Evaluating the Antiproliferative Effects of Tri(2-Furyl)- and Triphenylphosphine-Gold(I) Pyridyl- and Pyrimidine-Thiolate Complexes
Abstract
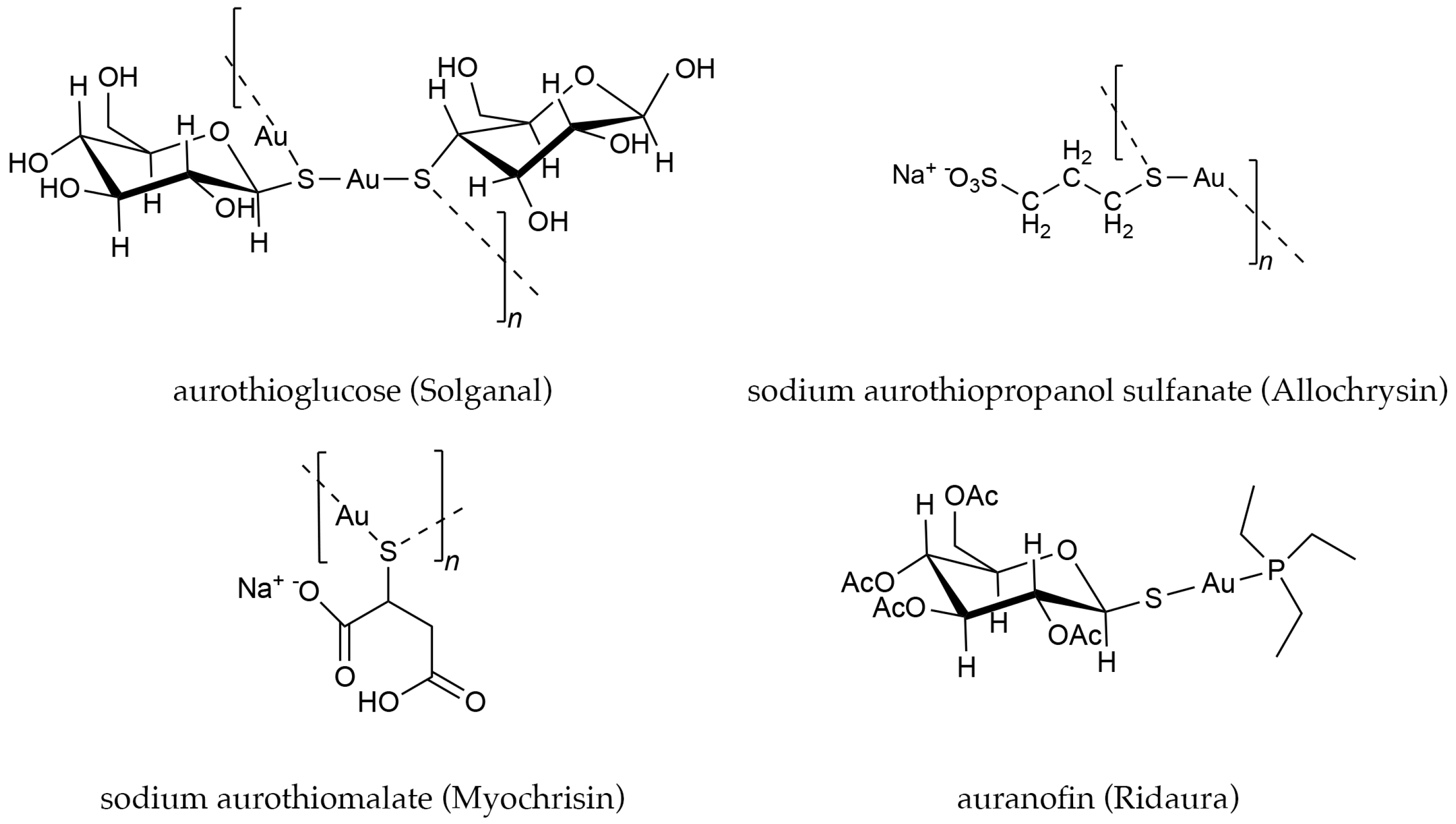
1. Introduction
2. Materials and Methods
2.1. Materials
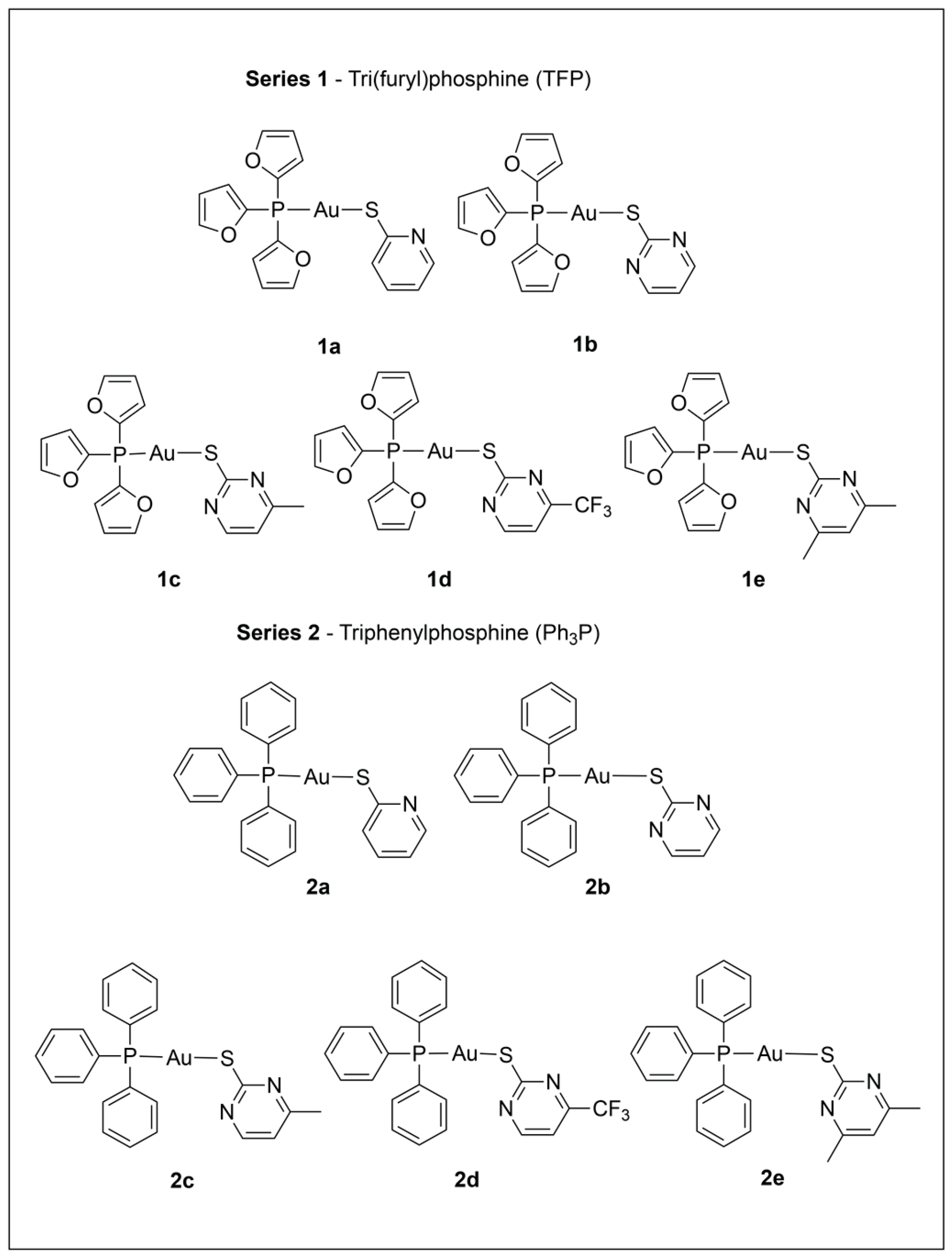
2.2. Synthesis and Characterization of Phosphine Gold(I) Thiolate Complexes
2.3. X-Ray Crystallography
2.4. ICP-MS
2.5. LC-MS
2.6. ESI-MS
2.7. UV-Vis
2.8. Cell Viability Measurements
3. Results and Discussion
3.1. Characterization and Assessment of Purity for Series 1 and 2
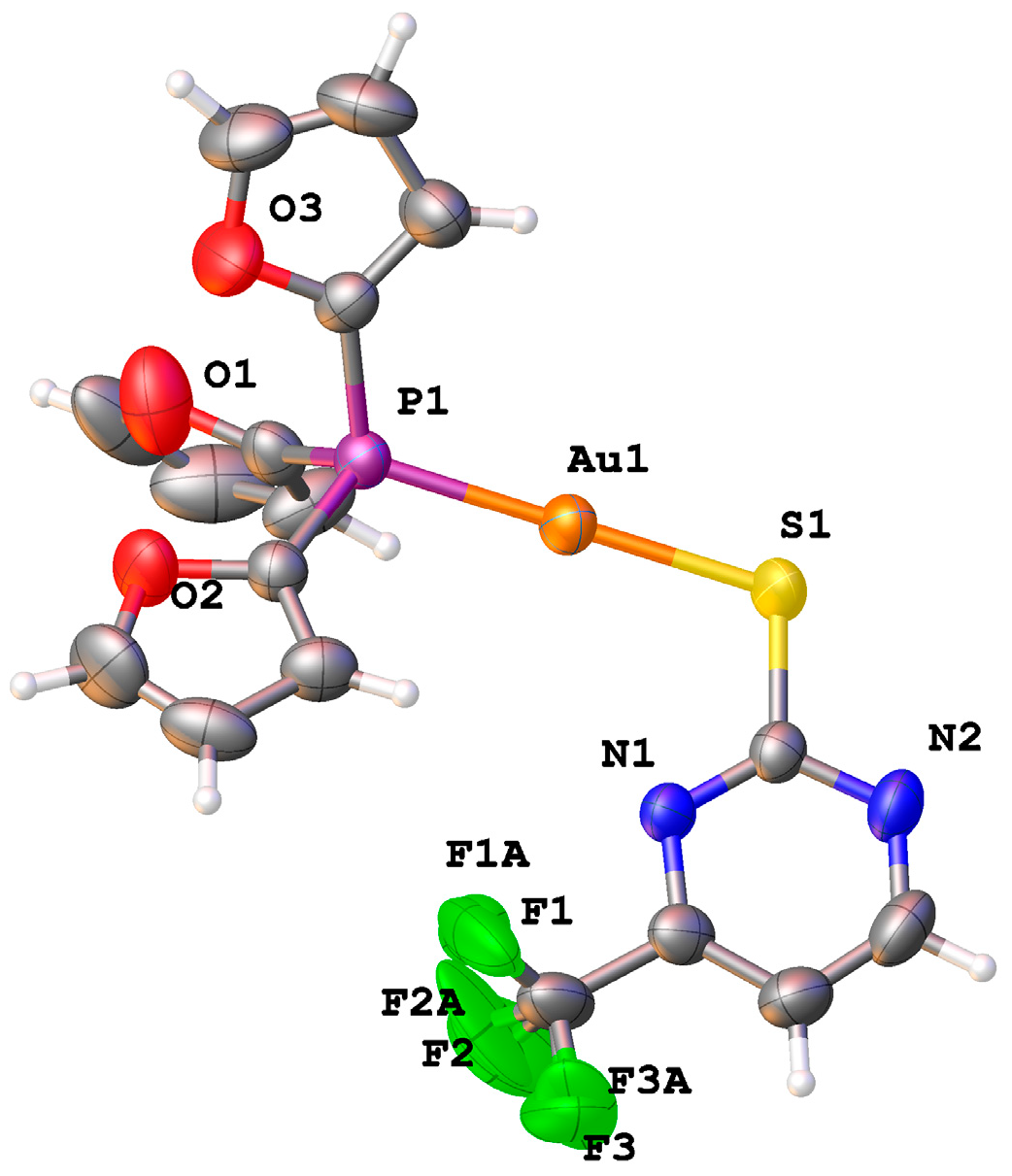
3.2. X-Ray Structure
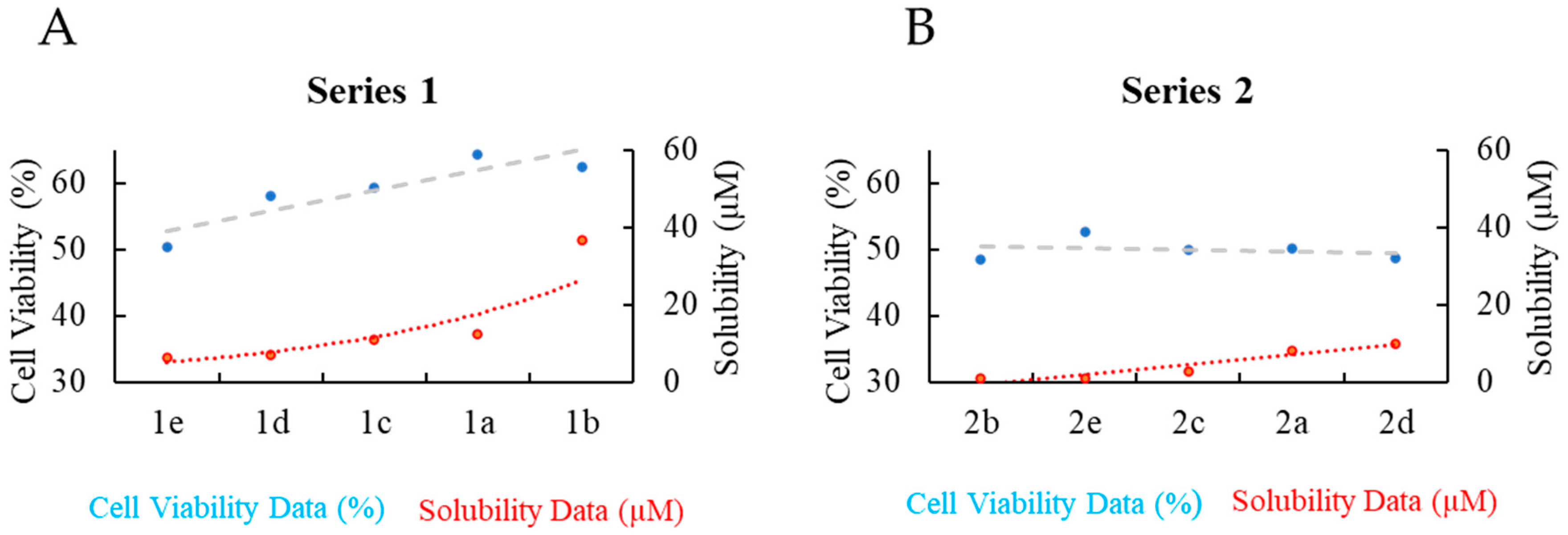
3.3. Solubility and Stability of Series 1 and 2 in Cell Media
3.4. Phase 1: Cytotoxicity of Series 1 and 2 Against the SK-BR-3 Breast Cancer Cell Line
3.5. Phase 2: Cytotoxicity and Selectivity of 1e and 2e for MDA-MB-231 and MCF7 Breast Cancer Cell Lines
3.6. Further Discussion on the Influence of 1% DMSO on the Cytotoxicity and Selectivity of 1e and 2e for MDA-MB-231 and MCF7 Breast Cancer Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Healy, M.L.; Lim, K.K.T.; Travers, R. Jacques Forestier (1890–1978) and gold therapy. Int. J. Rheum. Dis. 2009, 12, 145–148. [Google Scholar] [CrossRef]
- FDA. FDA Media 24 May 1985—Approval of Auranofin. Available online: https://www.fda.gov/media/177921/download (accessed on 13 May 2025).
- Sutton, B.M.; McGusty, E.; Walz, D.T.; DiMartino, M.J. Oral gold. Antiarthritic properties of alkylphosphinegold coordination complexes. J. Med. Chem. 1972, 15, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Clauss, G.; Fontes, J.V.; Oliveira, L.S.; Abbehausen, C. Oxidative Stress Mechanism by Gold Compounds: A Close Look at Total ROS Increase and the Inhibition of Antioxidant Enzymes. Chem. Asian J. 2025, 20, e202400792. [Google Scholar] [CrossRef] [PubMed]
- Hemmert, C.; Gornitzka, H.; Deraeve, C.; Stigliani, J.L. Current state of the art of gold complexes as antileishmanial agents. Coord. Chem. Rev. 2025, 528, 21. [Google Scholar] [CrossRef]
- Belza, J.; Trávníček, Z.; Vančo, J.; Čajan, M.; Hošek, J.; Dvořák, Z. Gold(I) N-Heterocyclic Carbene Complexes with 7-Azaindoles Demonstrates In Vitro Antiproliferative Effects on Ovarian Cancer Cells and Anti-inflammatory Activity. Organometallics 2024, 43, 1155–1164. [Google Scholar] [CrossRef]
- Seo, M.J.; Kim, I.Y.; Lee, D.M.; Park, Y.J.; Cho, M.-Y.; Jin, H.J.; Choi, K.S. Dual inhibition of thioredoxin reductase and proteasome is required for auranofin-induced paraptosis in breast cancer cells. Cell Death Dis. 2023, 14, 42. [Google Scholar] [CrossRef]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.; Sobreira, T.J.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 2016, 6, 22571–22584. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Aggarwal, S.; Celaje, J.J.A.; Ihara, S.; Ang, J.; Eremin, D.B.; Land, K.M.; Wrischnik, L.A.; Zhang, L.; Fokin, V.V.; et al. Gold(I) Phosphine Derivatives with Improved Selectivity as Topically Active Drug Leads to Overcome 5-Nitroheterocyclic Drug Resistance in Trichomonas vaginalis. J. Med. Chem. 2021, 64, 6608–6620. [Google Scholar] [CrossRef]
- Estrada-Ortiz, N.; Lopez-Gonzales, E.; Woods, B.; Stürup, S.; De Graaf, I.A.M.; Groothuis, G.M.M.; Casini, A. Ex vivo toxicological evaluation of experimental anticancer gold(i) complexes with lansoprazole-type ligands. Toxicol. Res. 2019, 8, 885–895. [Google Scholar] [CrossRef]
- Gambini, V.; Tilio, M.; Maina, E.W.; Andreani, C.; Bartolacci, C.; Wang, J.; Iezzi, M.; Ferraro, S.; Ramadori, A.T.; Simon, O.C.; et al. In vitro and in vivo studies of gold(I) azolate/phosphane complexes for the treatment of basal like breast cancer. Eur. J. Med. Chem. 2018, 155, 418–427. [Google Scholar] [CrossRef]
- NIH National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov (accessed on 14 February 2025).
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Galassi, R.; Luciani, L.; Wang, J.; Vincenzetti, S.; Cui, L.; Amici, A.; Pucciarelli, S.; Marchini, C. Breast Cancer Treatment: The Case of Gold(I)-Based Compounds as a Promising Class of Bioactive Molecules. Biomolecules 2022, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Landini, I.; Massai, L.; Cirri, D.; Gamberi, T.; Paoli, P.; Messori, L.; Mini, E.; Nobili, S. Structure-activity relationships in a series of auranofin analogues showing remarkable antiproliferative properties. J. Inorg. Biochem. 2020, 208, 111079. [Google Scholar] [CrossRef] [PubMed]
- Gamberi, T.; Chiappetta, G.; Fiaschi, T.; Modesti, A.; Sorbi, F.; Magherini, F. Upgrade of an Old Drug: Auranofin in Innovative Cancer Therapies to Overcome Drug Resistance and to Increase Drug Effectiveness. Med. Res. Rev. 2021, 42, 1111–1146. [Google Scholar] [CrossRef] [PubMed]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs RD 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Gromer, S.; Arscott, L.; Williams, C.J.; Schirmer, R.; Becker, K. Human placenta thioredoxin reductase—Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998, 273, 20096–20101. [Google Scholar] [CrossRef]
- Zhang, X.; Selvaraju, K.; Saei, A.A.; D’Arcy, P.; Zubarev, R.A.; Arnér, E.S.; Linder, S. Repurposing of auranofin: Thioredoxin reductase remains a primary target of the drug. Biochimie 2019, 162, 46–54. [Google Scholar] [CrossRef]
- Saei, A.A.; Gullberg, H.; Sabatier, P.; Beusch, C.M.; Johansson, K.; Lundgren, B.; Arvidsson, P.I.; Arner, E.S.J.; Zubarev, R.A. Comprehensive chemical proteomics for target deconvolution of the redox active drug auranofin. Redox Biol. 2020, 32, 101491. [Google Scholar] [CrossRef]
- Sabatier, P.; Beusch, C.M.; Gencheva, R.; Cheng, Q.; Zubarev, R.; Arner, E.S.J. Comprehensive chemical proteomics analyses reveal that the new TRi-1 and TRi-2 compounds are more specific thioredoxin reductase 1 inhibitors than auranofin. Redox Biol. 2021, 48, 102184. [Google Scholar] [CrossRef]
- Saei, A.A.; Lundin, A.; Lyu, H.; Gharibi, H.; Luo, H.Q.; Teppo, J.; Zhang, X.P.; Gaetani, M.; Vegvari, A.; Holmdahl, R.; et al. Multifaceted Proteome Analysis at Solubility, Redox, and Expression Dimensions for Target Identification. Adv. Sci. 2024, 15, 2401502. [Google Scholar] [CrossRef]
- Cui, X.Y.; Park, S.H.; Park, W.H. Anti-Cancer Effects of Auranofin in Human Lung Cancer Cells by Increasing Intracellular ROS Levels and Depleting GSH Levels. Molecules 2022, 27, 5207. [Google Scholar] [CrossRef] [PubMed]
- Hatem, E.; El Banna, N.; Heneman-Masurel, A.; Baïlle, D.; Vernis, L.; Riquier, S.; Golinelli-Cohen, M.-P.; Guittet, O.; Vallières, C.; Camadro, J.-M.; et al. Novel Insights into Redox-Based Mechanisms for Auranofin-Induced Rapid Cancer Cell Death. Cancers 2022, 14, 4864. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bouzakoura, S.; de Mey, S.; Jiang, H.; Law, K.; Dufait, I.; Corbet, C.; Verovski, V.; Gevaert, T.; Feron, O.; et al. Auranofin radiosensitizes tumor cells through targeting thioredoxin reductase and resulting overproduction of reactive oxygen species. Oncotarget 2017, 8, 35728–35742. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Elliott, A.G.; Kan, A.; Dinh, H.; Braese, S.; Bruce, A.E.; Bruce, M.R.; Chen, F.; Humaidy, D.; Jung, N.; et al. Metal Complexes as Antifungals?—From a Crowd-Sourced Compound Library to First In Vivo Experiments. JACS Au 2022, 2, 2277–2294. [Google Scholar] [CrossRef]
- Savin, N.; Erofeev, A.; Timoshenko, R.; Vaneev, A.; Garanina, A.; Salikhov, S.; Grammatikova, N.; Levshin, I.; Korchev, Y.; Gorelkin, P. Investigation of the Antifungal and Anticancer Effects of the Novel Synthesized Thiazolidinedione by Ion-Conductance Microscopy. Cells 2023, 12, 1666. [Google Scholar] [CrossRef]
- Weng, N.; Zhang, Z.; Tan, Y.; Zhang, X.; Wei, X.; Zhu, Q. Repurposing antifungal drugs for cancer therapy. J. Adv. Res. 2023, 48, 259–273. [Google Scholar] [CrossRef]
- Kadavakollu, S.; Stailey, C.; Kunapareddy, C.S.; White, S. Clotrimazole as a Cancer Drug: A Short Review. Med. Chem. 2014, 4, 722–724. [Google Scholar] [CrossRef]
- Pokharel, M.; Konarzewska, P.; Roberge, J.Y.; Han, G.-S.; Wang, Y.; Carman, G.M.; Xue, C. The Anticancer Drug Bleomycin Shows Potent Antifungal Activity by Altering Phospholipid Biosynthesis. Microbiol. Spectr. 2022, 10, e00862-22. [Google Scholar] [CrossRef]
- Andersen, N.G.; Keay, B.A. 2-Furyl Phosphines as Ligands for Transition-Metal-Mediated Organic Synthesis. Chem. Rev. 2001, 101, 997–1030. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gascón, S.; Rodríguez-Yoldi, M.J.; Laguna, M.; Cerrada, E. Novel Gold(I) Thiolate Derivatives Synergistic with 5-Fluorouracil as Potential Selective Anticancer Agents in Colon Cancer. Inorg. Chem. 2017, 56, 8562–8579. [Google Scholar] [CrossRef]
- Hao, L.; Mansour, M.A.; Lachicotte, R.J.; Gysling, H.J.; Eisenberg, R. A Gold(I) Mononuclear Complex and Its Association into Binuclear and Cluster Compounds by Hydrogen Bonding or Metal Ion Coordination. Inorg. Chem. 2000, 39, 5520–5529. [Google Scholar] [CrossRef] [PubMed]
- Onaka, S.; Yaguchi, M.; Yamauchi, R.; Ozeki, T.; Ito, M.; Sunahara, T.; Sugiura, Y.; Shiotsuka, M.; Nunokawa, K.; Horibe, M.; et al. The effect of carbon chain length of the diphosphine ligand on the aurophilic interaction. Synthesis and X-ray structural study for a series of Au(I) compounds with Ph2P–R–PPh2 and S-(CH2)n-py ligands. J. Organomet. Chem. 2005, 690, 57–68. [Google Scholar] [CrossRef]
- Cookson, P.D.; Tiekink, E.R.T. Triorganophosphinegold(I) complexes of pyridine-2-thionate and pyrimidine-2-thionate. J. Chem. Soc. Dalton Trans. 1993, 259–263. [Google Scholar] [CrossRef]
- Schulz Lang, E.; Fernandes, R.M., Jr.; Lemos, S.S.; Schulz Lang, L.; Burrow, R.A. (4,6-Dimethylpyrimidine-2-thiolato)(triphenylphosphine)gold(I). Acta Crystallogr. 2002, 58, m469–m470. [Google Scholar] [CrossRef]
- Hsieh, C.; Santell, R.; Haslam, S.; Helferich, W. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar]
- Pratelli, G.; Carlisi, D.; Di Liberto, D.; Notaro, A.; Giuliano, M.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Calvaruso, G.; De Blasio, A. MCL1 Inhibition Overcomes the Aggressiveness Features of Triple-Negative Breast Cancer MDA-MB-231 Cells. Int. J. Mol. Sci. 2023, 24, 11149. [Google Scholar] [CrossRef]
- Qu, Y.; Han, B.; Yu, Y.; Yao, W.; Bose, S.; Karlan, B.Y.; Giuliano, A.E.; Cui, X. Evaluation of MCF10A as a Reliable Model for Normal Human Mammary Epithelial Cells. PLoS ONE 2015, 10, e0131285. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Jamal, H.H.; Taheri, M.; Hajiesmaeili, M. A Comprehensive Review on Function of miR-15b-5p in Malignant and Non-Malignant Disorders. Front. Oncol. 2022, 12, 870996. [Google Scholar] [CrossRef]
- Uson, R.; Laguna, A.; Laguna, M.; Briggs, D.A.; Murray, H.H.; Fackler, J.P., Jr. (Tetrahydrothiophene)Gold(I) or Gold(III) Complexes. Inorg. Synth. 1989, 26, 85–91. [Google Scholar] [CrossRef]
- Jenkins, D.E.; Sykora, R.E.; Assefa, Z. Synthesis, X-ray crystallography, and photoluminescence studies of four coordinate gold(I) complexes with the weak Lewis base tri-2-furyl phosphine ligand. Inorg. Chim. Acta 2013, 406, 293–300. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Kazimi, S.G.T.; Iqbal, M.S.; Mulligan, C.C.; Frank Shaw, C.; Iram, F.; Stelmack, A.R.; Campbell, I.S. Ligand Exchange/Scrambling Study of Gold(I)-Phosphine Complexes in the Solid Phase by DESI-MS Analysis. J. Am. Soc. Mass Spectrom. 2019, 30, 2289–2296. [Google Scholar] [CrossRef]
- Seiji, W.; Takayuki, K.; Nobuko, K.; Motohiro, S.; Masami, N.; Yasushi, K.; Shozo, Y. Aggregation through the Quadrupole Interactions of Gold(I) Complex with Triphenylphosphine and Pentafluorobenzenethiolate. Chem. Lett. 2003, 32, 1070–1071. [Google Scholar] [CrossRef]
- Curado, N.; Dewaele-Le Roi, G.; Poty, S.; Lewis, J.S.; Contel, M. Trastuzumab gold-conjugates: Synthetic approach and in vitro evaluation of anticancer activities in breast cancer cell lines. Chem. Commun. 2019, 55, 1394–1397. [Google Scholar] [CrossRef]
- Langsjoen, R.M.; Auguste, A.J.; Rossi, S.L.; Roundy, C.M.; Penate, H.N.; Kastis, M.; Schnizlein, M.K.; Le, K.C.; Haller, S.L.; Chen, R.; et al. Host oxidative folding pathways offer novel anti-chikungunya virus drug targets with broad spectrum potential. Antivir. Res. 2017, 143, 246–251. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Shin, S.; An, H.G.; Kwon, T.U.; Baek, H.S.; Kwon, Y.J.; Chun, Y.J. Synergistic Induction of Apoptosis by the Combination of an Axl Inhibitor and Auranofin in Human Breast Cancer Cells. Biomol. Ther. 2020, 28, 473–481. [Google Scholar] [CrossRef]
- Mascarenhas, B.R.; Granda, J.L.; Freyberg, R.H. Gold metabolism in patients with rheumatoid arthritis treated with gold compounds—Reinvestigated. Arthritis Rheum. 1972, 15, 391–402. [Google Scholar] [CrossRef]
- Freyberg, R.H.; Block, W.D.; Levey, S. Metabolism, toxicity and manner of action of gold compounds used in the treatment of arthritis. I. Human plasma and synovial fluid concentration and urinary excretion of gold during and following treatment with gold sodium thiomalate, gold sodium thiosulfate, and colloidal gold sulfide. J. Clin. Investig. 1941, 20, 401–412. [Google Scholar] [CrossRef]
- Gottlieb, N.L. Metabolism and distribution of gold compounds. J. Rheumatol. Suppl. 1979, 5, 2–6. [Google Scholar] [PubMed]
- Graham, G.G.; Champion, G.D.; Ziegler, J.B. The cellular metabolism and effects of gold complexes. Met. Based Drugs 1994, 1, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Young, M.A.; Parkhurst, E.; Ouellette, M.; Kerr, M.E.; Ho, D.M.; Elder, R.C.; Bruce, A.E.; Bruce, M.R.M. Synthesis, Structure, and Electronic Spectroscopy of Neutral, Dinuclear Gold(I) Complexes. Gold(I)-Gold(I) Interactions in Solution and in the Solid State. Inorg. Chem. 1993, 32, 2506–2517. [Google Scholar] [CrossRef]
- Ackermann, M.; Pascariu, A.; Höcher, T.; Siehl, H.-U.; Berger, S. Electronic Properties of Furyl Substituents at Phosphorus and Their Influence on 31P NMR Chemical Shifts. J. Am. Chem. Soc. 2006, 128, 8434–8440. [Google Scholar] [CrossRef]
- Hill, A.P.; Young, R.J. Getting physical in drug discovery: A contemporary perspective on solubility and hydrophobicity. Drug Discov. Today 2010, 15, 648–655. [Google Scholar] [CrossRef]
- Yeo, C.; Ooi, K.; Tiekink, E. Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef]
- McKeage, M.J.; Berners-Price, S.J.; Galettis, P.; Bowen, R.J.; Brouwer, W.; Ding, L.; Zhuang, L.; Baguley, B.C. Role of lipophilicity in determining cellular uptake and antitumour activity of gold phosphine complexes. Cancer Chemother. Pharmacol. 2000, 46, 343–350. [Google Scholar] [CrossRef]
- Tamaian, R.; Mot, A.; Silaghi-Dumitrescu, R.; Ionut, I.; Stana, A.; Oniga, O.; Nastasa, C.; Benedec, D.; Tiperciuc, B. Study of the Relationships between the Structure, Lipophilicity and Biological Activity of Some Thiazolyl-carbonyl-thiosemicarbazides and Thiazolyl-azoles. Molecules 2015, 20, 22188–22201. [Google Scholar] [CrossRef]
- Hansch, C.; Steward, A.; Anderson, S.; Bentley, D. Prabolic Dependence of Drug Action upon Lipophilic Character as revealed by a Study of Hypnotics. J. Med. Chem. 1968, 11, 1–11. [Google Scholar] [CrossRef]
- Wetzel, C.; Kunz, P.C.; Kassack, M.U.; Hamacher, A.; Bohler, P.; Watjen, W.; Ott, I.; Rubbiani, R.; Spingler, B. Gold(I) complexes of water-soluble diphos-type ligands: Synthesis, anticancer activity, apoptosis and thioredoxin reductase inhibition. Dalton Trans. 2011, 40, 9212–9220. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Paulus, L.; Leger, D.Y.; Therrien, B.; Liagre, B. Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules 2022, 27, 4472. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Cetto, A.; Campbell, M.; Scoggins, S.; Stultz, L.; Hanson, P. DMSO reduces the cytotoxicity of anticancer ruthenium complex KP1019 in yeast. Micropubl. Biol. 2021, 2021, 10–17912. [Google Scholar] [CrossRef]
- De Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; De Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-α, IFN-γ, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef]
- Hanslick, J.L.; Lau, K.; Noguchi, K.K.; Olney, J.W.; Zorumski, C.F.; Mennerick, S.; Farber, N.B. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol. Dis. 2009, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.A.; Demir, S.; Aliyazicioglu, Y. In vitro Cytotoxic Effect of Ethanol and Dimethyl Sulfoxide on Various Human Cell Lines. KSU Tarim Doga Derg. 2020, 23, 1119–1124. [Google Scholar] [CrossRef]
- Sangweni, N.F.; Dludla, P.V.; Chellan, N.; Mabasa, L.; Sharma, J.R.; Johnson, R. The Implication of Low Dose Dimethyl Sulfoxide on Mitochondrial Function and Oxidative Damage in Cultured Cardiac and Cancer Cells. Molecules 2021, 26, 7305. [Google Scholar] [CrossRef]
- Garusinghe, G.S.P.; Bessey, S.M.; Boyd, C.; Aghamoosa, M.; Frederick, B.; Bruce, M.R.M.; Bruce, A.E. Identification of dimethyl sulfide in dimethyl sulfoxide and implications for metal-thiolate disulfide exchange reactions. RSC Adv. 2015, 5, 40603–40606. [Google Scholar] [CrossRef]
- Gil-Moles, M.; Olmos, M.E.; López-de-Luzuriaga, J.M.; Ott, I.; Gimeno, M.C. A dual approach to cancer treatment: Gold(I) terpyridine derivatives as DNA binders and inhibitors of mammalian thioredoxin reductase. Inorg. Chem. Front. 2024, 11, 4802–4814. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Robledo-Cadena, D.X.; Pacheco-Velázquez, S.C.; Vargas-Navarro, J.L.; Padilla-Flores, J.A.; Kaambre, T.; Moreno-Sánchez, R. Repurposing auranofin and meclofenamic acid as energy-metabolism inhibitors and anti-cancer drugs. PLoS ONE 2024, 19, e0309331. [Google Scholar] [CrossRef]
- Ye, D.J.; Kwon, Y.J.; Baek, H.S.; Cho, E.; Kwon, T.U.; Chun, Y.J. Combination treatment with auranofin and nutlin-3a induces synergistic cytotoxicity in breast cancer cells. J. Toxicol. Env. Health Part A 2019, 82, 626–637. [Google Scholar] [CrossRef]
- Fereidoonnezhad, M.; Ahmadi Mirsadeghi, H.; Abedanzadeh, S.; Yazdani, A.; Alamdarlou, A.; Babaghasabha, M.; Almansaf, Z.; Faghih, Z.; McConnell, Z.; Shahsavari, H.R.; et al. Synthesis and biological evaluation of thiolate gold(i) complexes as thioredoxin reductase (TrxR) and glutathione reductase (GR) inhibitors. New J. Chem. 2019, 43, 13173–13182. [Google Scholar] [CrossRef]
- Mizuno, M.; Matsuzaki, T.; Ozeki, N.; Katano, H.; Koga, H.; Takebe, T.; Yoshikawa, H.Y.; Sekiya, I. Cell membrane fluidity and ROS resistance define DMSO tolerance of cryopreserved synovial MSCs and HUVECs. Stem Cell Res. Ther. 2022, 13, 177. [Google Scholar] [CrossRef]
- Gironi, B.; Kahveci, Z.; McGill, B.; Lechner, B.-D.; Pagliara, S.; Metz, J.; Morresi, A.; Palombo, F.; Sassi, P.; Petrov, P.G. Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys. J. 2020, 119, 274–286. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nguyen, H.T.; Truong, K.D. Comparative cytotoxic effects of methanol, ethanol and DMSO on human cancer cell lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar] [CrossRef]
- Oommen, D.; Dodd, N.J.F.; Yiannakis, D.; Moyeed, R.; Jha, A.N. Linking genotoxicity and cytotoxicity with membrane fluidity: A comparative study in ovarian cancer cell lines following exposure to auranofin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 809, 43–49. [Google Scholar] [CrossRef]
| Complex | Solubility Limit (µM) |
|---|---|
| 1a; (TFP)Au(Spy) | 12.5 ± 0.09 |
| 1b; (TFP)Au(Spyrim) | 36.80 ± 0.50 |
| 1c; (TFP)Au(SMepyrim) | 11.04 ± 0.09 |
| 1d; (TFP)Au(SCF3pyrim) | 7.07 ± 0.06 |
| 1e; (TFP)Au(SMe2pyrim) | 6.59 ± 0.06 |
| 2a; (Ph3P)Au(Spy) | 8.20 ± 0.10 |
| 2b; (Ph3P)Au(Spyrim) | 1.07 ± 0.02 |
| 2c; (Ph3P)Au(SMepyrim) | 2.96 ± 0.04 |
| 2d; (Ph3P)Au(SCF3pyrim) | 10.00 ± 0.01 |
| 2e; (Ph3P)Au(SMe2pyrim) | 1.12 ± 0.02 |
| Auranofin | 43.6 ± 0.50 |
| Complex | Cell Viability (%) |
|---|---|
| 1a; (TFP)Au(Spy) | 64.4 ± 1.1 |
| 1b; (TFP)Au(Spyrim) | 62.5 ± 2.7 |
| 1c; (TFP)Au(SMepyrim) | 59.3 ± 2.5 |
| 1d; (TFP)Au(SCF3pyrim) | 58.1 ± 2.5 |
| 1e; (TFP)Au(SMe2pyrim) | 50.4 ± 1.9 |
| 2a; (Ph3P)Au(Spy) | 50.1 ± 2.0 |
| 2b; (Ph3P)Au(Spyrim) | 48.5 ± 1.7 |
| 2c; (Ph3P)Au(SMepyrim) | 51.9 ± 7.0 |
| 2d; (Ph3P)Au(SCF3pyrim) | 49.9 ± 2.3 |
| 2e; (Ph3P)Au(SMe2pyrim) | 48.7 ± 1.7 |
| Auranofin | 52.9 ± 1.3 |
| Cisplatin | 50.1 ± 3.8 |
| Cell Line | 1e (EC50 (µM)) | 2e (EC50 (µM)) |
|---|---|---|
| MDA-MB-231(breast cancer) | 0.07 ± 0.01 | 0.05 ± 0.01 |
| MCF7 (breast cancer) | 0.31 ± 0.03 | 0.11 ± 0.02 |
| MCF10A (non-transformed) | Cell Viability > 80% | Cell Viability > 80% |
| at doses ≥ 0.3–1.0 µM | at doses ≥ 0.3–1.0 µM | |
| HEK293T (non-transformed) | Cell Viability > 80% | Cell Viability > 80% |
| at doses ≥ 0.3–1.0 µM | at doses ≥ 0.3–1.0 µM |
| Cell Line | 1e (EC50 (µM) in 1% DMSO) | 2e (EC50 (µM) in 1% DMSO) |
|---|---|---|
| MDA-MB-231 (breast cancer) | 0.16 ± 0.02 | 0.19 ± 0.02 |
| MCF7 (breast cancer) | 0.07 ± 0.01 | 0.08 ± 0.01 |
| MCF10A (non-transformed) | 0.09 ± 0.01 | 0.15 ± 0.01 |
| HEK293T (non-transformed) | 0.20 ± 0.02 | 0.32 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wilhelm, K.L.; Pokhrel, S.; Stolpman, D.; Worth, C.; Mehta, S.; Villacob, R.A.; Zechmann, B.; Ahmad, A.A.L.; Taube, J.; Bruce, M.R.M.; et al. Evaluating the Antiproliferative Effects of Tri(2-Furyl)- and Triphenylphosphine-Gold(I) Pyridyl- and Pyrimidine-Thiolate Complexes. Biomolecules 2026, 16, 154. https://doi.org/10.3390/biom16010154
Wilhelm KL, Pokhrel S, Stolpman D, Worth C, Mehta S, Villacob RA, Zechmann B, Ahmad AAL, Taube J, Bruce MRM, et al. Evaluating the Antiproliferative Effects of Tri(2-Furyl)- and Triphenylphosphine-Gold(I) Pyridyl- and Pyrimidine-Thiolate Complexes. Biomolecules. 2026; 16(1):154. https://doi.org/10.3390/biom16010154
Chicago/Turabian StyleWilhelm, Kyle Logan, Shyam Pokhrel, Drew Stolpman, Charli Worth, Sonal Mehta, Raul A. Villacob, Bernd Zechmann, Ahmad A. L. Ahmad, Joseph Taube, Mitchell R. M. Bruce, and et al. 2026. "Evaluating the Antiproliferative Effects of Tri(2-Furyl)- and Triphenylphosphine-Gold(I) Pyridyl- and Pyrimidine-Thiolate Complexes" Biomolecules 16, no. 1: 154. https://doi.org/10.3390/biom16010154
APA StyleWilhelm, K. L., Pokhrel, S., Stolpman, D., Worth, C., Mehta, S., Villacob, R. A., Zechmann, B., Ahmad, A. A. L., Taube, J., Bruce, M. R. M., Bruce, A. E., & Solouki, T. (2026). Evaluating the Antiproliferative Effects of Tri(2-Furyl)- and Triphenylphosphine-Gold(I) Pyridyl- and Pyrimidine-Thiolate Complexes. Biomolecules, 16(1), 154. https://doi.org/10.3390/biom16010154







