin (Formlabs, Somerville, MA, US). The off-the-shelf components used in the robot assembly resulted in an estimated manufacturing cost of USD 700 [23], easing accessibility over proprietary solutions [5,6,7,9].
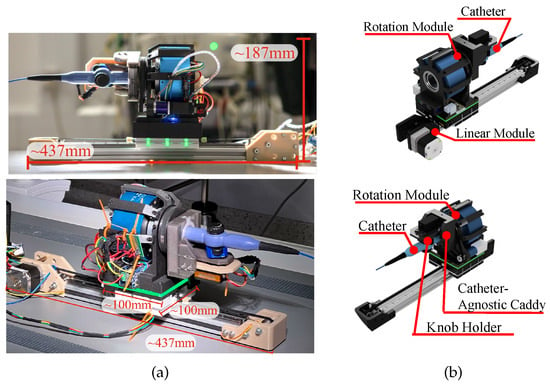
Figure 2.
Comparison of (a) manufactured robot and (b) 3D CAD rendering.
2.1.1. Catheter-Agnostic Handle Gripper
The catheter gripper in Figure 3a was molded to match the catheter handle geometries using medical-grade, two-part platinum silicone (Smooth-Sil™ 936, Smooth-On Inc., Macungie, PA, USA). The modeled catheter here a the bi-directional, tendon-driven Thermocool® SmartTouch™ SF Ablation Catheter (Biosense Webster Inc., Irvine, CA, USA). The silicone inserts are fitted on the inside of the gripping mechanism to conform to varying catheter handles (illustrated in Figure 3b), contributing to a universal, adaptable catheter interface, as demonstrated in Figure 4. Thumbscrews permit straightforward mounting and detachment of the disposable, single-use catheter from the rotation module. Anti-abrasion couplings made from the combination of thermoplastic polyurethane (TPU) and screws prevent damage during attachment to the servo, yet offer the required rigidity for stable manipulation.
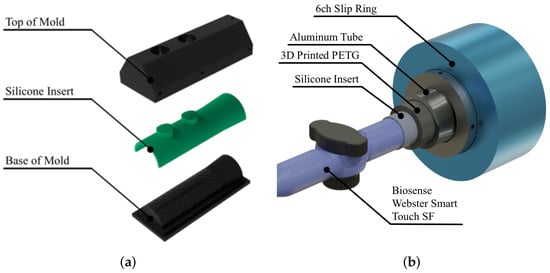
Figure 3.
Schematic of gripper mechanism. (a) Development of silicone mold based on catheter handle geometry. (b) Adaptive gripper assembly.
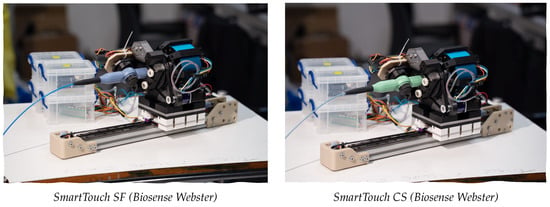
Figure 4.
Illustration of two different commercial ablation catheters in the catheter-agnostic gripping interface.
Part of the open-source designs contributed to the community is a set of two other catheters (Figure 5): an 8 mm nonirrigated catheter (Blazer™ II XP, Boston Scientific, Marlborough, MN) and a Webster™ CS Bi-Directional Catheter with EZ Steer™ (Biosense Webster, Inc., Irvine, CA, USA).

Figure 5.
Open-source CAD models of clinical catheters available at [23].
An ATI Nano17 force transducer (ATI Industrial Automation, Apex, NC, USA) was used to measure the necessary torque for knob actuation, which translates to bi-directional tip movement (Figure 6a). Using the turning handle, the knob was rotated 60° clockwise, briefly held in the neutral position, and then rotated 60° anti-clockwise. Figure 6b defines the maximum knob torque to be approximately 6 kgcm; therefore, a servo with a holding torque of 12 kgcm was secured to the catheter knob. The servo holds its position using 8-bit depth pulse-width modulation (PWM).
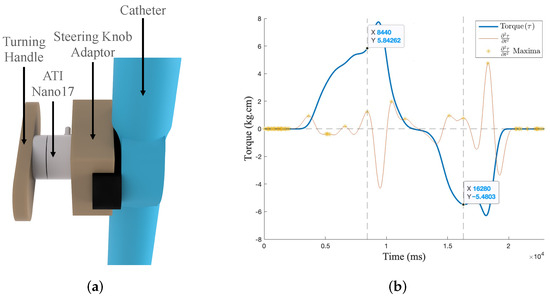
Figure 6.
Measurement of required torque to actuate knob. (a) Securement to knob. (b) Turning torque over time for ±60° knob rotation.
2.1.2. Range-of-Motion Characterization
The system affords a 220 mm insertion depth with unlimited axial rotation via a slip ring. The NEMA-8 bipolar (19:1 gearbox reduction) stepper motor (OMC Corporation Limited, Nanjing, China) driving the axial rotation of the catheter handle achieves 0.5° incremental rotations up to 9.375 rps. The servo provides ±60° knob actuation, which corresponds to bi-directional tip articulation with 0.5° precision. The 80 N axial driving force is supplied by the 4418M NEMA-17 bipolar stepper motor (Lin Engineering Inc., Morgan Hill, CA, USA) (0.415 Nm at 4.8 mm) in the linear module, and translates to velocities up to ±25 mm/s (20T GT2 gear at ±0.625 rps)—suited for cardiac navigation [25].
2.2. Software Architecture for Telerobotic Control
Figure 7 depicts the three abstraction levels—hardware, application, and control.
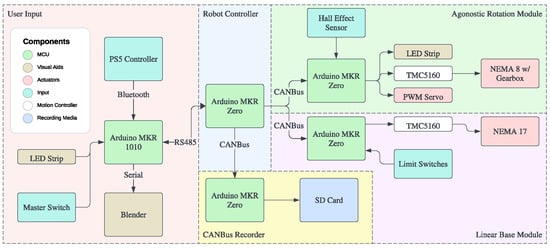
Figure 7.
Software architecture depicting the five phases of control: user input, robot controller, CANBus recorder, rotation module, and linear module. The communication protocols are identified with the arrows.
The hardware layer interfaces the components detailed in [23]. Motor controllers (Trinamic TMC5160) and a digital Hall effect sensor were leveraged to initialize the robot homing routine and determine the catheter configurations from the motor positions. A light-emitting diode (LED) strip in the carriage provides visual status indicators about the robot’s homing state. The robot was designed with wide operating voltage margins (12–36 V) to improve accessibility, but was only tested up to 20 V in this work. The typical voltage used in this work was 12 V.
The application layer consists of a graphical user interface (GUI), which runs on a host PC (AMD Ryzen 9 6900HX processor with 16 GB RAM) (Razer Inc., Irvine, CA, USA), on the open-source Blender 4.0 (Blender Foundation, Amsterdam, The Netherlands). The multi-threaded software renders a simulation of the robot hardware at 10 Hz by combining velocity commands from the joystick and module states. We describe the joystick controls in [23].
The communication layer was based on the Controller Area Network (CAN) Bus protocol, offering parallel processing in distributed controllers while connecting subsystems [26]. The protocol facilitates modular low-level communication (capable of running each of the robot’s onboard modules) with 10 Hz open-loop velocity control for smooth teleoperation (setup displayed in Figure 8). Its priority-based attribution ensures the avoidance of collisions while maintaining real-time, deterministic messaging.
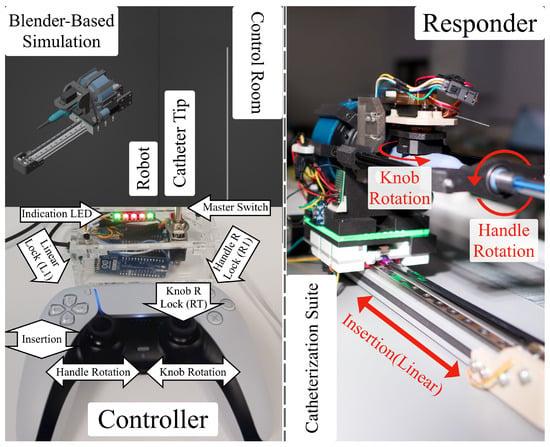
Figure 8.
Overview of clinician workstation for teleoperated catheter navigation from the control room of the catheterization lab.
Long-range data transmission occurs between the controller (located in the control room) and peripheral unit (located in the catheterization lab) through a full duplex RS-485 connection at 25 m and 115,200 cps (baud rate) over a standard CAT 6E Ethernet cable. The CANBus messages contain device-specific messages alongside the higher-level data aggregation managed by the RS-485 bus. To facilitate inter-module communication, the robot controller distributes the aggregated data from the RS-485 bus to subsets of device-specific commands over the CANBus, depending on module-specific messaging IDs. This hierarchy of the two buses ensures synchronized operations across various distributed modules without any disruption in the physical layer of information exchange between the buses.
An onboard datalogger was developed alongside the robot CANBus structure to facilitate post-processing of the three DoFs [27]. The positions recorded by the motor controllers were logged by the datalogger in real-time through a dedicated Arduino MKR Zero (Arduino LLC, Turin, Italy) connected to a CANBus transceiver, which saved the data to an SD card.
3. Operator’s Workstation: Visual Feedback and Catheter Modeling
The operator workstation, isolated from X-ray exposure (Figure 8), provides intuitive control and monitoring beyond the guidance of mapping and X-ray fluoroscopy imaging (Artis Q biplane, Siemens Healthcare GmbH, Forchheim, Germany) in the catheterization lab. The workstation consists of additional display screens streaming the robot/setup camera feed, a real-time robot state simulator (illustrated in Figure 9), and status indicators on the controllers for improved situational awareness.
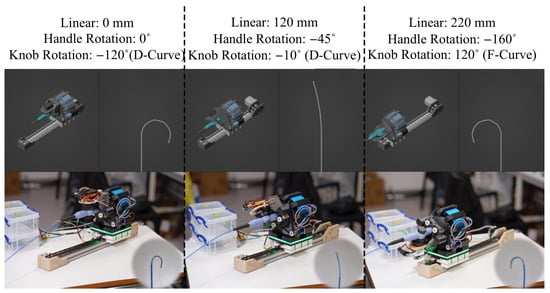
Figure 9.
Series of configurations demonstrating actuation with resulting tip articulation via linear translation, handle rotation, and knob rotation.
3.1. Catheter Tip Modeling
Hardware simulation delivers the manipulator’s reachability and speed characteristics, as well as its tip configuration, to the user. Visualization of the tip configuration is critical for clinicians. For simplification, rather than modeling localized curvature configurations, only the tip orientation was modeled, as the goal of this work was to introduce a foundation for an integrated operator’s workstation. There has been extensive research on this topic, including Fiber Bragg Grating (FBG)-based shape-sensing methods and their combination with neural networks [28]. In our work, articulation of the flexible distal section of the tendon-driven catheter was modeled by leveraging real-time PWM signals for servo control, translating them to a proportional tip-bending angle. Dead-zone effects, nonlinear hysteresis, and compliance along the catheter shaft were neglected. Blender rendered the tip articulation and robot state (illustrated in Figure 9) and Blender Python API was used for control.
3.2. Model-Based State Estimation
A Kalman filter fused the raw coordinate data recorded from the motor controllers, to reduce visualization jitter from encoder discretization and CANbus-induced time-varying random delays [29], optimizing user experience.
Table 1 compares four filters on the logged robot data: moving average, moving median, Kalman, and average-Kalman. The moving average calculates the mean over a sliding window (). The moving median finds the median in a window (). The Kalman filter estimates the forward state of a system by merging a motion model with noisy observations by computing an updated state estimate () at each timestep (k) using past measurements only. The average-Kalman filter applies a moving average filter () the the Kalman output for additional smoothing.

Table 1.
Performance comparison of four filters on joint positions logged by CANBus datalogger.
The results presented in Figure 10 depict the instability of the positions of the linear, axial, and knob actuators recorded by the datalogger (276 s of offline real data). A Kalman-filtered signal is overlayed, illustrating significantly reduced jitter.
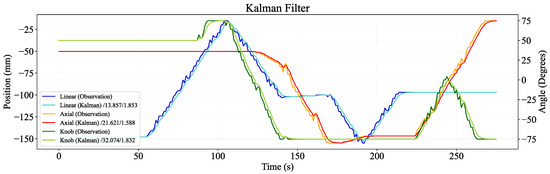
Figure 10.
Performance of Kalman filter applied to sample position data. The legend includes the filter results in the format ‘filter/moving variance/average absolute error’.
Two metrics were used: the moving variance and average absolute error. The results in Table 1 show that the Kalman filter had the lowest error across the three DoFs, with a mean of 1.76 mm and degrees, compared to a mean of 2.32 for the average-Kalman filter, and errors of over 2.0 mm and degrees for the average and median filters. This indicates that the prediction of the next state bypasses the lag of windowed averaging. However, the average-Kalman filter reduced the variance by a mean difference of 0.29 but was susceptible to a marginal lag. The Kalman filter relies solely on past data, making it most suitable for real-time usage. Additionally, the results from both metrics conclude that the Kalman filter exhibited superior performance, achieving smooth signal tracking.
A threaded architecture was employed to isolate communication, filtering, and rendering for smooth visualization at a fixed 10 Hz frame rate unaffected by discontinuous data.
4. System Evaluation
A pilot study quantified the workspace and usability of the modular robotic platform for manipulating an ablation catheter. Benchtop phantom trials served to objectively validate motion smoothness in Section 4.2 and performance in Section 4.3 in reaching target locations. Subjective clinical feedback is discussed in Section 4.4, capturing direct clinician responses regarding workflow integration factors such as the learning curve and visualization.
4.1. Experimental Setup
The robotic system was evaluated in a clinical environment using a cardiac phantom (Figure 11), manipulating the catheter using real-time X-ray fluoroscopy guidance (Artis Q biplane, Siemens Healthcare GmbH, Forchheim, Germany). The phantom was manufactured in Clear Resin (Formlabs Photopolymer Clear Resin) based on a magnetic resonance imaging (MRI) scan (1.5T, Philips Achieva, v3.2.2, Philips Healthcare, Best, The Netherlands) of an AF patient, modeling the left atrium (LA), right atrium (RA), and inlet pulmonary veins (PVs). Given that the workspace of the catheter is constrained within the LA, a trans-septal sheath was secured in the trans-septal puncture, providing the catheter with access from the RA to the LA.
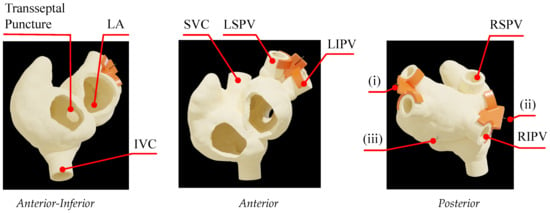
Figure 11.
Cardiac phantom of AF patient used in experimental evaluation, with Aurora sensors placed between the (i) left PVs, (ii) right PVs, and (iii) posterior wall of the interatrial septum.
The Aurora™ electromagnetic (EM) tracking system (NDI, Waterloo, ON, Canada) and four Micro 6-DoF sensors were used to localize the position of the catheter throughout the trials. Three reference sensors were secured to the phantom in the following places to locate the heart in the coordinate system of the Aurora: (i) on the left PVs fixture (orange clip attached to left superior (LSPV) and left inferior (LIPV)), (ii) on the right PVs fixture (clip attached to right superior (RSPV) and right inferior (RIPV)), and (iii) on the posterior wall of the biatrial structure along the interatrial septum (refer to Figure 11). A fourth sensor was placed on the catheter, which was fixed 3 mm from the tip. The EM system was interfaced to a PC (Core i9, 64GB RAM), capturing the position data at 25 Hz.
Three cardiologists and two cardiology trainees were enrolled in the study, with experience levels ranging from 0 to 25 years. Clinician experience levels defined the participants’ procedural skill levels: participants with under 10 years of experience were assigned to the intermediate group; otherwise, they were placed in the expert group. The participants had no prior experience with CardioXplorer and received basic standardized instructions.
Pulmonary vein isolation (PVI) forms the cornerstone of AF ablation to isolate PV triggers [2]; therefore, both left PVs were targeted for comparative navigation tests between manual and robotic trials. The targets reflect standard practice to objectively benchmark CardioXplorer in rendering manual expertise and accounting for clinical needs. Figure 12b depicts the confirmation of target achievement, which defined trial success. The starting position was the interatrial septum, with the participant moving the catheter to the LSPV, then moving back to the edge of the interatrial septum, and then moving to the LIPV, with the trajectory depicted in Figure 13.
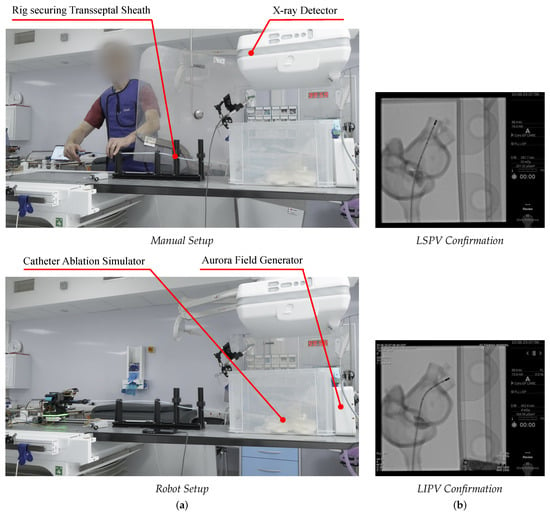
Figure 12.
Overview of experimental setup for (a) manual and robot trials. (b) Confirmation of target achievement in the LSPV and LIPV.
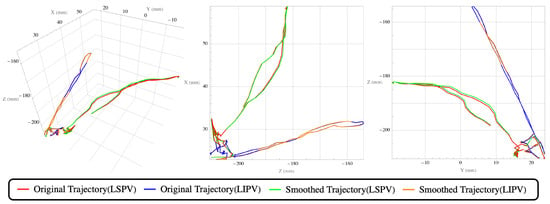
Figure 13.
Example trajectory (from the final robot trial of the cardiologist with 13 years of experience) of the catheter from the interatrial septum (starting position) to the LSPV (first target), back to the starting position, and then to the LIPV (second target).
4.2. Assessment of Motion Smoothness
PVI may involve methodical point-by-point ablation, warranting consistent, stable, and reliable catheter manipulation [2]. Therefore, the smoothness of the catheter trajectory within the LA offers an objective metric for skill assessment, comparing motion consistency. For this, we leveraged the mean tooltip motion jerk (J) [30,31], which can be obtained as follows:
The jerk was computed over the last 20 s (T) of the final attempts for manual and robot trials. Before jerk computation, a Gaussian filter ( samples) was applied to the noise from the recorded position data (the filtered trajectory is illustrated in Figure 13). Velocities between 0.5 and 10 mm/s were analyzed after excluding jitter and inactivity.
Table 2 summarizes the mean jerk for manual versus robotic ablation attempts, segmented by clinician experience, where lower jerk correlates to smoother catheter tip motion. An unpaired t-test assessed the differences between the two experience groups (using the last 250 frames from each participant) across the mean jerk metric—the null hypothesis assumes no difference between the two groups.

Table 2.
Summary of mean motion jerk of the last navigation attempt.
The mean motion jerk analysis revealed 35.2% (p = 4.50 × ) smoother robotic navigation for experts (≥10 years) compared to the intermediate group. Both groups achieved smoother robot motion compared to manual, with 42.2% (p = 2.69 × ) achieved by the experts and 13.6% (p = 1.77 × ) by the intermediate group (<10 years), indicating superior tip stability with the robot.
4.3. Assessment of Targeting Performance
An analysis of task duration (in Figure 14a) was conducted to evaluate targeting performance to the relevant PVs. The catheter was navigated from an initial septal position to the LSPV and LIPV sequentially, with the robot adding a mean overhead of 30.7 s for the most experienced cardiologist, compared to the additional 43.3 s taken by the least experienced participant in the manual approach relative to the robot, across the five trials. The large standard deviations across the participants suggest an initial learning curve (p = 0.005 (LS) and 0.003 (LI)).
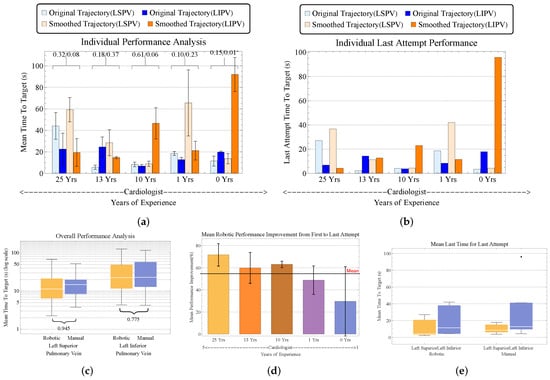
Figure 14.
Comparison of clinician performance from manual versus robotic targeting trials, analyzing metrics including individual achievement, time to target, and performance improvement over time.
Accessing the LIPV was more challenging across all the participants for both the manual and robot trials; however, the opposite is true for the manual trials conducted by cardiologists with 13 and 25 years of experience. Figure 14b,e depicts a decrease in inter-participant task duration between LIPV and LSPV of 19.21 s for manual and 8.65 s with the robot; this 55% reduction indicates that the robot eases targeting of the LIPV (p = 0.107 (LS) and 0.275 (LI)). The mean for the robot across the five trials from Figure 14c was 26.42 s ± 5.02, and it was 28.01 s ± 5.11 for the manual trials, leading us to conclude that the robotic approach was an average of 1.59 s faster than the manual approach (p = 0.902 (LS) and 0.69 (LI)). Moreover, the cardiac fellow (<1 year) performed fastest with the robot and had the lowest mean performance improvement (Figure 14d)—suggesting a shorter learning curve and lower cognitive load than for the less experienced participants (p = 0.05 (LS) and 0.02 (LI)).
4.4. Clinician Evaluation: Questionnaire
The questionnaire focused on evaluating the system’s usability and capabilities based on direct clinician experience. The elected multifaceted statements were devised through joint consultation with an experienced cardiologist (who did not partake in the experiments) and surgical robotics expert to encapsulate critical factors from both clinical and technological viewpoints, gathering quantitative and qualitative feedback. The quantitative questions leveraged five-point Likert rating scales to assess the total score, maneuver speed, maneuver sensitivity, and joystick control; these were used to stratify cases in the two performance groups.
The clinician responses (on a five-point scale) are summarized in Figure 15. The questionnaire and the responses can be found in our GitHub repository [23]. The mean rating of 4.2/5 ± 0.8 suggests a short learning curve with the system (Figure 15a), indicating ease of adoption following minimal training. The clinician’s experience with the joystick was rated at 4.2/5 ± 0.44, concluding intuitive navigation. Figure 15c visualizes the rated speed of critical maneuvers, with a mean overall rating of 3.9/5 ± 0.75 indicating efficient procedural performance. The rated overall level of maneuver sensitivity of CardioXplorer compared to the conventional approach was 3.9/5 ± 0.75, demonstrating precision in catheter manipulation.
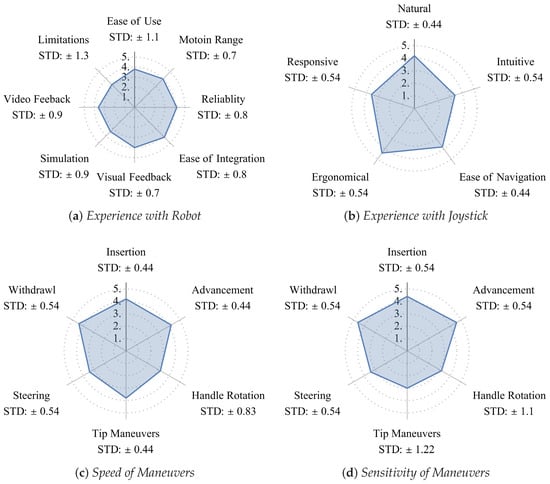
Figure 15.
Mean of clinician ratings on a 5-point Likert scale questionnaire. Response scales ranged from 1 (strongly disagree/very poor) to 5 (strongly agree/excellent).
5. Conclusions and Future Work
This article presents CardioXplorer, an open-source modular robotic system for catheter teleoperation. The robot inherits an advantage of the Amigo system in that it accommodates commercial catheters [9]. Collaboration with cardiologists through benchtop phantom experiments validated this method’s clinical usability (questionnaire analysis), workspace (targeting analysis), and smooth manipulation (mean motion jerk analysis). The latter was established from the robot results of the tooltip motion jerk and evaluation of the learning curve, which indicated incremental improvements in catheter manipulation by the intermediate group, approaching the performance of expert clinicians.
For most participants, the robot trials initially took longer than the manual ones; however, the results from the participants’ final attempts and conclusions drawn from other studies demonstrate an expectation that this difference will diminish with experience, accompanied by additional user training [32]. The analysis of the mean robotic performance improvement illustrated that the procedural times experienced in the robotic trials approached parity within a few attempts as the participants became more adept at using the joystick interface.
Nonetheless, these deductions are limited by the simplified physiology of the phantom, overlooking in vivo difficulties such as cardiac motion, as well as simplified catheter modeling. Future work on this modeling could incorporate image-based shape sensing with this simulator to validate the tip-bending position. Additionally, real-time catheter endpoint positioning can be used to provide closed-loop control.
Besides this, this study’s sample size limits the generalization of the results; however, given that this work serves as a proof-of-concept, future work will focus on more extensive evaluation over an extended period.
This work aims to accelerate progress in robotic catheter systems by lowering boundaries through open-source platforms. Ultimately, through the provision of a validated foundation for a catheter robot, the deliverables of this article will allow the community to dedicate resources to higher-level challenges in sensing, autonomy, and planning.
Author Contributions
Conceptualization, R.H. and K.R.; methodology, Z.X., A.M.Z., L.L. (Lukas Lindenroth), S.E.W., R.H. and K.R.; software, Z.X., A.M.Z., Y.H., Y.W. and Z.C.; validation, Z.X., A.M.Z., R.H. and K.R.; formal analysis, Z.X., A.M.Z., R.H. and K.R.; investigation, Z.X., A.M.Z., L.L. (Lisa Leung), C.B., S.S., S.E.W., J.B., C.A.R., J.W., A.A., R.H. and K.R.; resources, Z.X., A.M.Z., R.H. and K.R.; data curation, Z.X.; writing—original draft preparation, Z.X. and A.M.Z.; writing—review and editing, Z.X., A.M.Z., L.L. (Lisa Leung), S.E.W., R.H. and K.R.; visualization, Z.X. and A.M.Z.; supervision, R.H. and K.R.; project administration, R.H. and K.R.; funding acquisition, R.H. and K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] at King’s College London, the British Heart Foundation (BHF) Centre of Excellence at King’s College London, and the Department of Health and Social Care (DHSC) through the National Institute for Health and Care Research (NIHR) MedTech and Vitro Diagnostic Co-operative (MIC) award for Cardiovascular Diseases to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London. For the purpose of Open Access, the Author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Data Availability Statement
Github: CardioXplorer: An Open-Source Catheter Robot.
This project contains the following data:
- The hardware design files and bill of materials for the mechanical embedded components. These are in the form of CAD files that can be used for manufacturing.
- The embedded firmware code running on the microcontrollers with peripherals that control the system.
- The wiring diagram for each individual module.
- Documentation explaining the system design, assembly instructions, and usage guides.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Acknowledgments
We thank Antonia Pontiki and Laila Zeidan for their guidance and assistance with the experimental analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.; Crijns, H.J.G.; Damiano, R.J.; Davies, D.W.; DiMarco, J.; et al. News from the Heart Rhythm Society: 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS). EP Eur. 2012, 4, 632–696. [Google Scholar]
- Morillo, C.A.; Banerjee, A.; Perel, P.; Wood, D.; Jouven, X. Atrial fibrillation: The current epidemic. J. Geriatr. Cardiol. JGC 2017, 14, 195. [Google Scholar] [PubMed]
- Aagaard, P.; Natale, A.; Di Biase, L. Robotic navigation for catheter ablation: Benefits and challenges. Expert Rev. Med. Devices 2015, 12, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Bassil, G.; Markowitz, S.M.; Liu, C.F.; Thomas, G.; Ip, J.E.; Lerman, B.B.; Cheung, J.W. Robotics for catheter ablation of cardiac arrhythmias: Current technologies and practical approaches. J. Cardiovasc. Electrophysiol. 2020, 31, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Faddis, M.N.; Chen, J.; Osborn, J.; Talcott, M.; Cain, M.E.; Lindsay, B.D. Magnetic guidance system for cardiac electrophysiology: A prospective trial of safety and efficacy in humans. J. Am. Coll. Cardiol. 2003, 42, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Neuzil, P.; Malchano, Z.J.; Vijaykumar, R.; Cury, R.; Abbara, S.; Weichet, J.; McPherson, C.D.; Ruskin, J.N. View-synchronized robotic image-guided therapy for atrial fibrillation ablation: Experimental validation and clinical feasibility. Circulation 2007, 115, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhao, Y.; Zhang, J.; Li, H.; Li, K.; Zhang, J. The critical technologies of vascular interventional robotic catheterization: A Review. IEEE Sens. J. 2023, 23, 30051–30069. [Google Scholar] [CrossRef]
- Khan, E.M.; Frumkin, W.; Ng, G.A.; Neelagaru, S.; Abi-Samra, F.M.; Lee, J.; Giudici, M.; Gohn, D.; Winkle, R.A.; Sussman, J.; et al. First experience with a novel robotic remote catheter system: Amigo™ mapping trial. J. Interv. Card. Electrophysiol. 2013, 37, 121–129. [Google Scholar] [CrossRef]
- Ramadani, A.; Bui, M.; Wendler, T.; Schunkert, H.; Ewert, P.; Navab, N. A survey of catheter tracking concepts and methodologies. Med. Image Anal. 2022, 82, 102584. [Google Scholar] [CrossRef]
- Gupta, P.; Schomburg, J.; Krishna, S.; Adejoro, O.; Wang, Q.; Marsh, B.; Nguyen, A.; Genere, J.R.; Self, P.; Lund, E.; et al. Development of a classification scheme for examining adverse events associated with medical devices, specifically the DaVinci surgical system as reported in the FDA MAUDE database. J. Endourol. 2017, 31, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.C.; Thai, M.T.; Hoang, T.T.; Davies, J.; Phan, P.T.; Zhu, K.; Wu, L.; Brodie, M.A.; Tsai, D.; Ha, Q.P.; et al. Development of a soft robotic catheter for vascular intervention surgery. Sens. Actuators A Phys. 2023, 357, 114380. [Google Scholar] [CrossRef]
- Gao, A.; Liu, H.; Zou, Y.; Wang, Z.; Liang, M.; Wang, Z. A contact-aided asymmetric steerable catheter for atrial fibrillation ablation. IEEE Robot. Autom. Lett. 2017, 2, 1525–1531. [Google Scholar] [CrossRef]
- Le, H.M.; Do, T.N.; Phee, S.J. A survey on actuators-driven surgical robots. Sens. Actuators A Phys. 2016, 247, 323–354. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Teh, T.; Thai, M.T.; Phan, P.T.; Hoang, T.T.; Low, H.; Davies, J.; Nicotra, E.; Lovell, N.H.; Do, T.N. Bidirectional Soft Robotic Catheter for Arrhythmia Treatment. In Proceedings of the 2022 International Conference on Robotics and Automation (ICRA), Philadelphia, PA, USA, 23–27 May 2022; IEEE: New York, NY, USA, 2022; pp. 9579–9585. [Google Scholar]
- Hwang, J.; Kim, J.y.; Choi, H. A review of magnetic actuation systems and magnetically actuated guidewire-and catheter-based microrobots for vascular interventions. Intell. Serv. Robot. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Xu, Z.; Mower, C.E.; Wu, H.; Walker, Q.; Ayoade, O.; Cotic, N.; Behar, J.; Williams, S.; Arujuna, A.; et al. Design and Development of a Novel Force-Sensing Robotic System for the Transseptal Puncture in Left Atrial Catheter Ablation. In Proceedings of the 2023 IEEE International Conference on Robotics and Automation (ICRA), London, UK, 29 May–2 June 2023; IEEE: New York, NY, USA, 2023; pp. 6851–6858. [Google Scholar]
- Cercenelli, L.; Bortolani, B.; Marcelli, E. CathROB: A highly compact and versatile remote catheter navigation system. Appl. Bionics Biomech. 2017, 2017, 2712453. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.P.; Abah, C.; Velasco-Enriquez, M.; Herman, M.; Bennett, G.J.; Vela, E.A.; Walsh, C.J. The soft robotics toolkit: Strategies for overcoming obstacles to the wide dissemination of soft-robotic hardware. IEEE Robot. Autom. Mag. 2017, 24, 57–64. [Google Scholar] [CrossRef]
- Faure, F.; Duriez, C.; Delingette, H.; Allard, J.; Gilles, B.; Marchesseau, S.; Talbot, H.; Courtecuisse, H.; Bousquet, G.; Peterlik, I.; et al. Sofa: A multi-model framework for interactive physical simulation. In Soft Tissue Biomechanical Modeling for Computer Assisted Surgery; Springer: Berlin/Heidelberg, Germany, 2012; pp. 283–321. [Google Scholar]
- Coevoet, E.; Morales-Bieze, T.; Largilliere, F.; Zhang, Z.; Thieffry, M.; Sanz-Lopez, M.; Carrez, B.; Marchal, D.; Goury, O.; Dequidt, J.; et al. Software toolkit for modeling, simulation, and control of soft robots. Adv. Robot. 2017, 31, 1208–1224. [Google Scholar] [CrossRef]
- Yasa, O.; Toshimitsu, Y.; Michelis, M.Y.; Jones, L.S.; Filippi, M.; Buchner, T.; Katzschmann, R.K. An Overview of Soft Robotics. Annu. Rev. Control. Robot. Auton. Syst. 2023, 6, 1–29. [Google Scholar] [CrossRef]
- Xu, Z.; Zeidan, A.M.; He, Y.; Leung, L.; Byrne, C.; Sabu, S.; Wu, Y.; Chen, Z.; Williams, S.E.; Lindenroth, L.; et al. CardioXplorer: An Open-Source Modular Teleoperative Robotic Catheter Ablation System. 2023. Available online: https://github.com/ZhouyangX/CardioXplorer (accessed on 11 April 2024).
- Zhao, Y.; Mei, Z.; Luo, X.; Mao, J.; Zhao, Q.; Liu, G.; Wu, D. Remote vascular interventional surgery robotics: A literature review. Quant. Imaging Med. Surg. 2022, 12, 2552. [Google Scholar] [CrossRef]
- Kesner, S.B.; Howe, R.D. Robotic catheter cardiac ablation combining ultrasound guidance and force control. Int. J. Robot. Res. 2014, 33, 631–644. [Google Scholar] [CrossRef]
- Kiencke, U. A view of automotive control systems. IEEE Control Syst. Mag. 1988, 8, 11–19. [Google Scholar] [CrossRef]
- Palomino, J.; Cuty, E.; Huanachin, A. Development of a CAN Bus datalogger for recording sensor data from an internal combustion ECU. In Proceedings of the 2021 IEEE International Workshop of Electronics, Control, Measurement, Signals and their Application to Mechatronics (ECMSM), Liberec, Czech Republic, 21–22 June 2021; IEEE: New York, NY, USA, 2021; pp. 1–4. [Google Scholar]
- Jäckle, S.; García-Vázquez, V.; von Haxthausen, F.; Eixmann, T.; Sieren, M.M.; Schulz-Hildebrandt, H.; Hüttmann, G.; Ernst, F.; Kleemann, M.; Pätz, T. 3D catheter guidance including shape sensing for endovascular navigation. In Proceedings of the Medical Imaging 2020: Image-Guided Procedures, Robotic Interventions, and Modeling, Houston, TX, USA, 15–20 February 2020; SPIE: Bellingham, WA, USA, 2020; Volume 11315, pp. 21–29. [Google Scholar]
- Jiang, K.; Zhang, H.; Karimi, H.R.; Lin, J.; Song, L. Simultaneous input and state estimation for integrated motor-transmission systems in a controller area network environment via an adaptive unscented Kalman filter. IEEE Trans. Syst. Man Cybern. Syst. 2018, 50, 1570–1579. [Google Scholar] [CrossRef]
- Hogan, N.; Sternad, D. Sensitivity of smoothness measures to movement duration, amplitude, and arrests. J. Mot. Behav. 2009, 41, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Essa, I. Automated surgical skill assessment in RMIS training. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 731–739. [Google Scholar] [CrossRef]
- Kataria, V.; Berte, B.; Vandekerckhove, Y.; Tavernier, R.; Duytschaever, M. Remote magnetic versus manual navigation for radiofrequency ablation of paroxysmal atrial fibrillation: Long-term, controlled data in a large cohort. BioMed Res. Int. 2017, 2017, 6323729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).