Evaluations of Quinone/Hydroquinone Couples Acting as Two Hydrogen Atoms Antioxidants, Radical Quenchers, and Hydrogen Atom Abstractors
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermodynamic Parameters
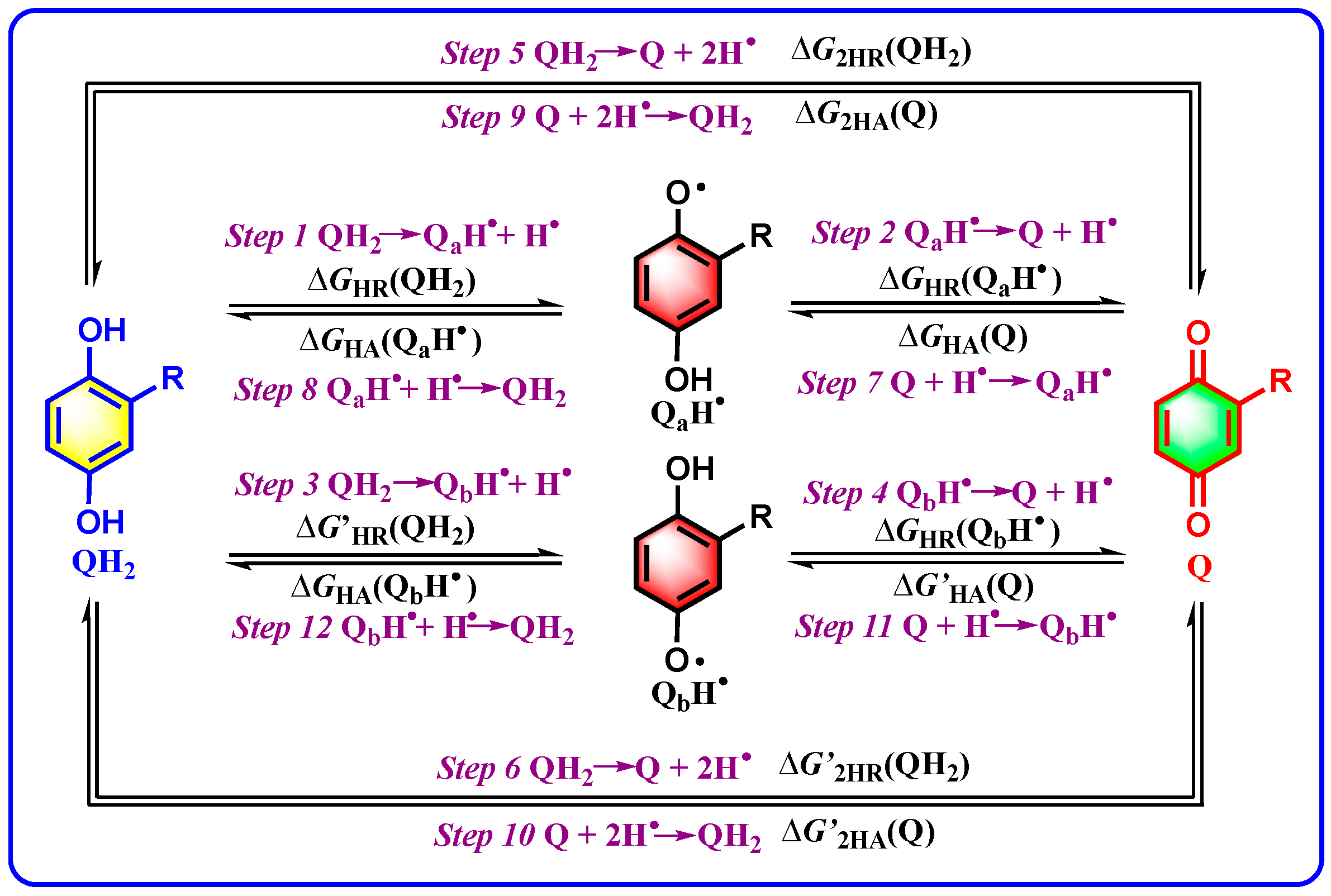
2.2. Calculation and Acquisition of Thermodynamic Data
3. Results
4. Discussion
4.1. Further Verification of Data Reliability
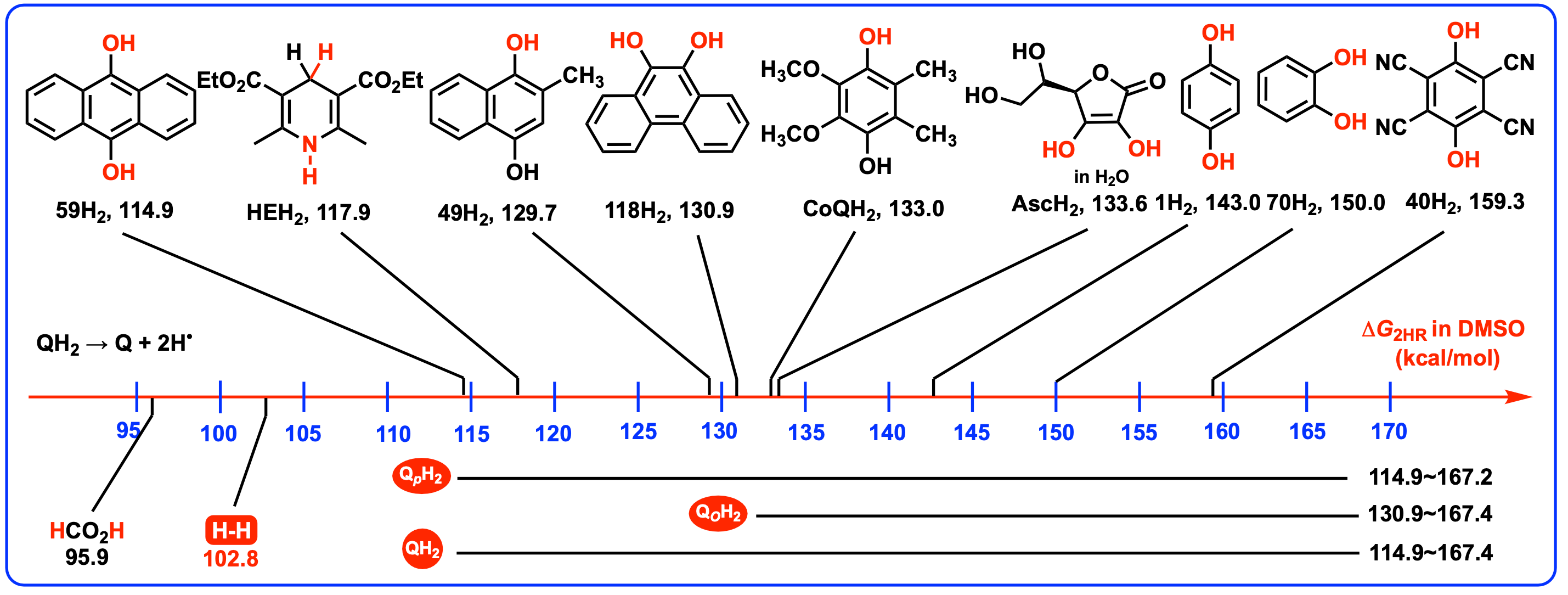
4.2. Thermodynamic Capabilities of QH2 Acting as Antioxidants by Releasing Two Hydrogen Atoms
4.3. Thermodynamic Capabilities of QH2 and QH• Releasing a Hydrogen Atom as Antioxidants
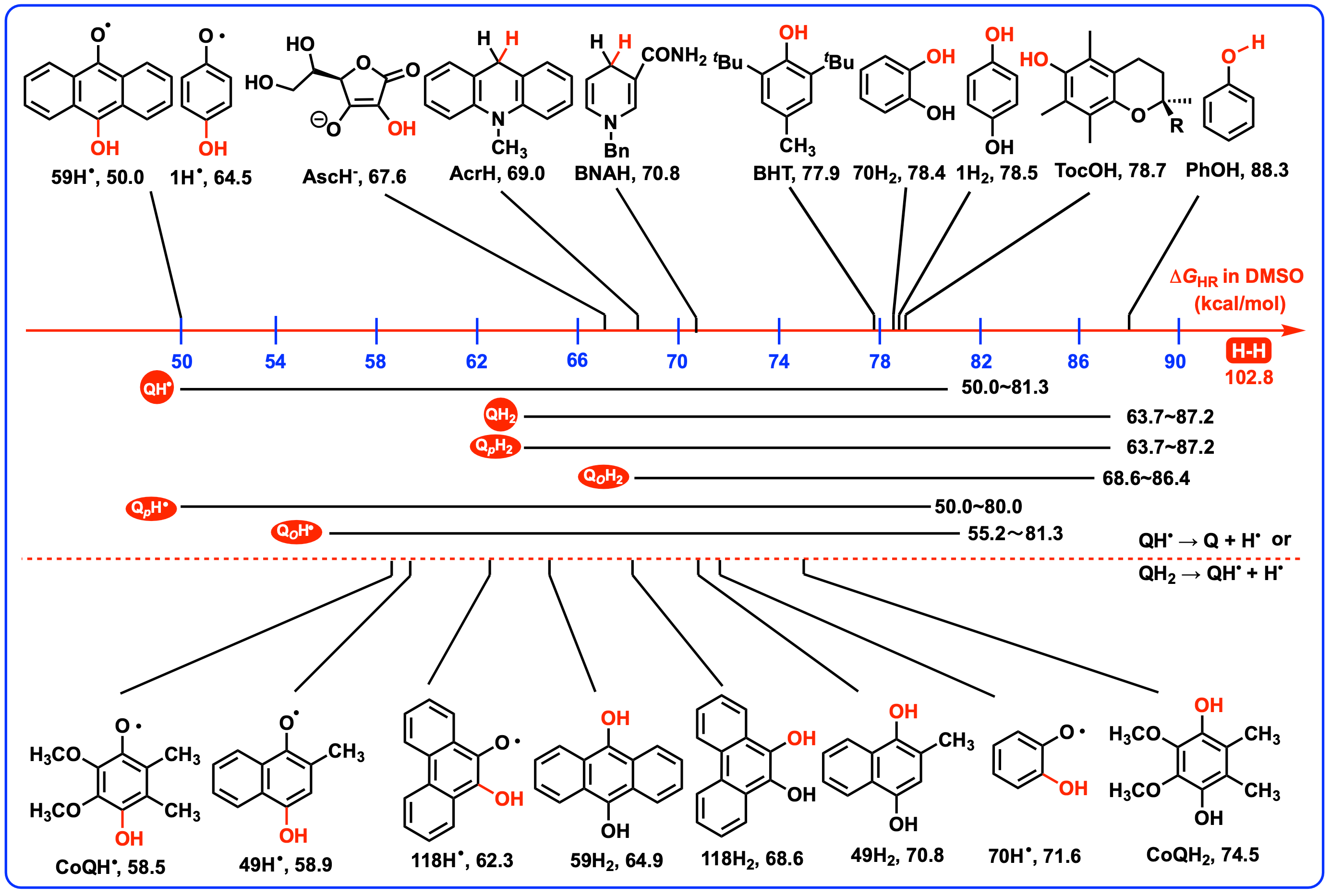
4.4. Thermodynamic Capabilities of QH2 and QH• Acting as Radical Quenchers
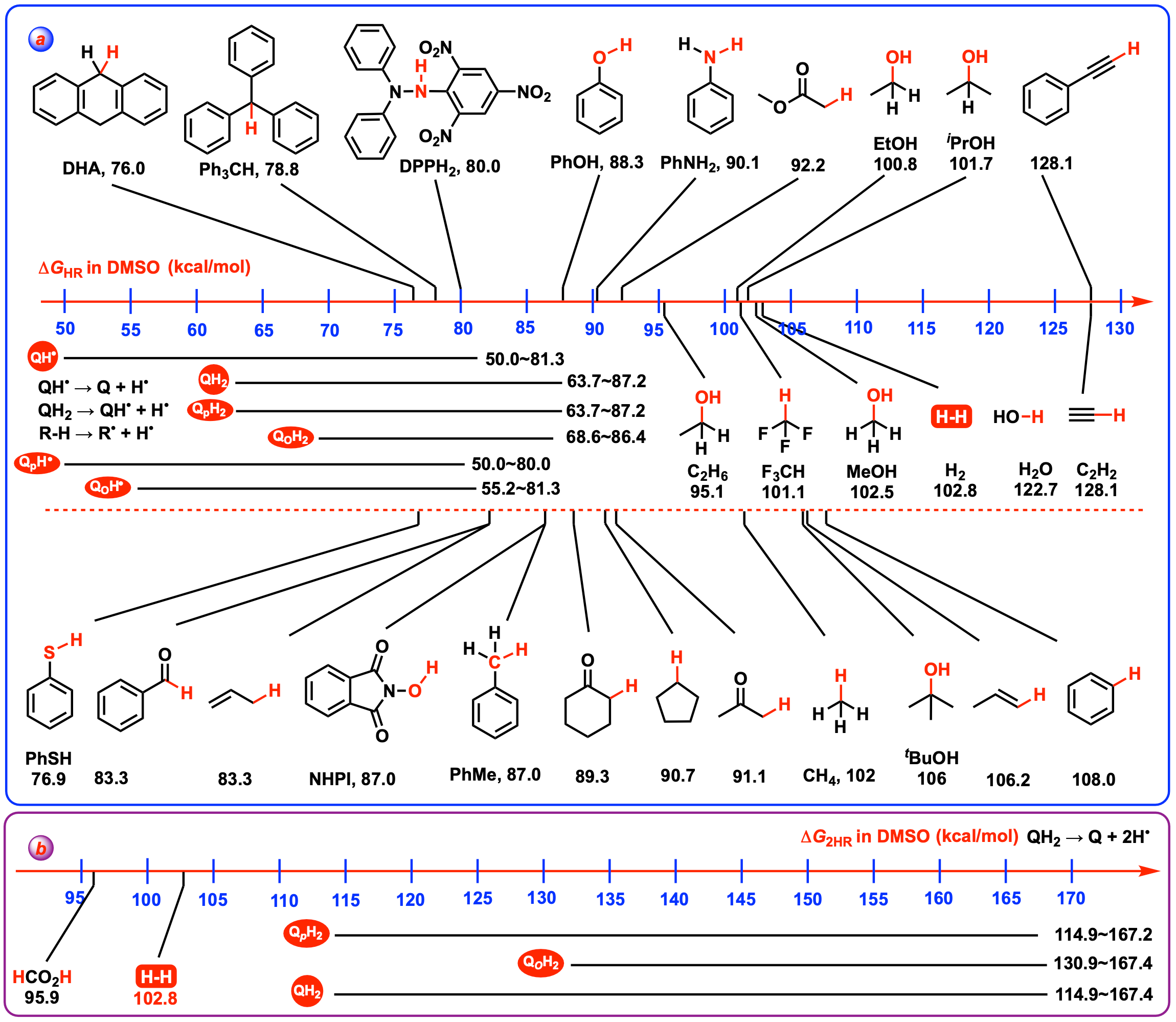
4.5. Thermodynamic Capabilities of Q and QH• Acting as Hydrogen Atoms Abstractors
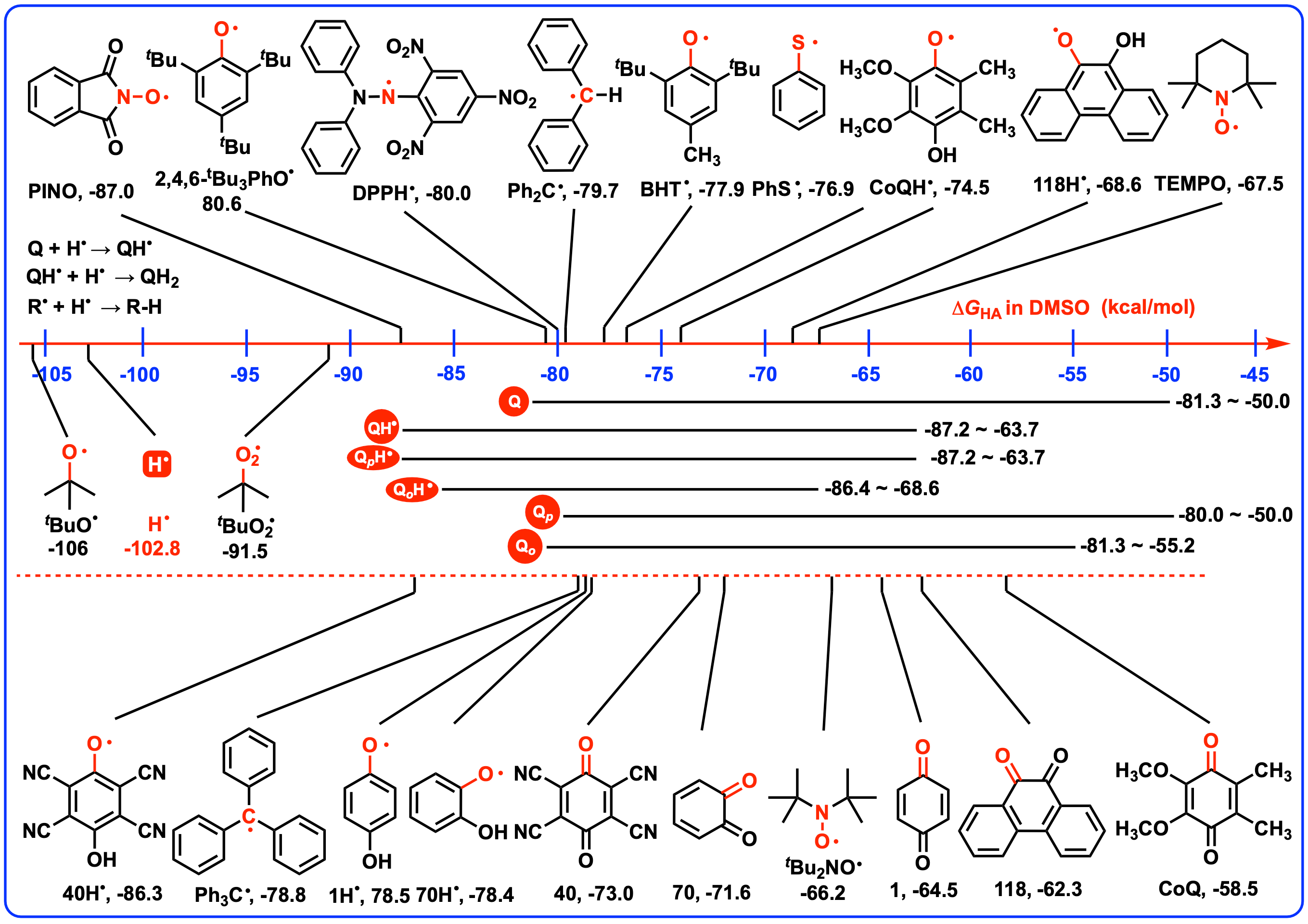
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogeski, I.; Gulaboski, R.; Kappl, R.; Mirceski, V.; Stefova, M.; Petreska, J.; Hoth, M. Calcium Binding and Transport by Coenzyme. Q. J. Am. Chem. Soc. 2011, 133, 9293–9303. [Google Scholar] [CrossRef]
- Wada, O.Z.; Rashid, N.; Wijten, P.; Thornalley, P.; Mckay, G.; Mackey, H.R. Evaluation of Cell Disruption Methods for Protein and Coenzyme Q10 Quantification in Purple Non-Sulfur Bacteria. Front. Microbiol. 2024, 15, 1324099. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, M.; Miao, Y.; Pei, K.; Lin, Z.; Liu, S.; Huang, X.; Wang, Y.; Lv, G. Research Progress on Quinone Compounds for the Treatment of Hepatocellular Carcinoma. Biomolecules 2025, 15, 1400. [Google Scholar] [CrossRef]
- Meka, A.K.; Gopalakrishna, A.; Iriarte-Mesa, C.; Rewatkar, P.; Qu, Z.; Wu, X.; Cao, Y.; Prasadam, I.; Janjua, T.I.; Kleitz, F.; et al. Influence of Pore Size and Surface Functionalization of Mesoporous Silica Nanoparticles on the Solubility and Antioxidant Activity of Confined Coenzyme Q10. Mol. Pharm. 2023, 20, 2966–2977. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A.; Daley, S. Pyrroloquinoline Quinone Chemistry, Biology, and Biosynthesis. Chem. Res. Toxicol. 2022, 35, 355–377. [Google Scholar] [CrossRef] [PubMed]
- López, J.; de la Cruz, F.; Alcaraz, Y.; Delgado, F.; Vázquez, M.A. Quinoid systems in chemistry and pharmacology. Med. Chem. Res. 2015, 24, 3599–3620. [Google Scholar] [CrossRef]
- Wendlandt, A.E.; Stahl, S.S. Quinone-Catalyzed Selective Oxidation of Organic Molecules. Angew. Chem. Int. Ed. 2015, 54, 14638–14658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, Y.; Zhang, L.; Luo, S. Oxidative Synthesis of Benzimidazoles, Quinoxalines, and Benzoxazoles from Primary Amines by ortho-Quinone Catalysis. Org. Lett. 2017, 19, 5629–5632. [Google Scholar] [CrossRef]
- Fan, W.; Zhao, X.; Deng, Y.; Chen, P.; Wang, F.; Liu, G. Electrophotocatalytic Decoupled Radical Relay Enables Highly Efficient and Enantioselective Benzylic C−H Functionalization. J. Am. Chem. Soc. 2022, 144, 21674–21682. [Google Scholar] [CrossRef]
- Giner, R.M.; Ríos, J.L.; Máñez, S. Antioxidant Activity of Natural Hydroquinones. Antioxidants 2022, 11, 343. [Google Scholar] [CrossRef]
- Kamau, P.; Jordan, R.B. Kinetic Study of the Oxidation of Catechol by Aqueous Copper(II). Inorg. Chem. 2002, 41, 3076–3083. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Wei, J.; Wang, H.; Zhong, F.; Zhai, H. Recent Advances in Catalytic Oxidative Reactions of Phenols and Naphthalenols. Org. Chem. Front. 2022, 9, 5395–5413. [Google Scholar] [CrossRef]
- Kumar, D.; Salam, A.; Sahu, T.K.; Sahoo, S.S.; Khan, T. DDQ-Catalyzed Oxidative C(sp3)–H Functionalization of Aryltetralins and Subsequent Chemoselective Oxidative Demethylation to Access Dihydronaphthalenes and Dihydronaphthoquinones. J. Org. Chem. 2021, 86, 15096–15116. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Pan, Z.; Hu, Z.; Li, M.; Hu, X.; Jin, L.; Sun, N.; Hu, B.; Shen, Z. Metal-Free Aerobic Oxidative C−O Coupling of C(sp3)−H with Carboxylic Acids Catalyzed by DDQ/tert-Butyl nitrite. Eur. J. Org. Chem. 2019, 33, 5650–5655. [Google Scholar] [CrossRef]
- Nakayama, K.; Okada, Y. Arene C−H Amination with N-Heteroarenes by Catalytic DDQ Photocatalysis. J. Org. Chem. 2023, 88, 5913–5922. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Yu, G.; Wena, C.; Huo, Y. DDQ-Mediated Direct C(sp3)–H Phosphorylation of Xanthene Derivatives. Org. Chem. Front. 2018, 5, 2652–2656. [Google Scholar] [CrossRef]
- Kang, L.-S.; Xia, H.-L.; Zeng, C.-C.; Hu, L.-M.; Little, R.D. Electrochemical Synthesis of Benzoxazoles Mediated by 2,3-Dichloro-5,6-Dicyano-p-hydroquinone (DDH) as a Redox Catalyst. Electroanal. Chem. 2016, 767, 13–17. [Google Scholar] [CrossRef]
- Twilton, J.; Johnson, M.R.; Sidana, V.; Franke, M.C.; Bottecchia, C.; Lehnherr, D.; Lévesque, F.; Knapp, S.M.M.; Wang, L.; Gerken, J.B.; et al. Quinone-Mediated Hydrogen Anode for Non-aqueous Reductive Electrosynthesis. Nature 2023, 623, 71–76. [Google Scholar] [CrossRef]
- Preger, Y.; Gerken, J.B.; Biswas, S.; Anson, C.W.; Johnson, M.R.; Root, T.W.; Stahl, S.S. Quinone-Mediated Electrochemical O2 Reduction Accessing High Power Density with an Off-Electrode Co-N/C Catalyst. Joule 2018, 2, 2722–2731. [Google Scholar] [CrossRef]
- Alligrant, T.M.; Alvarez, J.C. The Role of Intermolecular Hydrogen Bonding and Proton Transfer in Proton-Coupled Electron Transfer. J. Phys. Chem. C 2011, 115, 10797–10805. [Google Scholar] [CrossRef]
- Preger, Y.; Johnson, M.R.; Biswas, S.; Anson, C.W.; Root, T.W.; Stahl, S.S. Anthraquinone-Mediated Fuel Cell Anode with an Off-Electrode Heterogeneous Catalyst Accessing High Power Density when Paired with a Mediated Cathode. ACS Energy Lett. 2020, 5, 1407–1412. [Google Scholar] [CrossRef]
- Alsharif, M.A.; Raja, Q.A.; Majeed, N.A.; Jassas, R.S.; Alsimaree, A.A.; Sadiq, A.; Naeem, N.; Mughal, E.U.; Alsantali, R.I.; Moussa, Z.; et al. DDQ as a Versatile and Easily Recyclable Oxidant: A Systematic Review. RSC Adv. 2021, 11, 29826–29858. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Mori, H.; Imahori, H.; Suenobu, T.; Araki, Y.; Ito, O.; Kadish, K.M. Scandium Ion-Promoted Photoinduced Electron-Transfer Oxidation of Fullerenes and Derivatives by p-Chloranil and p-Benzoquinone. J. Am. Chem. Soc. 2001, 123, 12458–12465. [Google Scholar] [CrossRef]
- Kim, S.; Matsubara, R.; Hayashi, M. Activated Carbon-Promoted Dehydrogenation of Hydroquinones to Benzoquinones, Naphthoquinones, and Anthraquinones under Molecular Oxygen Atmosphere. J. Org. Chem. 2019, 84, 2997–3003. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Amorati, R.; Valgimigli, L.; Sambri, L. 1-Methyl-1,4-cyclohexadiene as a Traceless Reducing Agent for the Synthesis of Catechols and Hydroquinones. J. Org. Chem. 2019, 84, 13655–13664. [Google Scholar] [CrossRef] [PubMed]
- Luca, O.R.; Wang, T.; Konezny, S.J.; Batista, V.S.; Crabtree, R.H. DDQ as an Electrocatalyst for Amine Dehydrogenation, A Model System for Virtual Hydrogen Storage. New J. Chem. 2011, 35, 998–999. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Wang, C.-H.; Liang, H. Scales of Oxidation Potentials, pKa, and BDE of Various Hydroquinones and Catechols in DMSO. J. Org. Chem. 2010, 75, 7240–7257. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.-B.; Qian, B.-C.; Fu, Y.-H.; Zhu, X.-Q. Thermodynamics of the Elementary Steps of Organic Hydride Chemistry Determined in Acetonitrile and their Applications. Org. Chem. Front. 2022, 9, 6001–6062. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Bruno, J.W.; Enyinnaya, E. Hydride Affinities of Arylcarbenium Ions and Iminium Ions in Dimethyl Sulfoxide and Acetonitrile. J. Org. Chem. 1998, 63, 4671–4678. [Google Scholar] [CrossRef]
- Shen, G.-B.; Qian, B.-C.; Fu, Y.-H.; Zhu, X.-Q. Discovering and Evaluating the Reducing Abilities of Polar Alkanes and Related Family Members as Organic Reductants Using Thermodynamics. J. Org. Chem. 2022, 87, 9357–9374. [Google Scholar] [CrossRef]
- Warren, J.J.; Tronic, T.A.; Mayer, J.M. Thermochemistry of Proton−Coupled Electron Transfer Reagents and Its Implications. Chem. Rev. 2010, 110, 6961–7001. [Google Scholar] [CrossRef]
- Ilic, S.; Kadel, U.P.; Basdogan, Y.; Keith, J.A.; Glusac, K.D. Thermodynamic Hydricities of Biomimetic Organic Hydride Donors. J. Am. Chem. Soc. 2018, 140, 4569–4579. [Google Scholar] [CrossRef]
- Cheng, J.-P.; Lu, Y.; Zhu, X.-Q.; Sun, Y.; Bi, F.; He, J. Heterolytic and Homolytic N−H Bond Dissociation Energies of 4−Substituted Hantzsch 2,6−Dimethyl−1,4−dihydropyridines and the Effect of One−Electron Transfer on the N−H Bond Activation. J. Org. Chem. 2000, 65, 3853–3857. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Yang, J.-D.; Liu, Y.; Zhang, L.; Luo, S.; Cheng, J.-P. Holistic Prediction of pKa in Diverse Solvents Based on Machine Learning Approach. Angew. Chem. Int. Ed. 2020, 59, 19282–19291. [Google Scholar] [CrossRef]
- Tang, R.; Shao, Z.; Wang, J.; Liu, Z.; Li, Y.-M.; Shen, Y. Iron(II)-Catalyzed Radical Addition to Aldimines with Hantzsch Ester as a Two-Hydrogen Atom Donor. J. Org. Chem. 2019, 84, 8177–8184. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.; Litwinienko, G.; Lin, S.; MacLean, P.D.; Barclay, L.R.C.; Ingold, K.U. The L-type Calcium Channel Blockers, Hantzsch 1,4-Dihydropyridines, Are Not Peroxyl Radical-Trapping, Chain Breaking Antioxidants. Chem. Res. Toxicol. 2006, 19, 79–85. [Google Scholar] [CrossRef]
- Qin, Y.; Zhu, L.; Luo, S. Organocatalysis in Inert C−H Bond Functionalization. Chem. Rev. 2017, 117, 9433–9520. [Google Scholar] [CrossRef]
- Zhang, J.; Rueping, M. Metallaphotoredox Catalysis for sp3 C–H Functionalizations through Hydrogen Atom Transfer (HAT). Chem. Soc. Rev. 2023, 52, 4099–4120. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D.; Fagnoni, M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C−H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Peng, X.; Tang, Z.; Yang, W.; Deng, W.; Yin, S.-F.; Kambe, N.; Qiu, R. DTBP-Mediated Cross-Dehydrogenative Coupling of 3-Aryl Benzofuran-2(3H)-ones with Toluenes/Phenols for All-Carbon Quaternary Centers. RSC Adv. 2022, 12, 35215–35220. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Roy, S.; Bhowmik, A.; Sarkar, W.; Mondal, I.; Mishra, A.; Saha, S.J.; Karmakar, S.; Deb, I. A Radical–Radical Cross-Coupling Reaction of Xanthene with Sulfonyl Hydrazides: Facile Access to Xanthen-9-Sulfone Derivatives. Chem. Commun. 2022, 58, 2902–2905. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Ni, Z.; Wang, S.; Wu, J.; Pan, Y. TBPB-Promoted Metal-Free Synthesis of Thiophosphinate/Phosphonothioate by Direct P–S Bond Coupling. Green Chem. 2015, 17, 314–319. [Google Scholar] [CrossRef]
- Bosque, I.; Chinchilla, R.; Gonzalez-Gomez, J.C.; Guijarro, D.; Alonso, F. Cross-Dehydrogenative Coupling Involving Benzylic and Allylic C–H Bonds. Org. Chem. Front. 2020, 7, 1717–1742. [Google Scholar] [CrossRef]
- Nutting, J.E.; Rafiee, M.; Stahl, S.S. Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-Oxyl (PINO), and Related N-Oxyl Species: Electrochemical Properties and Their Use in Electrocatalytic Reactions. Chem. Rev. 2018, 118, 4834–4885. [Google Scholar] [CrossRef]
- Paveliev, S.A.; Segida, O.O.; Dvoretskiy, A.; Dzyunov, M.M.; Fedorova, U.V.; Terent’ev, A.O. Electrifying Phthalimide-N-Oxyl (PINO) Radical Chemistry: Anodically Induced Dioxygenation of Vinyl Arenes with N-Hydroxyphthalimide. J. Org. Chem. 2021, 86, 18107–18116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cui, Y.; Jiao, N. Metal-free TEMPO-catalyzed Oxidative C–C Bond Formation from Csp3–H Bonds Using Molecular Oxygen as the Oxidant. Chem. Commun. 2012, 48, 4498–4500. [Google Scholar] [CrossRef]
- Lv, H.; Laishram, R.D.; Yang, Y.; Li, J.; Xu, D.; Zhan, Y.; Luo, Y.; Su, Z.; More, S.; Fan, B. TEMPO Catalyzed Oxidative Dehydrogenation of Hydrazobenzenes to Azobenzenes. Org. Biomol. Chem. 2020, 18, 3471–3474. [Google Scholar] [CrossRef]
- Leifert, D.; Studer, A. Organic Synthesis Using Nitroxides. Chem. Rev. 2023, 123, 10302–10380. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Seidel, F.W.; Jin, X.; Nozaki, K.J. TEMPO as a Hydrogen Atom Transfer Catalyst for Aerobic Dehydrogenation of Activated Alkanes to Alkenes. Org. Chem. 2022, 87, 12733–12740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, G.; Wang, X.; Ou, Y.; Huo, Y. Catalyst-Free Direct C(sp3)–H Sulfenylation of Xanthene Derivatives Using Air as Oxidant. Green Chem. 2019, 21, 798–802. [Google Scholar] [CrossRef]
- Liu, X.; Shen, Y.; Lu, C.; Jian, Y.; Xia, S.; Gao, Z.; Zheng, Y.; An, Y.; Wang, Y. Visible-Light-Driven PhSSPh-Catalysed Regioselective Hydroborylation of a,b-Unsaturated Carbonyl Compounds with NHC-Boranes. Chem. Commun. 2022, 58, 8380–8383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-A.; Palani, V.; Seim, A.E.; Wang, Y.; Wang, K.J.; Wendlandt, A.E. Stereochemical Editing Logic Powered by the Epimerization of Unactivated Tertiary Stereocenters. Science 2022, 378, 383–390. [Google Scholar] [CrossRef]
- Shah, R.; Farmer, L.A.; Zilka, O.; Van Kessel, A.T.M.; Pratt, D.A. Beyond DPPH: Use of Fluorescence-Enabled Inhibited Autoxidation to Predict Oxidative Cell Death Rescue. Cell Chem. Biol. 2019, 26, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Sasada, R.; Chiba, K.; Gotoh, H. Effect of Side Chain Functional Groups on The DPPH Radical Scavenging Activity of Bisabolane-Type Phenols. Antioxidants 2019, 8, 65. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In Vitro Antioxidant Activity of Kyoho Grape Extracts in DPPH and ABTS Assays: Estimation Methods for EC50 Using Advanced Statistical Programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical Re-Evaluation of DPPH Assay: Presence of Pigments Affects the Results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Celiz, G.; Renfige, M.; Finetti, M. Spectral Analysis Allows Using The DPPH* UV–Vis Assay to Estimate Antioxidant Activity of Colored Compounds. Chem. Pap. 2020, 74, 3101–3109. [Google Scholar] [CrossRef]
| Hydroquinones Releasing 2H• or H2 | Quinones Accepting 2H• or H2 | ||||
|---|---|---|---|---|---|
| Step X | Definitions | Parameters | Step X | Definitions | Parameters |
| Step 1 | QH2 → QaH• + H• | ΔGHR(QH2) | Step 7 | QaH• + H• → QH2 | ΔGHA(QaH•) |
| Step 2 | QaH• → Q + H• | ΔGHR(QaH•) | Step 8 | Q + H• → QaH• | ΔGHA(Q) |
| Step 3 | QH2 → QbH• + H• | ΔG′HR(QH2) | Step 9 | QbH• + H• → QH2 | ΔGHA(QbH•) |
| Step 4 | QbH• → Q + H• | ΔGHR(QbH•) | Step 10 | Q + H• → QbH• | ΔG′HA(Q) |
| Step 5 | QH2 → Q + 2H• | ΔG2HR(QH2) | Step 11 | Q + 2H• → QH2 | ΔG2HA(Q) |
| Step 6 | QH2 → Q + 2H• | ΔG′2HR(QH2) | Step 12 | Q + 2H• → QH2 | ΔG′2HA(Q) |
| Equation X | Expressions | Data Sources |
|---|---|---|
| (1) | ΔGHR(QH2) = −ΔGHA(QaH•) = ΔHHR(QH2) − 4.9 kcal/mol | ΔHHR(QH2) were calculated by DFT [27] |
| (2) | ΔGHR(QaH•) = −ΔGHA(Q) = ΔHHR(QaH•) − 4.9 kcal/mol | ΔHHR(QaH•) were calculated by DFT [27] |
| (3) | ΔG′HR(QH2) = −ΔGHA(QbH•) = ΔH′HR(QH2) − 4.9 kcal/mol | ΔH′HR(QH2) were calculated by DFT [27] |
| (4) | ΔGHR(QbH•) = −ΔG′HA(Q) = ΔHHR(QbH•) − 4.9 kcal/mol | ΔHHR(QbH•) were calculated by DFT [27] |
| (5) | ΔG2HR(QH2) = −ΔG2HA(Q) = ΔGHR(QH2) + ΔGHR(QaH•) | derived in this work based on Hess’s law |
| (6) | ΔG′2HR(QH2) = −ΔG′2HA(Q) = ΔG′HR(QH2) + ΔGHR(QbH•) | derived in this work based on Hess’s law |
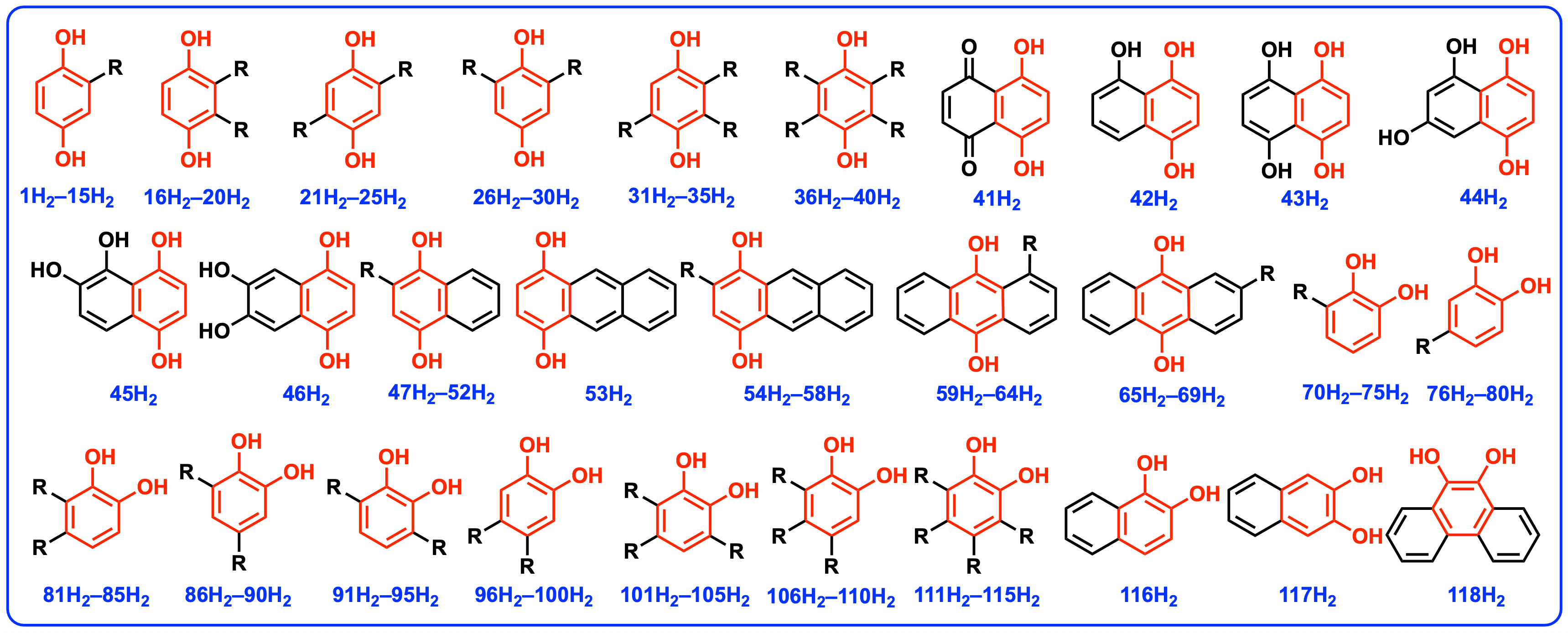
| NO. | R | ΔGHR(QH2) Step 1 | ΔG′HR(QH2) Step 3 | ΔGHR(QaH•) Step 2 | ΔGHR(QbH•) Step 4 | ΔG2HR(QH2) Step 5 | ΔG′2HR(QH2) Step 6 |
|---|---|---|---|---|---|---|---|
| −ΔGHA(QaH•) Step 8 | −ΔGHA(QbH•) Step 12 | −ΔGHA(Q) Step 7 | −ΔG′HA(Q) Step 11 | −ΔG2HA(Q) Step 9 | −ΔG′2HA(Q) Step 10 | ||
| 1 | H | 78.5 | − | 64.5 | − | 143.0 | − |
| 2 | N(Me)2 | 70.6 | 76.7 | 64.1 | 57.9 | 134.7 | 134.6 |
| 3 | NH2 | 67.5 | 74.1 | 64.8 | 58.2 | 132.3 | 132.3 |
| 4 | OMe | 79.1 | 76.8 | 60.1 | 62.3 | 139.2 | 139.1 |
| 5 | OH | 73.2 | 78.0 | 65.4 | 60.6 | 138.6 | 138.6 |
| 6 | SH | 74.9 | 79.2 | 65.9 | 61.6 | 140.8 | 140.8 |
| 7 | CH3 | 76.6 | 77.4 | 63.2 | 62.4 | 139.8 | 139.8 |
| 8 | SiH3 | 76.9 | 79.0 | 65.8 | 63.6 | 142.7 | 142.6 |
| 9 | F | 78.0 | 79.5 | 66.0 | 64.5 | 144.0 | 144.0 |
| 10 | Cl | 79.8 | 78.2 | 63.9 | 65.5 | 143.7 | 143.7 |
| 11 | Br | 78.4 | 79.7 | 66.3 | 65.1 | 144.7 | 144.8 |
| 12 | CHO | 83.7 | 85.0 | 68.8 | 67.4 | 152.5 | 152.4 |
| 13 | CO2Me | 82.7 | 80.8 | 67.5 | 69.4 | 150.2 | 150.2 |
| 14 | CF3 | 78.3 | 81.6 | 67.6 | 64.4 | 145.9 | 146.0 |
| 15 | CN | 78.8 | 82.4 | 69.4 | 65.9 | 148.2 | 148.3 |
| 16 | OMe | 76.9 | − | 64.4 | − | 141.3 | − |
| 17 | CH3 | 75.4 | − | 61.0 | − | 136.4 | − |
| 18 | F | 78.8 | − | 65.1 | − | 143.9 | − |
| 19 | Cl | 79.1 | − | 64.6 | − | 143.7 | − |
| 20 | CN | 83.0 | − | 69.8 | − | 152.8 | − |
| 21 | OMe | 73.5 | − | 60.6 | − | 134.1 | − |
| 22 | CH3 | 75.9 | − | 61.0 | − | 136.9 | − |
| 23 | F | 78.5 | − | 64.9 | − | 143.4 | − |
| 24 | Cl | 79.4 | − | 64.9 | − | 144.3 | − |
| 25 | CN | 82.1 | − | 69.0 | − | 151.1 | − |
| 26 | OMe | 80.3 | 79.4 | 54.1 | 55.0 | 134.4 | 134.4 |
| 27 | CH3 | 75.1 | 77.5 | 61.8 | 59.4 | 136.9 | 136.9 |
| 28 | F | 70.0 | 73.6 | 67.1 | 63.4 | 137.1 | 137.0 |
| 29 | Cl | 81.1 | 77.6 | 63.1 | 66.6 | 144.2 | 144.2 |
| 30 | CN | 80.4 | 85.6 | 72.3 | 67.1 | 152.7 | 152.7 |
| 31 | OMe | 77.3 | 78.7 | 57.2 | 55.8 | 134.5 | 134.5 |
| 32 | CH3 | 76.1 | 74.7 | 57.8 | 59.2 | 133.9 | 133.9 |
| 33 | F | 77.8 | 79.5 | 65.7 | 64.0 | 143.5 | 143.5 |
| 34 | Cl | 80.2 | 78.2 | 63.6 | 65.6 | 143.8 | 143.8 |
| 35 | CN | 83.6 | 85.5 | 72.2 | 70.2 | 155.8 | 155.7 |
| 36 | OMe | 74.9 | − | 60.0 | − | 134.9 | − |
| 37 | CH3 | 74.0 | − | 57.0 | − | 131.0 | − |
| 38 | F | 78.7 | − | 65.0 | − | 143.7 | − |
| 39 | Cl | 79.2 | − | 64.2 | − | 143.4 | − |
| 40 | CN | 86.3 | − | 73.0 | − | 159.3 | − |
| 41 | − | 87.2 | − | 80.0 | − | 167.2 | − |
| 42 | − | 67.6 | 72.9 | 67.6 | 62.2 | 135.2 | 135.1 |
| 43 | − | 65.9 | − | 56.2 | − | 122.1 | − |
| 44 | − | 67.2 | 72.3 | 65.3 | 60.2 | 132.5 | 132.5 |
| 45 | − | 65.1 | 72.0 | 66.9 | 60.0 | 132.0 | 132.0 |
| 46 | − | 71.8 | − | 59.3 | − | 131.1 | − |
| 47 | H | 72.8 | − | 57.9 | − | 130.7 | − |
| 48 | OMe | 69.8 | 70.4 | 62.1 | 61.5 | 131.9 | 131.9 |
| 49 | CH3 | 70.8 | 72.5 | 58.9 | 57.3 | 129.7 | 129.8 |
| 50 | F | 72.0 | 73.3 | 59.9 | 58.5 | 131.9 | 131.8 |
| 51 | Cl | 72.3 | 73.9 | 60.7 | 59.1 | 133.0 | 133.0 |
| 52 | CN | 72.8 | 77.0 | 64.4 | 60.3 | 137.2 | 137.3 |
| 53 | − | 70.0 | − | 58.1 | − | 128.1 | − |
| 54 | OMe | 70.1 | 67.6 | 52.1 | 54.7 | 122.2 | 122.3 |
| 55 | CH3 | 68.2 | 69.7 | 56.9 | 55.5 | 125.1 | 125.2 |
| 56 | F | 69.5 | 70.2 | 57.3 | 56.7 | 126.8 | 126.9 |
| 57 | Cl | 69.6 | 71.4 | 59.0 | 57.2 | 128.6 | 128.6 |
| 58 | CN | 69.9 | 74.5 | 63.1 | 58.4 | 133.0 | 132.9 |
| 59 | H | 64.9 | − | 50.0 | − | 114.9 | − |
| 60 | OMe | 69.5 | 64.6 | 51.8 | 56.7 | 121.3 | 121.3 |
| 61 | CH3 | 64.3 | 64.5 | 51.7 | 51.4 | 116.0 | 115.9 |
| 62 | F | 66.3 | 65.1 | 52.6 | 53.8 | 118.9 | 118.9 |
| 63 | Cl | 66.5 | 65.2 | 52.4 | 53.7 | 118.9 | 118.9 |
| 64 | CN | 64.4 | 65.9 | 53.5 | 51.9 | 117.9 | 117.8 |
| 65 | OMe | 63.7 | 63.8 | 51.3 | 51.2 | 115.0 | 115.0 |
| 66 | CH3 | 64.3 | 64.4 | 51.7 | 51.6 | 116.0 | 116.0 |
| 67 | F | 65.7 | 65.4 | 52.3 | 52.6 | 118.0 | 118.0 |
| 68 | Cl | 65.5 | 65.6 | 52.8 | 52.7 | 118.3 | 118.3 |
| 69 | CN | 65.5 | 66.5 | 54.5 | 53.5 | 120.0 | 120.0 |
| 70 | H | 78.4 | − | 71.6 | − | 150.0 | − |
| 71 | OMe | 78.2 | 81.1 | 68.4 | 65.6 | 146.6 | 146.7 |
| 72 | CH3 | 76.9 | 77.8 | 71.3 | 70.3 | 148.2 | 148.1 |
| 73 | F | 77.9 | 81.8 | 72.8 | 68.9 | 150.7 | 150.7 |
| 74 | Cl | 78.0 | 79.6 | 72.5 | 70.9 | 150.5 | 150.5 |
| 75 | CN | 78.0 | 79.5 | 73.7 | 72.3 | 151.7 | 151.8 |
| 76 | OMe | 74.9 | 72.8 | 66.8 | 68.9 | 141.7 | 141.7 |
| 77 | CH3 | 77.2 | 75.9 | 69.8 | 71.0 | 147.0 | 146.9 |
| 78 | F | 78.0 | 77.1 | 70.7 | 71.5 | 148.7 | 148.6 |
| 79 | Cl | 79.0 | 78.1 | 71.3 | 72.2 | 150.3 | 150.3 |
| 80 | CN | 81.9 | 81.8 | 75.0 | 75.0 | 156.9 | 156.8 |
| 81 | OMe | 78.5 | 76.9 | 66.6 | 68.1 | 145.1 | 145.0 |
| 82 | CH3 | 77.1 | 76.6 | 69.6 | 70.1 | 146.7 | 146.7 |
| 83 | F | 77.9 | 78.4 | 71.9 | 71.4 | 149.8 | 149.8 |
| 84 | Cl | 78.2 | 79.0 | 72.0 | 71.2 | 150.2 | 150.2 |
| 85 | CN | 81.6 | 82.8 | 77.5 | 76.4 | 159.1 | 159.2 |
| 86 | OMe | 73.9 | 79.9 | 65.1 | 59.1 | 139.0 | 139.0 |
| 87 | CH3 | 76.1 | 77.0 | 69.5 | 68.6 | 145.6 | 145.6 |
| 88 | F | 76.8 | 79.9 | 72.9 | 69.8 | 149.7 | 149.7 |
| 89 | Cl | 77.4 | 80.0 | 73.2 | 70.6 | 150.6 | 150.6 |
| 90 | CN | 81.3 | 82.7 | 76.8 | 75.4 | 158.1 | 158.1 |
| 91 | OMe | 80.1 | − | 65.5 | − | 145.6 | − |
| 92 | CH3 | 75.7 | − | 70.3 | − | 146.0 | − |
| 93 | F | 79.1 | − | 72.5 | − | 151.6 | − |
| 94 | Cl | 79.1 | − | 72.0 | − | 151.1 | − |
| 95 | CN | 80.6 | − | 74.5 | − | 155.1 | − |
| 96 | OMe | 77.2 | − | 55.2 | − | 132.4 | − |
| 97 | CH3 | 75.1 | − | 69.5 | − | 144.6 | − |
| 98 | F | 77.2 | − | 70.3 | − | 147.5 | − |
| 99 | Cl | 78.8 | − | 72.5 | − | 151.3 | − |
| 100 | CN | 84.8 | − | 77.6 | − | 162.4 | − |
| 101 | OMe | 78.2 | 74.6 | 65.7 | 69.2 | 143.9 | 143.8 |
| 102 | CH3 | 75.2 | 73.8 | 68.4 | 69.9 | 143.6 | 143.7 |
| 103 | F | 79.7 | 78.0 | 71.2 | 72.8 | 150.9 | 150.8 |
| 104 | Cl | 79.4 | 78.4 | 71.3 | 72.2 | 150.7 | 150.6 |
| 105 | CN | 83.9 | 83.7 | 77.8 | 78.0 | 161.7 | 161.7 |
| 106 | OMe | 76.0 | 77.7 | 60.5 | 58.8 | 136.5 | 136.5 |
| 107 | CH3 | 73.9 | 74.4 | 69.2 | 68.7 | 143.1 | 143.1 |
| 108 | F | 76.8 | 78.7 | 71.9 | 70.0 | 148.7 | 148.7 |
| 109 | Cl | 78.2 | 80.0 | 73.2 | 71.4 | 151.4 | 151.4 |
| 110 | CN | 84.4 | 85.5 | 79.9 | 78.9 | 164.3 | 164.4 |
| 111 | OMe | 73.7 | − | 64.4 | − | 138.1 | − |
| 112 | CH3 | 73.7 | − | 68.7 | − | 142.4 | − |
| 113 | F | 78.3 | − | 71.6 | − | 149.9 | − |
| 114 | Cl | 79.0 | − | 72.2 | − | 151.2 | − |
| 115 | CN | 86.4 | − | 81.0 | − | 167.4 | − |
| 116 | − | 71.1 | 73.1 | 66.3 | 64.3 | 137.4 | 137.4 |
| 117 | − | 78.9 | − | 81.3 | − | 160.2 | − |
| 118 | − | 68.6 | − | 62.3 | − | 130.9 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, J.-K.; Zhu, X.-Q.; Shen, G.-B. Evaluations of Quinone/Hydroquinone Couples Acting as Two Hydrogen Atoms Antioxidants, Radical Quenchers, and Hydrogen Atom Abstractors. Biomolecules 2025, 15, 1606. https://doi.org/10.3390/biom15111606
Chen X, Wang J-K, Zhu X-Q, Shen G-B. Evaluations of Quinone/Hydroquinone Couples Acting as Two Hydrogen Atoms Antioxidants, Radical Quenchers, and Hydrogen Atom Abstractors. Biomolecules. 2025; 15(11):1606. https://doi.org/10.3390/biom15111606
Chicago/Turabian StyleChen, Xiaotang, Jun-Ke Wang, Xiao-Qing Zhu, and Guang-Bin Shen. 2025. "Evaluations of Quinone/Hydroquinone Couples Acting as Two Hydrogen Atoms Antioxidants, Radical Quenchers, and Hydrogen Atom Abstractors" Biomolecules 15, no. 11: 1606. https://doi.org/10.3390/biom15111606
APA StyleChen, X., Wang, J.-K., Zhu, X.-Q., & Shen, G.-B. (2025). Evaluations of Quinone/Hydroquinone Couples Acting as Two Hydrogen Atoms Antioxidants, Radical Quenchers, and Hydrogen Atom Abstractors. Biomolecules, 15(11), 1606. https://doi.org/10.3390/biom15111606






