TrkB Agonist Treatment Decreases Hippocampal Testosterone Contents in a Sex-Dependent Manner Following Neonatal Hypoxia and Ischemia
Abstract
1. Introduction
2. Materials and Methods
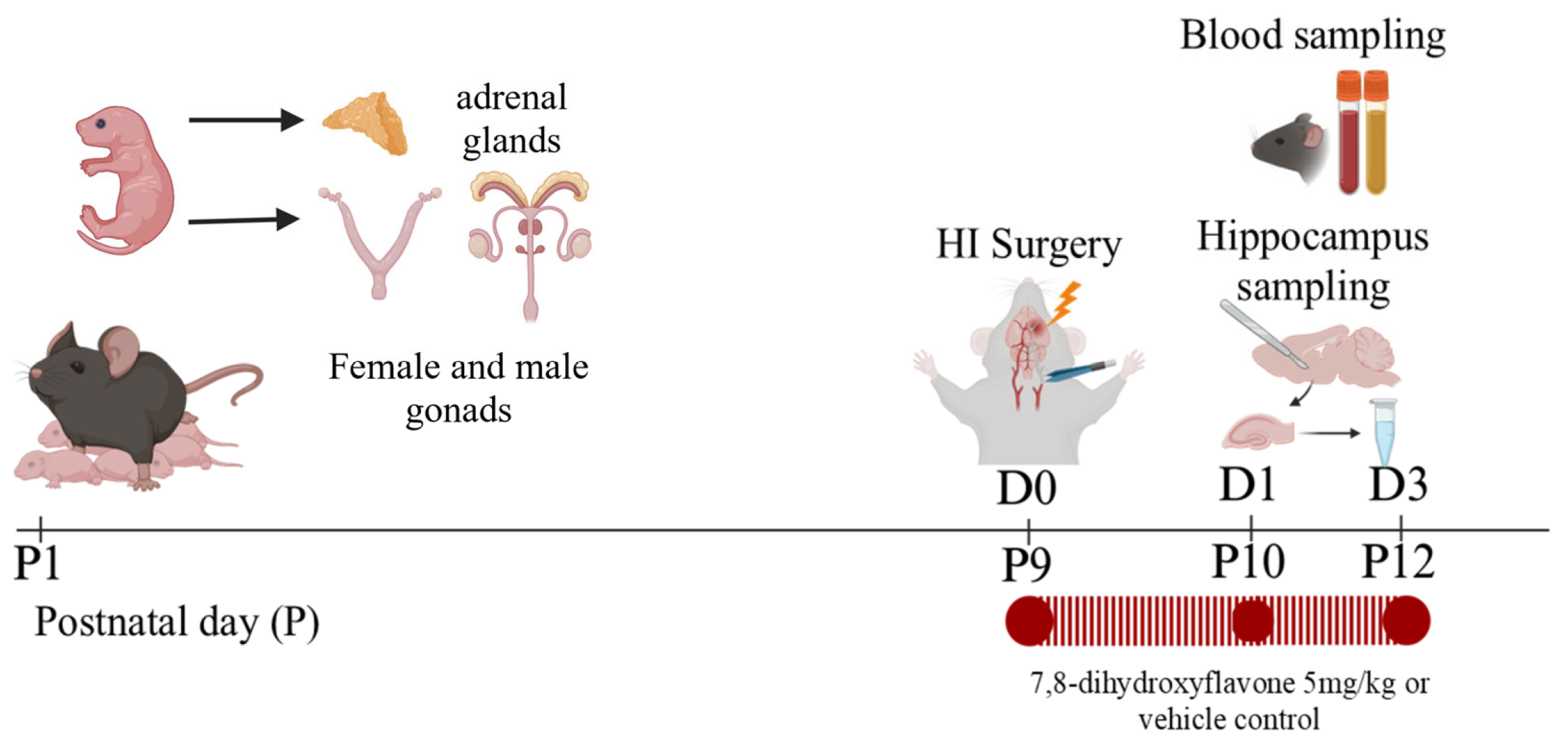
2.1. Animal Use
2.2. Induction of Neonatal HI
2.3. Drug Administration
2.4. Blood Sampling and Hippocampal Tissue Extraction
2.5. LCMS-MS Measurement
2.6. Statistical Analysis
3. Results
3.1. Multivariate Analysis for T
3.2. T Contents in Plasma and Hippocampi in Sham Mice
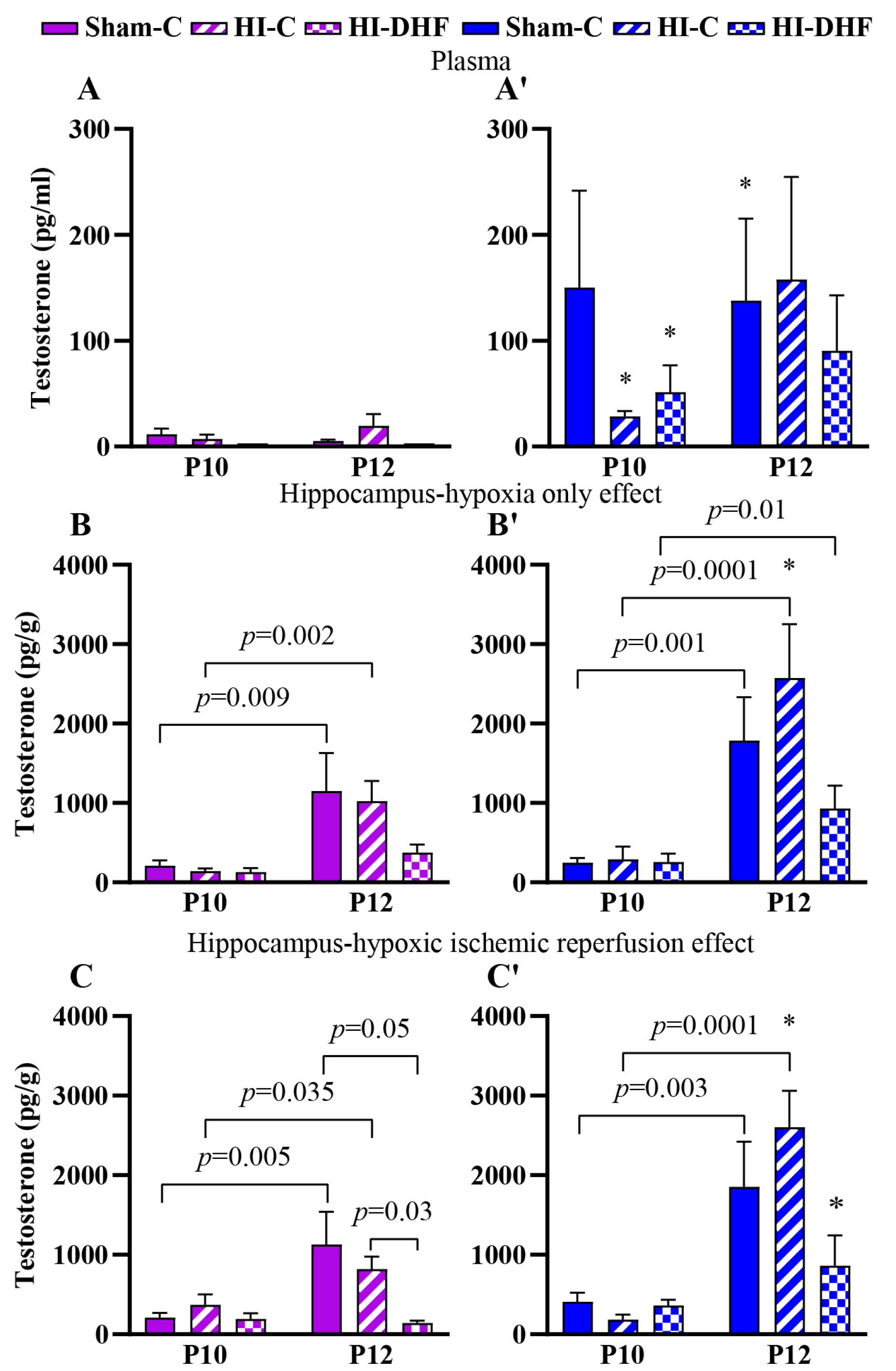
3.3. Effect of HO and DHF on Hippocampal T Contents
3.4. Effect of HI and DHF on Hippocampal T Contents
3.5. Multivariate Analysis for E2
3.6. E2 Contents in Plasma and Hippocampi in Sham Mice
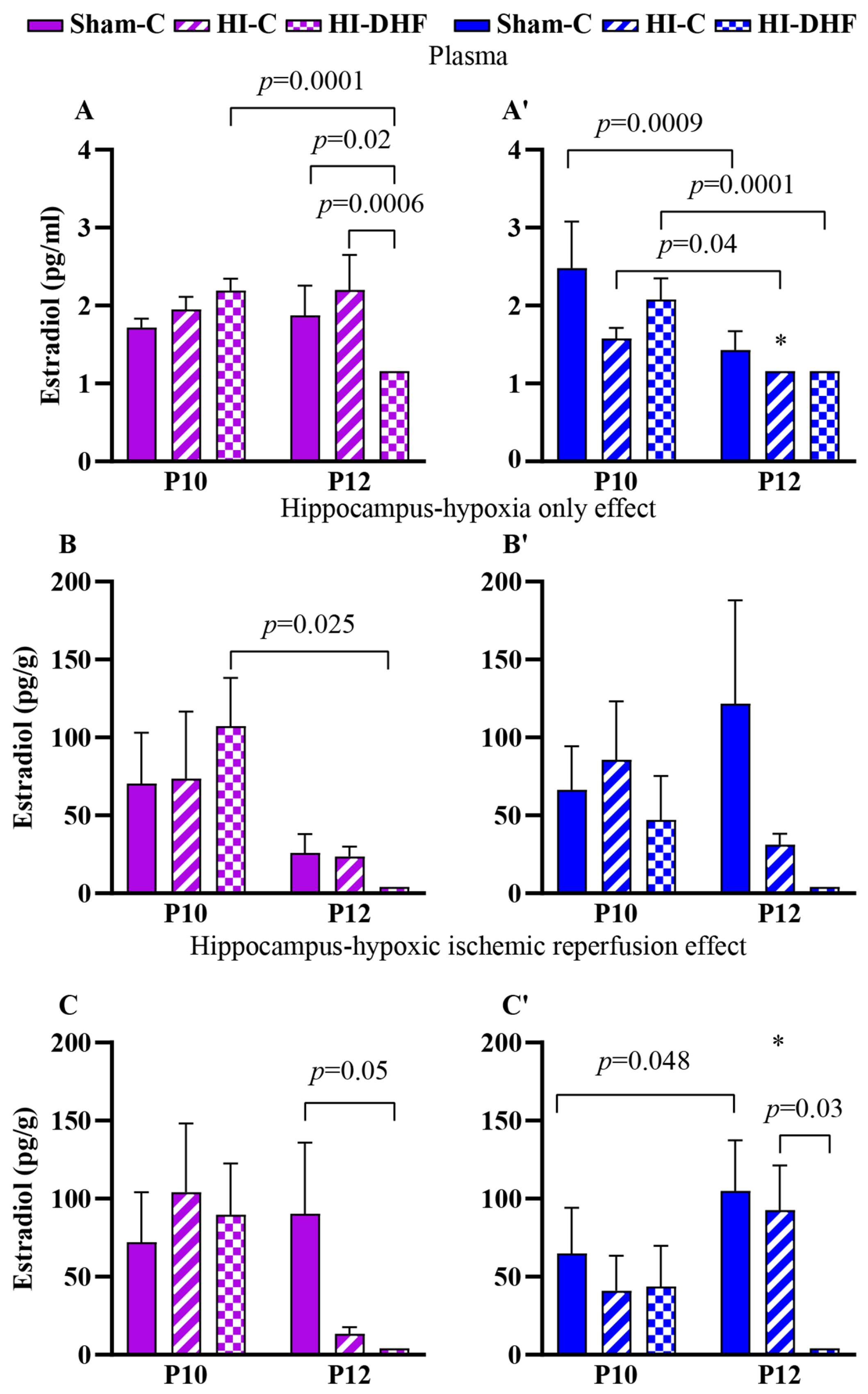
3.7. Effect of HO and DHF on Hippocampal E2 Content
3.8. Effect of HI and DHF on Hippocampal E2 Content
3.9. Multivariate Analysis for P4
3.10. P4 Content in Plasma and Hippocampi in Sham Mice
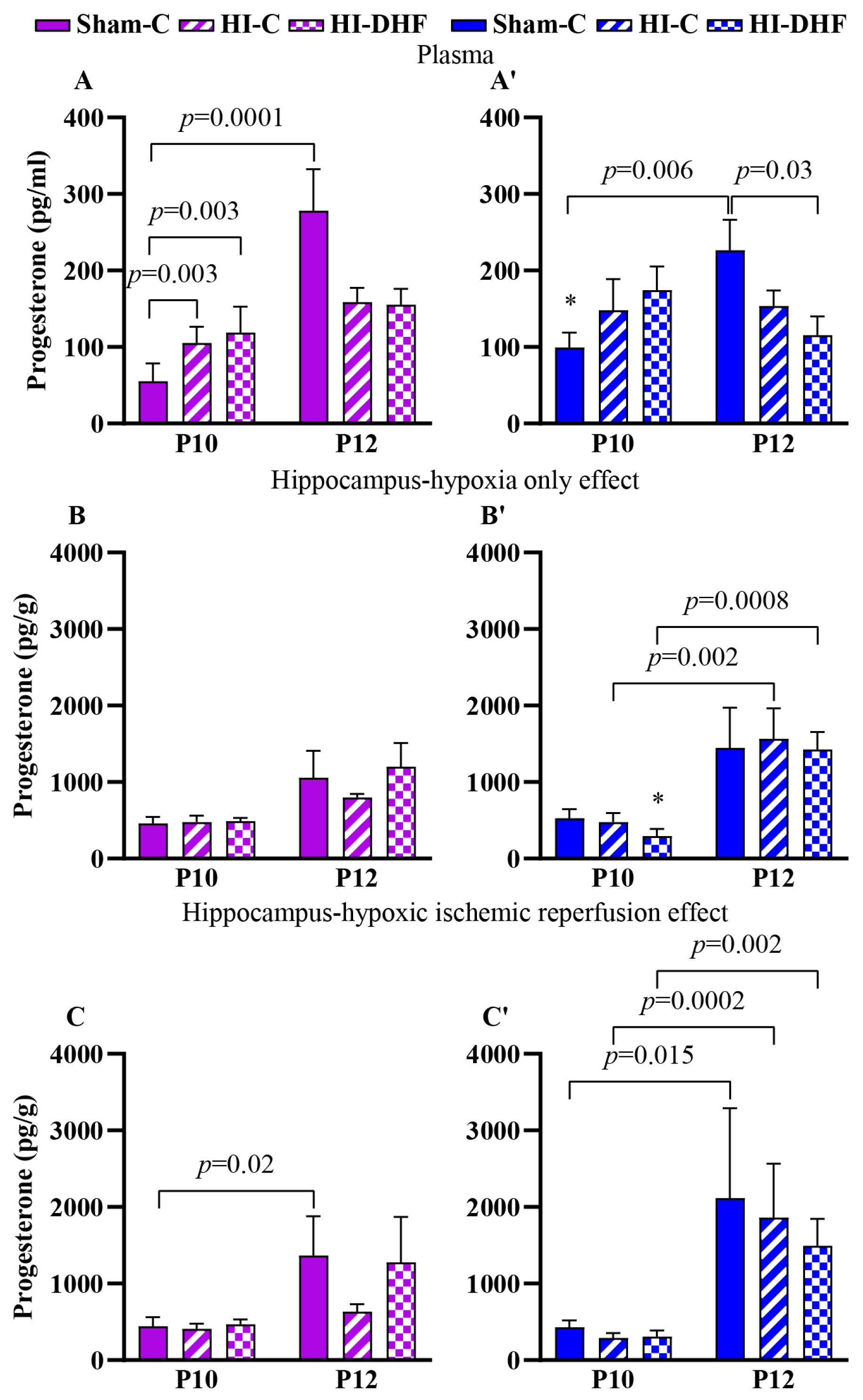
3.11. Effect of HO and DHF on Hippocampal P4 Content
3.12. Effect of HI and DHF on Hippocampal P4 Content
3.13. Multivariate Analysis for CORT
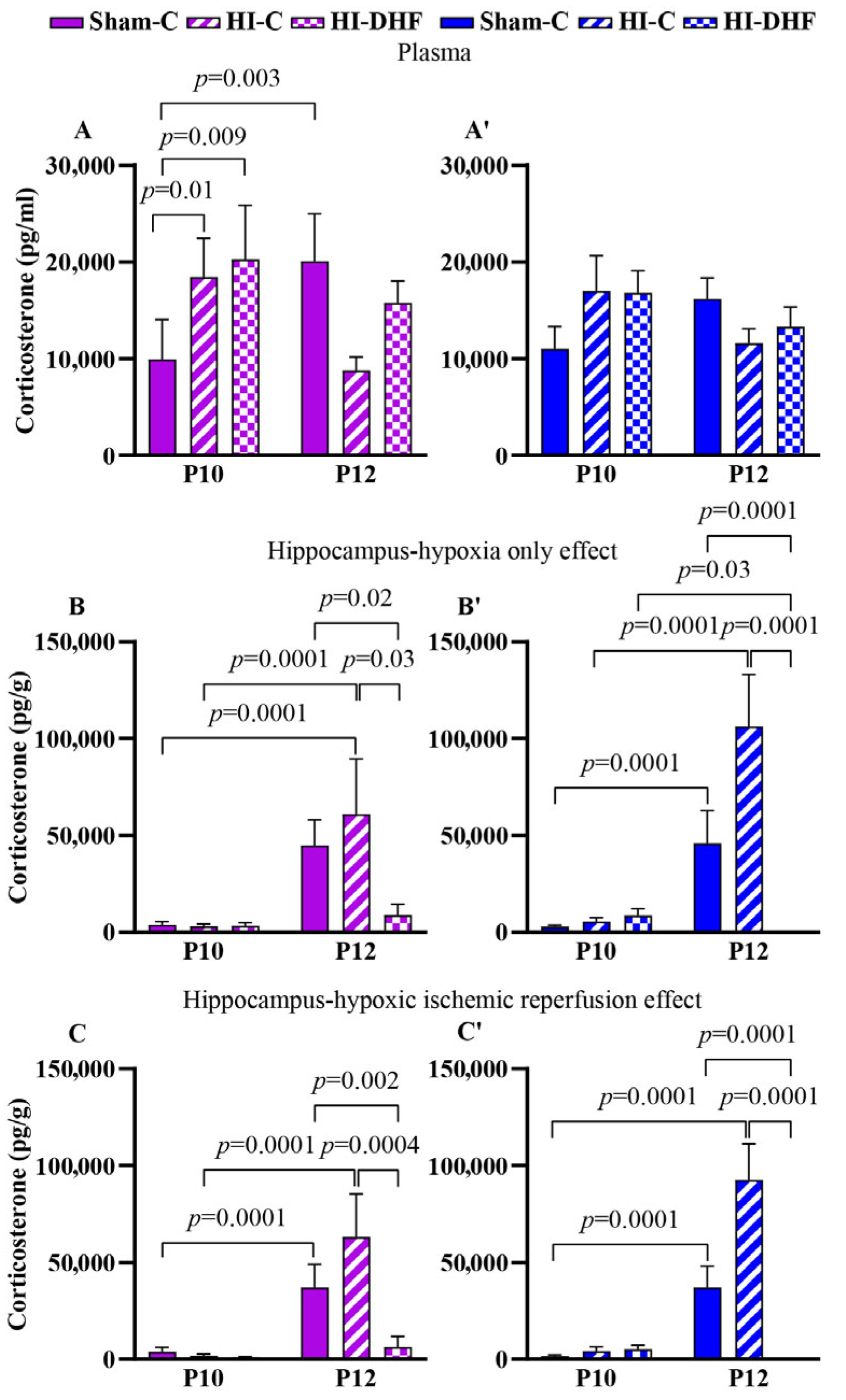
3.14. CORT Contents in Plasma and Hippocampi in Sham Mice
3.15. Effect of HO and DHF on Hippocampal CORT Contents
3.16. Effect of HI and DHF on Hippocampal CORT Contents
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HI | Hypoxia–ischemia |
| HO | Hypoxia only |
| P | Postnatal day |
| TrkB | Tyrosine kinase B receptor |
| DHF | 7,8 dihydroxyflavone |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| ERα | Estrogen receptor alpha |
| T | Testosterone |
| E2 | Estradiol |
| P4 | Progesterone |
| CORT | Corticosterone |
| CL | Contralateral |
| IL | Ipsilateral |
References
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Greco, P.; Nencini, G.; Piva, I.; Scioscia, M.; Volta, C.A.; Spadaro, S.; Neri, M.; Bonaccorsi, G.; Greco, F.; Cocco, I.; et al. Pathophysiology of hypoxic-ischemic encephalopathy: A review of the past and a view on the future. Acta Neurol. Belg 2020, 120, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Ferriero, D.M. Neonatal brain injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef]
- Lawn, J.E.; Osrin, D.; Adler, A.; Cousens, S. Four million neonatal deaths: Counting and attribution of cause of death. Paediatr. Perinat. Epidemiol. 2008, 22, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Chalak, L.F.; Pruszynski, J.E.; Spong, C.Y. Sex Vulnerabilities to Hypoxia-Ischemia at Birth. JAMA Netw. Open 2023, 6, e2326542. [Google Scholar] [CrossRef]
- Johnston, M.V.; Hadgberg, H. Sex and the pathogenesis of cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Ritzel, R.; Xu, Y.; McCullough, L.D.; Liu, F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflamm. 2015, 12, 32. [Google Scholar] [CrossRef]
- Hill, C.A.; Fitch, R.H. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: Implications for sex-specific neuroprotection in clinical neonatal practice. Neurol. Res. Int. 2012, 2012, 867531. [Google Scholar] [CrossRef]
- Uluc, K.; Kendigelen, P.; Fidan, E.; Zhang, L.; Chanana, V.; Kintner, D.; Akture, E.; Song, C.; Ye, K.; Sun, D.; et al. TrkB receptor agonist 7, 8 dihydroxyflavone triggers profound gender- dependent neuroprotection in mice after perinatal hypoxia and ischemia. CNS Neurol. Disord. Drug Targets 2013, 12, 360–370. [Google Scholar] [CrossRef]
- Cikla, U.; Chanana, V.; Kintner, D.B.; Udho, E.; Eickhoff, J.; Sun, W.; Marquez, S.; Covert, L.; Otles, A.; Shapiro, R.A.; et al. ERalpha Signaling Is Required for TrkB-Mediated Hippocampal Neuroprotection in Female Neonatal Mice after Hypoxic Ischemic Encephalopathy(1,2,3). eNeuro 2016, 3, e0025-15. [Google Scholar] [CrossRef]
- Chanana, V.; Hackett, M.; Deveci, N.; Aycan, N.; Ozaydin, B.; Cagatay, N.S.; Hanalioglu, D.; Kintner, D.B.; Corcoran, K.; Yapici, S.; et al. TrkB-mediated sustained neuroprotection is sex-specific and Eralpha-dependent in adult mice following neonatal hypoxia ischemia. Biol. Sex Differ. 2024, 15, 1. [Google Scholar] [CrossRef]
- Chanana, V.; Zafer, D.; Kintner, D.B.; Chandrashekhar, J.H.; Eickhoff, J.; Ferrazzano, P.A.; Levine, J.E.; Cengiz, P. TrkB-mediated neuroprotection in female hippocampal neurons is autonomous, estrogen receptor alpha-dependent, and eliminated by testosterone: A proposed model for sex differences in neonatal hippocampal neuronal injury. Biol. Sex Differ. 2024, 15, 30. [Google Scholar] [CrossRef]
- Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocrinol. 2019, 55, 100787. [Google Scholar] [CrossRef]
- Spritzer, M.D.; Roy, E.A.; Calhoun, K.M.K.; Schneider-Lynch, Z.E.; Panella, L.; Michaelcheck, C.; Qian, A.; Kelly, E.D.; Barr, H.; Hall, E.; et al. Effects of Testosterone and Its Major Metabolites upon Different Stages of Neuron Survival in the Dentate Gyrus of Male Rats. Biomolecules 2025, 15, 542. [Google Scholar] [CrossRef] [PubMed]
- Konkle, A.T.; McCarthy, M.M. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 2011, 152, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, T.S.; Hussain, Z.; Fitch, R.H. Sex Differences in Brain Injury and Repair in Newborn Infants: Clinical Evidence and Biological Mechanisms. Front. Pediatr. 2019, 7, 211. [Google Scholar] [CrossRef]
- Yang, S.H.; Perez, E.; Cutright, J.; Liu, R.; He, Z.; Day, A.L.; Simpkins, J.W. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J. Appl. Physiol. 2002, 92, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; Singh, M.; Su, C.; Cunningham, R.L. Effects of Oxidative Stress and Testosterone on Pro-Inflammatory Signaling in a Female Rat Dopaminergic Neuronal Cell Line. Endocrinology 2016, 157, 2824–2835. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Wang, R.; Tang, H.; Dong, Y.; Chan, A.; Sareddy, G.R.; Vadlamudi, R.K.; Brann, D.W. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol. Cell. Endocrinol. 2014, 389, 84–91. [Google Scholar] [CrossRef]
- Raghava, N.; Das, B.C.; Ray, S.K. Neuroprotective effects of estrogen in CNS injuries: Insights from animal models. Neurosci. Neuroecon 2017, 6, 15–29. [Google Scholar] [CrossRef]
- Nakano, T.; Hurn, P.D.; Herson, P.S.; Traystman, R.J. Testosterone exacerbates neuronal damage following cardiac arrest and cardiopulmonary resuscitation in mouse. Brain Res. 2010, 1357, 124–130. [Google Scholar] [CrossRef]
- Hill, C.A.; Threlkeld, S.W.; Fitch, R.H. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int. J. Dev. Neurosci. 2011, 29, 381–388. [Google Scholar] [CrossRef]
- de Assis, G.G.; de Sousa, M.B.C.; Murawska-Cialowicz, E. Sex Steroids and Brain-Derived Neurotrophic Factor Interactions in the Nervous System: A Comprehensive Review of Scientific Data. Int. J. Mol. Sci. 2025, 26, 2532. [Google Scholar] [CrossRef]
- Lannigan, D.A. Estrogen receptor phosphorylation. Steroids 2003, 68, 1–9. [Google Scholar] [CrossRef]
- Wong, J.; Woon, H.G.; Weickert, C.S. Full length TrkB potentiates estrogen receptor alpha mediated transcription suggesting convergence of susceptibility pathways in schizophrenia. Mol. Cell. Neurosci. 2011, 46, 67–78. [Google Scholar] [CrossRef]
- Jodhka, P.K.; Kaur, P.; Underwood, W.; Lydon, J.P.; Singh, M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology 2009, 150, 3162–3168. [Google Scholar] [CrossRef]
- Cole, R.; Pascal, L.E.; Wang, Z. The classical and updated models of androgen receptor nucleocytoplasmic trafficking. Am. J. Clin. Exp. Urol. 2021, 9, 287–291. [Google Scholar] [PubMed]
- Yang, L.Y.; Verhovshek, T.; Sengelaub, D.R. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology 2004, 145, 161–168. [Google Scholar] [CrossRef]
- Di Donato, M.; Bilancio, A.; Auricchio, F.; Castoria, G.; Migliaccio, A. Androgens and NGF Mediate the Neurite-Outgrowth through Inactivation of RhoA. Cells 2023, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Vannucci, S.J. Perinatal hypoxic-ischemic brain damage: Evolution of an animal model. Dev. Neurosci. 2005, 27, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Scarlett, C.O.; Yapici, S.; Ferrazzano, P.; Cengiz, P. Pharmacokinetics of 7,8-dihydroxyflavone in neonatal mice with hypoxia-ischemia related brain injury. Front. Pharmacol. 2024, 15, 1508696. [Google Scholar] [CrossRef]
- Chanana, V.; Tumturk, A.; Kintner, D.; Udho, E.; Ferrazzano, P.; Cengiz, P. Sex Differences in Mouse Hippocampal Astrocytes after In-Vitro Ischemia. J. Vis. Exp. JoVE 2016, e53695. [Google Scholar] [CrossRef]
- Beaudoin, G.M., 3rd; Lee, S.H.; Singh, D.; Yuan, Y.; Ng, Y.G.; Reichardt, L.F.; Arikkath, J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 2012, 7, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Cikla, U.; Chanana, V.; Kintner, D.B.; Covert, L.; Dewall, T.; Waldman, A.; Rowley, P.; Cengiz, P.; Ferrazzano, P. Suppression of microglia activation after hypoxia-ischemia results in age-dependent improvements in neurologic injury. J. Neuroimmunol. 2016, 291, 18–27. [Google Scholar] [CrossRef]
- McQuillen, P.S.; Ferriero, D.M. Selective vulnerability in the developing central nervous system. Pediatr. Neurol. 2004, 30, 227–235. [Google Scholar] [CrossRef]
- Morken, T.S.; Brekke, E.; Haberg, A.; Wideroe, M.; Brubakk, A.M.; Sonnewald, U. Altered astrocyte-neuronal interactions after hypoxia-ischemia in the neonatal brain in female and male rats. Stroke A J. Cereb. Circ. 2014, 45, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, N.C.; Sohrabji, F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol. Dis. 2016, 85, 245–253. [Google Scholar] [CrossRef]
- Liu, M.; Oyarzabal, E.A.; Yang, R.; Murphy, S.J.; Hurn, P.D. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. J. Neurosci. Methods 2008, 171, 214–217. [Google Scholar] [CrossRef]
- Bertin, J.; Dury, A.Y.; Ke, Y.; Ouellet, J.; Labrie, F. Accurate and sensitive liquid chromatography/tandem mass spectrometry simultaneous assay of seven steroids in monkey brain. Steroids 2015, 98, 37–48. [Google Scholar] [CrossRef]
- Munley, K.M.; Wade, K.L.; Pradhan, D.S. Uncovering the seasonal brain: Liquid chromatography-tandem mass spectrometry (LC-MS/MS) as a biochemical approach for studying seasonal social behaviors. Horm. Behav. 2022, 142, 105161. [Google Scholar] [CrossRef]
- Kenealy, B.P.; Kapoor, A.; Guerriero, K.A.; Keen, K.L.; Garcia, J.P.; Kurian, J.R.; Ziegler, T.E.; Terasawa, E. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 19051–19059. [Google Scholar] [CrossRef]
- Skalski, H.J.; Arendt, A.R.; Harkins, S.K.; MacLachlan, M.; Corbett, C.J.M.; Goy, R.W.; Kapoor, A.; Hostetter, G.; Chandler, R.L. Key Considerations for Studying the Effects of High-Fat Diet on the Nulligravid Mouse Endometrium. J. Endocr. Soc. 2024, 8, bvae104. [Google Scholar] [CrossRef] [PubMed]
- Pillerova, M.; Borbelyova, V.; Hodosy, J.; Riljak, V.; Renczes, E.; Frick, K.M.; Tothova, L. On the role of sex steroids in biological functions by classical and non-classical pathways. An update. Front. Neuroendocrinol. 2021, 62, 100926. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.; Herbison, A.E. Hypothalamic control of the male neonatal testosterone surge. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150115. [Google Scholar] [CrossRef] [PubMed]
- Fester, L.; Rune, G.M. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2015, 1621, 162–169. [Google Scholar] [CrossRef]
- Cabrera, O.H.; Gulvezan, T.; Symmes, B.; Quillinan, N.; Jevtovic-Todorovic, V. Sex differences in neurodevelopmental abnormalities caused by early-life anaesthesia exposure: A narrative review. Br. J. Anaesth. 2020, 124, e81–e91. [Google Scholar] [CrossRef]
- Aubrecht, T.G.; Jenkins, R.; Magalang, U.J.; Nelson, R.J. Influence of gonadal hormones on the behavioral effects of intermittent hypoxia in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R489–R499. [Google Scholar] [CrossRef]
- Brockmann, M.D.; Kukovic, M.; Schonfeld, M.; Sedlacik, J.; Hanganu-Opatz, I.L. Hypoxia-ischemia disrupts directed interactions within neonatal prefrontal-hippocampal networks. PLoS ONE 2013, 8, e83074. [Google Scholar] [CrossRef]
- Waddell, J.; Hanscom, M.; Shalon Edwards, N.; McKenna, M.C.; McCarthy, M.M. Sex differences in cell genesis, hippocampal volume and behavioral outcomes in a rat model of neonatal HI. Exp. Neurol. 2016, 275, 285–295. [Google Scholar] [CrossRef]
- Durán-Carabali, L.E.; Arcego, D.M.; Sanches, E.F.; Odorcyk, F.K.; Marques, M.R.; Tosta, A.; Reichert, L.; Carvalho, A.S.; Dalmaz, C.; Netto, C.A. Preventive and therapeutic effects of environmental enrichment in Wistar rats submitted to neonatal hypoxia-ischemia. Behav. Brain Res. 2019, 359, 485–497. [Google Scholar] [CrossRef]
- Mabry, S.; Wilson, E.N.; Bradshaw, J.L.; Gardner, J.J.; Fadeyibi, O.; Vera, E., Jr.; Osikoya, O.; Cushen, S.C.; Karamichos, D.; Goulopoulou, S.; et al. Sex and age differences in social and cognitive function in offspring exposed to late gestational hypoxia. Biol. Sex Differ. 2023, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.; Duong, P.; Trieu, J.; Cunningham, R.L. Androgens modulate chronic intermittent hypoxia effects on brain and behavior. Horm. Behav. 2018, 106, 62–73. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, Q.; Kong, D.; Zhou, C.; Zhou, J.; Han, J.; Zhou, Y.; Jin, G.; Hua, X.; Wang, J.; et al. Gender difference in the effect of progesterone on neonatal hypoxic/ischemic brain injury in mouse. Exp. Neurol. 2018, 306, 190–198. [Google Scholar] [CrossRef]
- Annink, K.V.; de Vries, L.S.; Groenendaal, F.; Eijsermans, R.; Mocking, M.; van Schooneveld, M.M.J.; Dudink, J.; van Straaten, H.L.M.; Benders, M.; Lequin, M.; et al. Mammillary body atrophy and other MRI correlates of school-age outcome following neonatal hypoxic-ischemic encephalopathy. Sci. Rep. 2021, 11, 5017. [Google Scholar] [CrossRef]
- Stamenova, V.; Nicola, R.; Aharon-Peretz, J.; Goldsher, D.; Kapeliovich, M.; Gilboa, A. Long-term effects of brief hypoxia due to cardiac arrest: Hippocampal reductions and memory deficits. Resuscitation 2018, 126, 65–71. [Google Scholar] [CrossRef]
- van Handel, M.; Swaab, H.; de Vries, L.S.; Jongmans, M.J. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: A review. Eur. J. Pediatr. 2007, 166, 645–654. [Google Scholar] [CrossRef]
- Chang, H.A.; Wang, Y.H.; Tung, C.S.; Yeh, C.B.; Liu, Y.P. 7,8-Dihydroxyflavone, a Tropomyosin-Kinase Related Receptor B Agonist, Produces Fast-Onset Antidepressant-Like Effects in Rats Exposed to Chronic Mild Stress. Psychiatry Investig. 2016, 13, 531–540. [Google Scholar] [CrossRef][Green Version]
- Andero, R.; Daviu, N.; Escorihuela, R.M.; Nadal, R.; Armario, A. 7,8-dihydroxyflavone, a TrkB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus 2012, 22, 399–408. [Google Scholar] [CrossRef]
- Barfield, E.T.; Gourley, S.L. Adolescent Corticosterone and TrkB Pharmaco-Manipulations Sex-Dependently Impact Instrumental Reversal Learning Later in Life. Front. Behav. Neurosci. 2017, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Barfield, E.T.; Gerber, K.J.; Zimmermann, K.S.; Ressler, K.J.; Parsons, R.G.; Gourley, S.L. Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure. PLoS Biol. 2017, 15, e2003000. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kao, Y.C.; Laughton, C.A. Binding characteristics of aromatase inhibitors and phytoestrogens to human aromatase. J. Steroid Biochem. Mol. Biol. 1997, 61, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mak, P.; Tchoudakova, A.; Callard, G.; Chen, S. Different catalytic properties and inhibitor responses of the goldfish brain and ovary aromatase isozymes. Gen. Comp. Endocrinol. 2001, 123, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sareddy, G.R.; Wang, J.; Wang, R.; Li, Y.; Dong, Y.; Zhang, Q.; Liu, J.; O’Connor, J.C.; Xu, J.; et al. Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 2792–2809. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Bilancio, A.; D’Amato, L.; Claudiani, P.; Oliviero, M.A.; Barone, M.V.; Auricchio, A.; Appella, E.; Migliaccio, A.; Auricchio, F.; et al. Cross-talk between androgen receptor/filamin A and TrkA regulates neurite outgrowth in PC12 cells. Mol. Biol. Cell 2015, 26, 2858–2872. [Google Scholar] [CrossRef]
| Sham-C | HI-C | HI-DHF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P10 | P12 | p-Value | P10 | P12 | p-Value | P10 | P12 | p-Value | |||
| Testosterone | Female | Plasma | 11.6 ± 5.3 (8) | 5.3 ± 1.2 * (9) | 0.64 | 7.1 ± 4.2 * (8) | 19.6 ± 11.1 (9) | 0.27 | 2.9 ± 0.0 * (8) | 2.9 ± 0.0 (14) | 1.00 |
| CL | 211 ± 67.9 (8) | 1152 ± 476 (9) | 0.01 | 140 ± 36.9 (5) | 1023 ± 253 * (10) | 0.002 | 130.0 ± 48.6 (8) | 376 ± 102 (3) | 0.057 | ||
| IL | 210 ± 58.4 (8) | 1129 ± 414 (8) | 0.005 | 370. ± 133 (6) | 821 ± 157 * (9) | 0.04 | 195 ± 68.9 (8) | 142 ± 30.5 * (3) | 0.94 | ||
| Male | Plasma | 150 ± 91.3 (8) | 137.8 ± 77.6 * (12) | 0.59 | 28.5 ± 5.1 * (8) | 157.7 ± 97 (14) | 0.75 | 51.5 ± 25.3 * (8) | 90.4 ± 52.6 (14) | 0.08 | |
| CL | 249 ± 58.2 (8) | 1788 ± 545 (8) | 0.001 | 292 ± 1601 (8) | 2574 ± 678 * (7) | <0.0001 | 255 ± 107 (8) | 931 ± 287 (3) | 0.01 | ||
| IL | 409 ± 114.7 (8) | 1853 ± 570.1 (9) | 0.003 | 186 ± 60.8 (8) | 2601 ± 460 * (7) | <0.0001 | 363 ± 72.9 (8) | 861 ± 380 * (3) | 0.16 | ||
| Estradiol | Female | Plasma | 1.7 ± 0.1 (8) | 1.9 ± 0.4 (9) | 0.8821 | 2.0 ± 0.2 (8) | 2.2 ± 0.5 * (9) | 1.00 | 2.2 ± 0.2 (8) | 1.2 ± 0.0 (14) | <0.0001 |
| CL | 70.5 ± 32.5 (8) | 26.0 ± 12.1 (9) | 0.80 | 73.6 ± 43.0 (5) | 23.7 ± 6.4 (10) | 0.89 | 107 ± 30.9 (8) | 4.1 ± 0.0 (3) | 0.03 | ||
| IL | 72.1 ± 31.9 (8) | 90.3 ± 45.7 (8) | 0.28 | 104.2 ± 44.1 (6) | 13.5 ± 4.2 * (9) | 0.15 | 89.8 ± 32.8 (8) | 4.1 ± 0.0 (3) | 0.07 | ||
| Male | Plasma | 2.5 ± 0.6 (8) | 1.4 ± 0.2 (12) | 0.0009 | 1.6 ± 0.1 (8) | 1.2 ± 0.0 * (14) | 0.04 | 2.1 ± 0.3 (8) | 1.2 ± 0.0 (14) | 0.0001 | |
| CL | 66.4 ± 28.1 (8) | 122 ± 66.5 (9) | 0.36 | 85.7 ± 37.5 (8) | 31.3 ± 7.1 (7) | 0.88 | 47.1 ± 28.2 (8) | 4.1 ± 0.0 (3) | 0.36 | ||
| IL | 64.9 ± 29.3 (8) | 105 ± 32.4 (9) | 0.05 | 41.0 ± 22.5 (8) | 92.7 ± 28.5 * (7) | 0.06 | 43.8 ± 26.0 (8) | 4.1 ± 0.0 (3) | 0.37 | ||
| Progesterone | Female | Plasma | 55.4 ± 23.2 * (8) | 278 ± 54 (9) | <0.0001 | 105 ± 21.6 (8) | 158 ± 18.7 (9) | 0.14 | 119 ± 33 (8) | 155 ± 20.8 (14) | 0.19 |
| CL | 460 ± 85.2 (8) | 1057 ± 351.4 (9) | 0.10 | 477 ± 82.8 (5) | 799 ± 48.5 (10) | 0.26 | 488 ± 44.1 * (8) | 1203 ± 307 (3) | 0.16 | ||
| IL | 445 ± 117.2 (8) | 1368 ± 512.2 (8) | 0.02 | 410 ± 67.8 (6) | 633 ± 96.9 (9) | 0.39 | 467 ± 62.9 (8) | 1278 ± 594 (3) | 0.15 | ||
| Male | Plasma | 99.4 ± 19.5 * (8) | 227 ± 39.5 (12) | 0.007 | 148 ± 40.6 (8) | 153 ± 20.3 (14) | 0.59 | 175 ± 30.8 (8) | 115 ± 24.7 (14) | 0.13 | |
| CL | 526 ± 119 (8) | 1448 ± 525 (9) | 0.096 | 476 ± 118 (8) | 1567 ± 398.8 (7) | 0.002 | 294 ± 92.0 * (8) | 1426 ± 231 (3) | 0.0008 | ||
| IL | 431 ± 88.1 (8) | 2115 ± 1174 (9) | 0.015 | 290 ± 63.8 (8) | 1863 ± 703.9 (7) | 0.0002 | 307 ± 81.1 (8) | 1496 ± 350 (3) | 0.002 | ||
| Corticosterone | Female | Plasma | 9917 ± 4145 (8) | 20,068 ± 4915 (9) | 0.003 | 18,446 ± 4001 (8) | 8788 ± 1397 (9) | 0.08 | 20,244 ± 5602 (8) | 15,778 ± 2244 (14) | 0.51 |
| CL | 3811 ± 1673 (8) | 44,778 ± 13,265(9) | <0.0001 | 2891 ± 1364 (5) | 60,889 ± 28,627 (9) | <0.0001 | 3331 ± 1572 (8) | 8768 ± 5829 (3) | 0.23 | ||
| IL | 3939 ± 2114 (8) | 37,027 ± 11,984(8) | <0.0001 | 1780 ± 1066 (6) | 63,159 ± 22,218 (9) | <0.0001 | 1040 ± 326 (8) | 6248 ± 5534 (3) | 0.27 | ||
| Male | Plasma | 11,040 ± 2289(8) | 16,207 ± 2161(12) | 0.16 | 17,005 ± 3651(8) | 11,603 ± 1509 (14) | 0.60 | 16,819 ± 2253 (8) | 13,294 ± 2058 (14) | 0.31 | |
| CL | 2808 ± 836 (8) | 45,767 ± 17,085 (9) | <0.0001 | 5509 ± 2020 (8) | 106,172 ± 26,835 (7) | <0.0001 | 8699 ± 3528 (8) | 714 ± 0.0 (3) | 0.03 | ||
| IL | 1650 ± 614 (8) | 37,119 ± 10,967 (9) | <0.0001 | 4248 ± 2252 (8) | 92,522 ± 18,835 (7) | <0.0001 | 5187 ± 2118 (8) | 714 ± 0.0 (3) | 0.12 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Aycan, N.; Isik, I.; Cagatay, N.S.; Cetin, F.; Valdes-Arciniega, T.J.; Ozaydin, B.; Yapici, S.; Goy, R.W.; Collo, L.; Zhao, Q.; et al. TrkB Agonist Treatment Decreases Hippocampal Testosterone Contents in a Sex-Dependent Manner Following Neonatal Hypoxia and Ischemia. Biomolecules 2026, 16, 180. https://doi.org/10.3390/biom16020180
Aycan N, Isik I, Cagatay NS, Cetin F, Valdes-Arciniega TJ, Ozaydin B, Yapici S, Goy RW, Collo L, Zhao Q, et al. TrkB Agonist Treatment Decreases Hippocampal Testosterone Contents in a Sex-Dependent Manner Following Neonatal Hypoxia and Ischemia. Biomolecules. 2026; 16(2):180. https://doi.org/10.3390/biom16020180
Chicago/Turabian StyleAycan, Nur, Irem Isik, Nur Sena Cagatay, Feyza Cetin, Teresita J. Valdes-Arciniega, Burak Ozaydin, Sefer Yapici, Robinson W. Goy, Luc Collo, Qianqian Zhao, and et al. 2026. "TrkB Agonist Treatment Decreases Hippocampal Testosterone Contents in a Sex-Dependent Manner Following Neonatal Hypoxia and Ischemia" Biomolecules 16, no. 2: 180. https://doi.org/10.3390/biom16020180
APA StyleAycan, N., Isik, I., Cagatay, N. S., Cetin, F., Valdes-Arciniega, T. J., Ozaydin, B., Yapici, S., Goy, R. W., Collo, L., Zhao, Q., Eickhoff, J., Ferrazzano, P., Levine, J. E., Kapoor, A., & Cengiz, P. (2026). TrkB Agonist Treatment Decreases Hippocampal Testosterone Contents in a Sex-Dependent Manner Following Neonatal Hypoxia and Ischemia. Biomolecules, 16(2), 180. https://doi.org/10.3390/biom16020180






