The Therapeutic Potential of Nanoparticles to Reduce Inflammation in Atherosclerosis
Abstract
1. Introduction
2. Treatments Targeting Inflammation in Atherogenesis
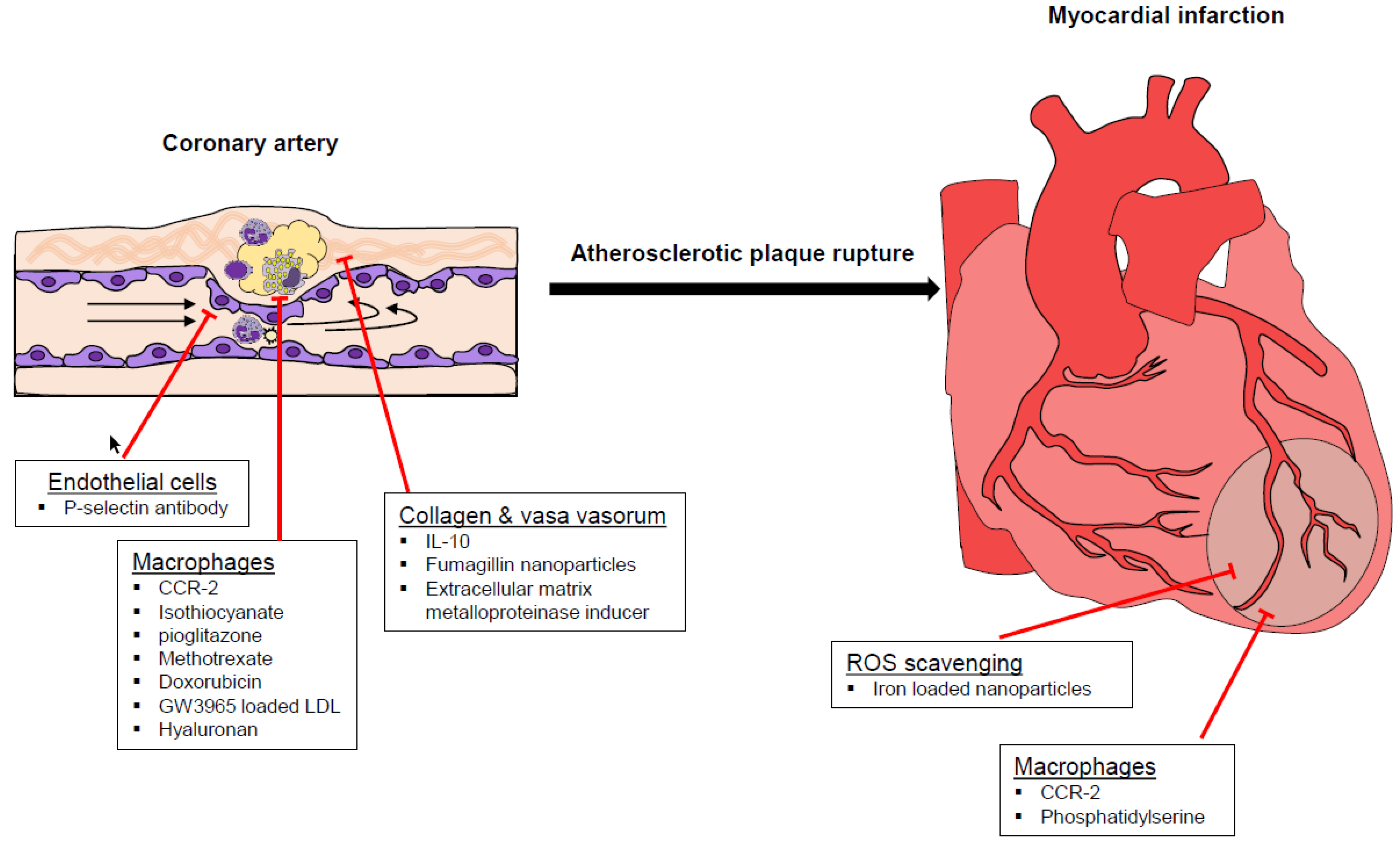
3. The Potential of Nanoparticles as to Prevent and Treat Atherosclerosis and Related Complications
| Nanoparticle | Target | Outcome | Ref |
|---|---|---|---|
| siRNA | |||
| siRNA targeting CCR2 | Monocytes, macrophages. | Reduction of atherosclerosis Attenuated infarct inflammation, post-infarction left ventricular remodeling | [51,52] |
| Sulphate-based nanoparticles | |||
| Nanoparticles loaded with fluorescein isothiocyanate and/or pioglitazone. | Monocytes, macrophages. | Modified polarity of monocytes in the periphery. Decreased development of inflammatory macrophages. Destabilized atherosclerotic plaque and rupture. | [56] |
| Lipid-based nanoparticles | |||
| Lipid coated nanoparticles loaded with MTX | Macrophages, foam cells | Decreased plaque coverage in the aortic arch | [40] |
| Library of LDL mimicking nanoparticles loaded with GW3965 | Monocytes and Macrophages for reversing cholesterol efflux. | Decreased total lipids in aortic macrophages. Decreased monocyte number. | [66] |
| Lipid core nanoparticles carrying MTX and/or PTX | Macrophages | Decreased size of the plaque and of intima area. Reduced number of macrophages in aortic lesions. Downregulation of MMP-9 and TNF-α. | [20] |
| Liposomal nanoparticles loaded with prednisolone | Macrophage lipid loading, ER stress and apoptosis | Lipotoxicity | [59] |
| Lipid core nanoparticles carrying doxorubicin | Macrophages | Anti-inflammatory and anti-proliferating effects | [60] |
| Liposomes presenting PS | Macrophages | Shift toward anti-inflammatory phenotype with consequent improvement of myocardial healing | [65] |
| Glycosaminoglycan | |||
| Hyaluronan nanoparticles | Atherosclerotic plaque, macrophages | Decreased size of the atherosclerotic lesions. Decreased macrophage number. Increased collagen content. | [58,67] |
| Other approaches | |||
| Nanoparticles loaded with the EMMPRIN (extracellular matrix metalloproteinase inducer) Ldlr, low density lipoprotein receptor) binding peptide AP-9. | EMMPRIN | Ameliorated heart contractility. Decreased cardiac necrosis. Decreased levels of MMP-2 and MMP-9 | [68] |
| Nanoparticles containing IL-10 and targeting peptide collagen IV | Collagen IV | Reduced oxidative stress in lesions. Stabilized atherosclerotic plaques. | [69] |
| Magnetic microbubbles modified with P-selectin antibody | Endothelial cells | Leukocyte rolling | [61] |
| Fumagillin nanoparticles | Vasa vasorum | Reduced neovascularization | [62,63] |
| Iron oxide–cerium oxide core–shell nanoparticles | Macrophages | ROS scavenging with reduced atherosclerotic burden and improved myocardial healing | [64] |
4. Future Perspectives in the Application of Nanoparticles in the Prevention and Treatment of Atherosclerosis
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ApoE−/− | apolipoprotein E(Apoe) knockout |
| CCR | C-C chemokine receptor |
| CT | computed tomography |
| CV | cardiovascular |
| ER | endothelial reticulum |
| gGT | gamma-glutamyl transferase |
| HFD | high fat diet |
| IL-10 | interleukin-10 |
| LDL | low-density lipoprotein |
| Ldlr | low density lipoprotein receptor |
| MMP | matrix metalloproteinase |
| MMP | matrix metalloproteinase |
| MRI | magnetic resonance imaging |
| MTX | methotrexate |
| NIR | near-infrared |
| OI | optical imaging |
| PEG | polyethylene glycol |
| PET | positron emission tomography |
| PPARγ | peroxisome proliferator-activated receptor |
| PS | phosphatidylserine |
| PTX | Paclitaxel |
| RES | reticuloendothelial system |
| ROS | Reactive oxygen species |
| SHP-1 | Src homology region 2 domain-containing tyrosine phosphatase-1 |
| siRNA | small interfering RNA |
| SPECT | single photon emission tomography |
| TIMP3 | tissue inhibitor of metalloproteinase 3 |
| TNF | tumor necrosis factor |
| US-PA | ultrasound and photoacoustic |
| VCAM-1 | vascular cell adhesion molecule 1 |
| Zr | Zirconium |
References
- Shoenfeld, Y.; Sherer, Y.; Harats, D. Atherosclerosis as an infectious, inflammatory and autoimmune disease. Trends Immunol. 2001, 22, 293–295. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. New Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Bejarano, J.; Navarro-Marquez, M.; Morales-Zavala, F.; Morales, J.O.; Garcia-Carvajal, I.; Araya-Fuentes, E.; Flores, Y.; Verdejo, H.E.; Castro, P.F.; Lavandero, S.; et al. Nanoparticles for diagnosis and therapy of atherosclerosis and myocardial infarction: Evolution toward prospective theranostic approaches. Theranostics 2018, 8, 4710–4732. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, Z.; Sepahvand, F.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell Physiol. 2018, 233, 2116–2132. [Google Scholar] [CrossRef] [PubMed]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Fereidouni, M.; Barreto, G.; Sahebkar, A. NLRP3 inflammasome as a treatment target in atherosclerosis: A focus on statin therapy. Int. Immunopharmacol. 2019, 73, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.; Gutnikov, S.; Warlow, C. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 2003, 34, 514–523. [Google Scholar] [CrossRef]
- Everett, B.M.; Pradhan, A.D.; Solomon, D.H.; Paynter, N.; MacFadyen, J.; Zaharris, E.; Gupta, M.; Clearfield, M.; Libby, P.; Hasan, A.A. Rationale and design of the Cardiovascular Inflammation Reduction Trial: A test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 2013, 166, 199–207. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arterioscler. Thromb Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef]
- Parizadeh, S.M.R.; Azarpazhooh, M.R.; Moohebati, M.; Nematy, M.; Ghayour-Mobarhan, M.; Tavallaie, S.; Rahsepar, A.A.; Amini, M.; Sahebkar, A.; Mohammadi, M.; et al. Simvastatin therapy reduces prooxidant-antioxidant balance: Results of a placebo-controlled cross-over trial. Lipids 2011, 46, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Kotani, K.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Jones, S.R.; Ray, K.K.; Blaha, M.J.; Rysz, J.; Toth, P.P.; et al. Statin therapy reduces plasma endothelin-1 concentrations: A meta-analysis of 15 randomized controlled trials. Atherosclerosis 2015, 241, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, C.; Mikhailidis, D.P.; Undas, A.; Lip, G.Y.H.; Muntner, P.; Bittner, V.; Ray, K.K.; Watts, G.F.; Hovingh, G.K.; et al. Association between statin use and plasma d-dimer levels: A systematic review and meta-analysis of randomised controlled trials. Thromb. Haemost. 2015, 114, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Coomes, E.; Chan, E.S.; Reiss, A.B. Methotrexate in atherogenesis and cholesterol metabolism. Cholesterol 2011. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; van Rijs, S.M.; et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014. [Google Scholar] [CrossRef]
- Tsujita, K.; Sugiyama, S.; Sumida, H.; Shimomura, H.; Yamashita, T.; Yamanaga, K.; Komura, N.; Sakamoto, K.; Oka, H.; Nakao, K.; et al. Impact of Dual Lipid-Lowering Strategy With Ezetimibe and Atorvastatin on Coronary Plaque Regression in Patients With Percutaneous Coronary Intervention: The Multicenter Randomized Controlled PRECISE-IVUS Trial. J. Am. Coll. Cardiol. 2015, 66, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P.J.A.h.j. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 2011, 162, 597–605. [Google Scholar] [CrossRef]
- Cervadoro, A.; Palomba, R.; Vergaro, G.; Cecchi, R.; Menichetti, L.; Decuzzi, P.; Emdin, M.; Luin, S. Targeting Inflammation With Nanosized Drug Delivery Platforms in Cardiovascular Diseases: Immune Cell Modulation in Atherosclerosis. Front. Bioeng. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Gomes, F.L.T.; Maranhao, R.C.; Tavares, E.R.; Carvalho, P.O.; Higuchi, M.L.; Mattos, F.R.; Pitta, F.G.; Hatab, S.A.; Kalil-Filho, R.; Serrano, C.V., Jr. Regression of Atherosclerotic Plaques of Cholesterol-Fed Rabbits by Combined Chemotherapy With Paclitaxel and Methotrexate Carried in Lipid Core Nanoparticles. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 561–569. [Google Scholar] [CrossRef]
- Bulgarelli, A.; Dias, A.A.M.; Caramelli, B.; Maranhao, R.C. Treatment With Methotrexate Inhibits Atherogenesis in Cholesterol-Fed Rabbits. J. Cardiovasc. Pharm. 2012, 59, 308–314. [Google Scholar] [CrossRef]
- Narasimhulu, C.A.; Fernandez-Ruiz, I.; Selvarajan, K.; Jiang, X.; Sengupta, B.; Riad, A.; Parthasarathy, S.J.C.o.i.p. Atherosclerosis—Do we know enough already to prevent it? Curr. Opin. Pharmacol. 2016, 27, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Tabas, I.; Fredman, G.; Fisher, E.A. Inflammation and its Resolution as Determinants of Acute Coronary Syndromes. Circ. Res. 2014, 114, 1867–1879. [Google Scholar] [CrossRef]
- Belcastro, E.; Franzini, M.; Cianchetti, S.; Lorenzini, E.; Masotti, S.; Fierabracci, V.; Pucci, A.; Pompella, A.; Corti, A. Monocytes/macrophages activation contributes to b-gamma-glutamyltransferase accumulation inside atherosclerotic plaques. J. Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, A.; Qadri, F.; Leukel, L.; Yilmaz, R.; Fontaine, J.F.; Sihn, G.; Bader, M.; Ahluwalia, A.; Duchene, J. CXCL5 limits macrophage foam cell formation in atherosclerosis. J. Clin. Invest. 2013, 123, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.A.C.; Reis, S. Temperature-responsive polymeric nanospheres containing methotrexate and gold nanoparticles: A multi-drug system for theranostic in rheumatoid arthritis. Coll. Surf. B 2015, 133, 378–387. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Cheraghi, M.; Negahdari, B.; Daraee, H.; Eatemadi, A. Heart targeted nanoliposomal/nanoparticles drug delivery: An updated review. Biomed. Pharmacother. 2017, 86, 316–323. [Google Scholar] [CrossRef]
- Matoba, T.; Koga, J.; Nakano, K.; Egashira, K.; Tsutsui, H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol. 2017, 70, 206–211. [Google Scholar] [CrossRef]
- Allen, S.; Liu, Y.G.; Scott, E. Engineering Nanomaterials to Address Cell-Mediated Inflammation in Atherosclerosis. Regen Eng. Transl. Med. 2016, 2, 37–50. [Google Scholar] [CrossRef]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef]
- Pentecost, A.E.; Lurier, E.B.; Spiller, K.L. Nanoparticulate Systems for Controlling Monocyte/Macrophage Behavior. In Microscale Technologies for Cell Engineering; Springer: New York, NY, USA, 2016; pp. 291–304. [Google Scholar]
- Jokerst, J.V.; Gambhir, S.S. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef]
- Di Mascolo, D.; Lyon, C.J.; Aryal, S.; Ramirez, M.R.; Wang, J.; Candeloro, P.; Guindani, M.; Hsueh, W.A.; Decuzzi, P. Rosiglitazone-loaded nanospheres for modulating macrophage-specific inflammation in obesity. J. Control. Release 2013, 170, 460–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Koradia, A.; Kamato, D.; Popat, A.; Little, P.J.; Ta, H.T. Treatment of atherosclerotic plaque: Perspectives on theranostics. J. Pharm. Pharmacol. 2019, 71, 1029–1043. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Kim, Y.; Lobatto, M.E.; Kawahara, T.; Lee Chung, B.; Mieszawska, A.J.; Sanchez-Gaytan, B.L.; Fay, F.; Senders, M.L.; Calcagno, C.; Becraft, J.; et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc. Natl. Acad. Sci. USA 2014, 111, 1078–1083. [Google Scholar] [CrossRef]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Atukorale, P.U.; Covarrubias, G.; Bauer, L.; Karathanasis, E. Vascular targeting of nanoparticles for molecular imaging of diseased endothelium. Adv. Drug Deliv. Rev. 2017, 113, 141–156. [Google Scholar] [CrossRef]
- Stigliano, C.; Ramirez, M.R.; Singh, J.V.; Aryal, S.; Key, J.; Blanco, E.; Decuzzi, P.J.A.H.M. Methotraxate-Loaded Hybrid Nanoconstructs Target Vascular Lesions and Inhibit Atherosclerosis Progression in ApoE−/− Mice. Adv. Healthc. Mater. 2017. [Google Scholar] [CrossRef]
- Hossain, S.S.; Zhang, Y.; Fu, X.; Brunner, G.; Singh, J.; Hughes, T.J.; Shah, D.; Decuzzi, P. Magnetic resonance imaging-based computational modelling of blood flow and nanomedicine deposition in patients with peripheral arterial disease. J. R. Soc. Interface 2015. [Google Scholar] [CrossRef]
- Moore, T.L.; Hauser, D.; Gruber, T.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A.; Lyck, R. Cellular Shuttles: Monocytes/Macrophages Exhibit Transendothelial Transport of Nanoparticles under Physiological Flow. ACS Appl. Mater. Interfaces 2017, 9, 18501–18511. [Google Scholar] [CrossRef]
- Key, J.; Palange, A.L.; Gentile, F.; Aryal, S.; Stigliano, C.; Di Mascolo, D.; De Rosa, E.; Cho, M.; Lee, Y.; Singh, J.; et al. Soft Discoidal Polymeric Nanoconstructs Resist Macrophage Uptake and Enhance Vascular Targeting in Tumors. ACS Nano 2015, 9, 11628–11641. [Google Scholar] [CrossRef]
- Palomba, R.; Palange, A.L.; Rizzuti, I.F.; Ferreira, M.; Cervadoro, A.; Barbato, M.G.; Canale, C.; Decuzzi, P. Modulating Phagocytic Cell Sequestration by Tailoring Nanoconstruct Softness. Acs Nano 2018, 12, 1433–1444. [Google Scholar] [CrossRef]
- Schottler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailander, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Mima, Y.; Abu Lila, A.S.; Shimizu, T.; Ukawa, M.; Ando, H.; Kurata, Y.; Ishida, T. Ganglioside inserted into PEGylated liposome attenuates anti-PEG immunity. J. Control. Release 2017, 250, 20–26. [Google Scholar] [CrossRef]
- Ranalli, A.; Santi, M.; Capriotti, L.; Voliani, V.; Porciani, D.; Beltram, F.; Signore, G. Peptide-Based Stealth Nanoparticles for Targeted and pH-Triggered Delivery. Bioconjug. Chem. 2017, 28, 627–635. [Google Scholar] [CrossRef]
- Santi, M.; Maccari, G.; Mereghetti, P.; Voliani, V.; Rocchiccioli, S.; Ucciferri, N.; Luin, S.; Signore, G. Rational Design of a Transferrin-Binding Peptide Sequence Tailored to Targeted Nanoparticle Internalization. Bioconjug. Chem. 2017, 28, 471–480. [Google Scholar] [CrossRef]
- Barchet, T.M.; Amiji, M.M. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin. Drug Deliv. 2009, 6, 211–225. [Google Scholar] [CrossRef]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica-gold nanoparticles for atheroprotective management of plaques: Results of the NANOM-FIM trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef]
- Majmudar, M.D.; Keliher, E.J.; Heidt, T.; Leuschner, F.; Truelove, J.; Sena, B.F.; Gorbatov, R.; Iwamoto, Y.; Dutta, P.; Wojtkiewicz, G. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 2013, 127, 2038–2046. [Google Scholar] [CrossRef]
- Kao, C.-W.; Wu, P.-T.; Liao, M.-Y.; Chung, I.-J.; Yang, K.-C.; Tseng, W.-Y.; Yu, J. Magnetic nanoparticles conjugated with peptides derived from monocyte chemoattractant protein-1 as a tool for targeting atherosclerosis. Pharmaceutics 2018, 10, 62. [Google Scholar] [CrossRef]
- Majmudar, M.D.; Yoo, J.; Keliher, E.J.; Truelove, J.J.; Iwamoto, Y.; Sena, B.; Dutta, P.; Borodovsky, A.; Fitzgerald, K.; Di Carli, M.F.; et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 2013, 112, 755–761. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Kim, C.W.; Seo, J.W.; Kumar, S.; Son, D.J.; Gagnon, M.K.; Ingham, E.S.; Ferrara, K.W.; Jo, H. Multifunctional Nanoparticles Facilitate Molecular Targeting and miRNA Delivery to Inhibit Atherosclerosis in ApoE(-/-) Mice. ACS Nano 2015, 9, 8885–8897. [Google Scholar] [CrossRef]
- Kim, D.; Hong, J.; Moon, H.-H.; Nam, H.Y.; Mok, H.; Jeong, J.H.; Kim, S.W.; Choi, D.; Kim, S.H. Anti-apoptotic cardioprotective effects of SHP-1 gene silencing against ischemia–reperfusion injury: Use of deoxycholic acid-modified low molecular weight polyethyleneimine as a cardiac siRNA-carrier. J. Control. Release 2013, 168, 125–134. [Google Scholar] [CrossRef]
- Nakashiro, S.; Matoba, T.; Umezu, R.; Koga, J.; Tokutome, M.; Katsuki, S.; Nakano, K.; Sunagawa, K.; Egashira, K. Pioglitazone-Incorporated Nanoparticles Prevent Plaque Destabilization and Rupture by Regulating Monocyte/Macrophage Differentiation in ApoE-/- Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 491–500. [Google Scholar] [CrossRef]
- Leite, A.C.A.; Solano, T.V.; Tavares, E.R.; Maranhao, R.C. Use of Combined Chemotherapy with Etoposide and Methotrexate, both Associated to Lipid Nanoemulsions for Atherosclerosis Treatment in Cholesterol-fed Rabbits. Cardiovasc. Drug Ther. 2015, 29, 15–22. [Google Scholar] [CrossRef]
- Park, D.; Cho, Y.; Goh, S.-H.; Choi, Y. Hyaluronic acid–polypyrrole nanoparticles as pH-responsive theranostics. Chem. Commun. 2014, 50, 15014–15017. [Google Scholar] [CrossRef]
- Van der Valk, F.M.; Schulte, D.M.; Meiler, S.; Tang, J.; Zheng, K.H.; Van den Bossche, J.; Seijkens, T.; Laudes, M.; de Winther, M.; Lutgens, E. Liposomal prednisolone promotes macrophage lipotoxicity in experimental atherosclerosis. Nanomed. Nanotechnol. 2016, 12, 1463–1470. [Google Scholar] [CrossRef]
- Meneghini, B.C.; Tavares, E.R.; Guido, M.C.; Tavoni, T.M.; Stefani, H.A.; Kalil-Filho, R.; Maranhão, R.C. Lipid core nanoparticles as vehicle for docetaxel reduces atherosclerotic lesion, inflammation, cell death and proliferation in an atherosclerosis rabbit model. Vasc. Pharmacol. 2019, 115, 46–54. [Google Scholar] [CrossRef]
- Wu, W.; Feng, X.; Yuan, Y.; Liu, Y.; Li, M.; Bin, J.; Xiao, Y.; Liao, W.; Liao, Y.; Zhang, W. Comparison of magnetic microbubbles and dual-modified microbubbles targeted to P-selectin for imaging of acute endothelial inflammation in the abdominal aorta. Mol. Imaging Biol. 2017, 19, 183–193. [Google Scholar] [CrossRef]
- Winter, P.M.; Neubauer, A.M.; Caruthers, S.D.; Harris, T.D.; Robertson, J.D.; Williams, T.A.; Schmieder, A.H.; Hu, G.; Allen, J.S.; Lacy, E.K. Endothelial ανβ3 integrin–targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2103–2109. [Google Scholar] [CrossRef]
- Winter, P.M.; Caruthers, S.D.; Zhang, H.; Williams, T.A.; Wickline, S.A.; Lanza, G.M. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc. Imaging 2008, 1, 624–634. [Google Scholar] [CrossRef]
- Mauricio, M.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.; Rocha, M.; Vila, J.; Victor, V. Nanoparticles in medicine: A focus on vascular oxidative stress. Oxid Med. Cell Longev. 2018. [Google Scholar] [CrossRef]
- Harel-Adar, T.; Mordechai, T.B.; Amsalem, Y.; Feinberg, M.S.; Leor, J.; Cohen, S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc. Natl. Acad. Sci. USA 2011, 108, 1827–1832. [Google Scholar] [CrossRef]
- Tang, J.; Baxter, S.; Menon, A.; Alaarg, A.; Sanchez-Gaytan, B.L.; Fay, F.; Zhao, Y.; Ouimet, M.; Braza, M.S.; Longo, V.A.; et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl Acad. Sci. USA 2016. [Google Scholar] [CrossRef]
- Beldman, T.J.; Senders, M.L.; Alaarg, A.; Perez-Medina, C.; Tang, J.; Zhao, Y.; Fay, F.; Deichmoller, J.; Born, B.; Desclos, E.; et al. Hyaluronan Nanoparticles Selectively Target Plaque-Associated Macrophages and Improve Plaque Stability in Atherosclerosis. ACS Nano 2017, 11, 5785–5799. [Google Scholar] [CrossRef]
- Cuadrado, I.; Piedras, M.J.; Herruzo, I.; Turpin Mdel, C.; Castejon, B.; Reventun, P.; Martin, A.; Saura, M.; Zamorano, J.L.; Zaragoza, C. EMMPRIN-Targeted Magnetic Nanoparticles for In Vivo Visualization and Regression of Acute Myocardial Infarction. Theranostics 2016, 6, 545–557. [Google Scholar] [CrossRef]
- Kamaly, N.; Fredman, G.; Fojas, J.J.R.; Subramanian, M.; Choi, W.I.; Zepeda, K.; Vilos, C.; Yu, M.Y.; Gadde, S.; Wu, J.; et al. Targeted Interleukin-10 Nanotherapeutics Developed with.a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis. ACS Nano 2016, 10, 5280–5292. [Google Scholar] [CrossRef]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailander, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar] [CrossRef]
- Veseli, B.E.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lin, H.Y.; Chan, Y.W.; Li, K.H.; To, O.T.; Yan, B.P.; Liu, T.; Li, G.; Wong, W.T.; Keung, W.; et al. Mouse models of atherosclerosis: A historical perspective and recent advances. Lipids Health Dis. 2017. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdavi Gorabi, A.; Kiaie, N.; Reiner, Ž.; Carbone, F.; Montecucco, F.; Sahebkar, A. The Therapeutic Potential of Nanoparticles to Reduce Inflammation in Atherosclerosis. Biomolecules 2019, 9, 416. https://doi.org/10.3390/biom9090416
Mahdavi Gorabi A, Kiaie N, Reiner Ž, Carbone F, Montecucco F, Sahebkar A. The Therapeutic Potential of Nanoparticles to Reduce Inflammation in Atherosclerosis. Biomolecules. 2019; 9(9):416. https://doi.org/10.3390/biom9090416
Chicago/Turabian StyleMahdavi Gorabi, Armita, Nasim Kiaie, Željko Reiner, Federico Carbone, Fabrizio Montecucco, and Amirhossein Sahebkar. 2019. "The Therapeutic Potential of Nanoparticles to Reduce Inflammation in Atherosclerosis" Biomolecules 9, no. 9: 416. https://doi.org/10.3390/biom9090416
APA StyleMahdavi Gorabi, A., Kiaie, N., Reiner, Ž., Carbone, F., Montecucco, F., & Sahebkar, A. (2019). The Therapeutic Potential of Nanoparticles to Reduce Inflammation in Atherosclerosis. Biomolecules, 9(9), 416. https://doi.org/10.3390/biom9090416








