Dehydroandrographolide Alleviates Oxidative Stress, Inflammatory Response, and Pyroptosis in DSS-Induced Colitis Mice by Modulating Nrf2 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. DA Reagent
2.2. Animal Model
2.3. Disease Activity Index
2.4. Hematoxylin and Eosin (H&E) and Immunohistochemistry
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Western Blot
2.8. Real-Time Quantitative PCR
2.9. Reactive Oxygen Species (ROS) Detection
2.10. MDA, GSH, and LDH Detection
- -
- Malondialdehyde (MDA) Assay
- -
- Glutathione (GSH) Assay
- -
- Lactate Dehydrogenase (LDH) Assay
2.11. ELISA Analysis
2.12. Statistical Analysis
3. Results
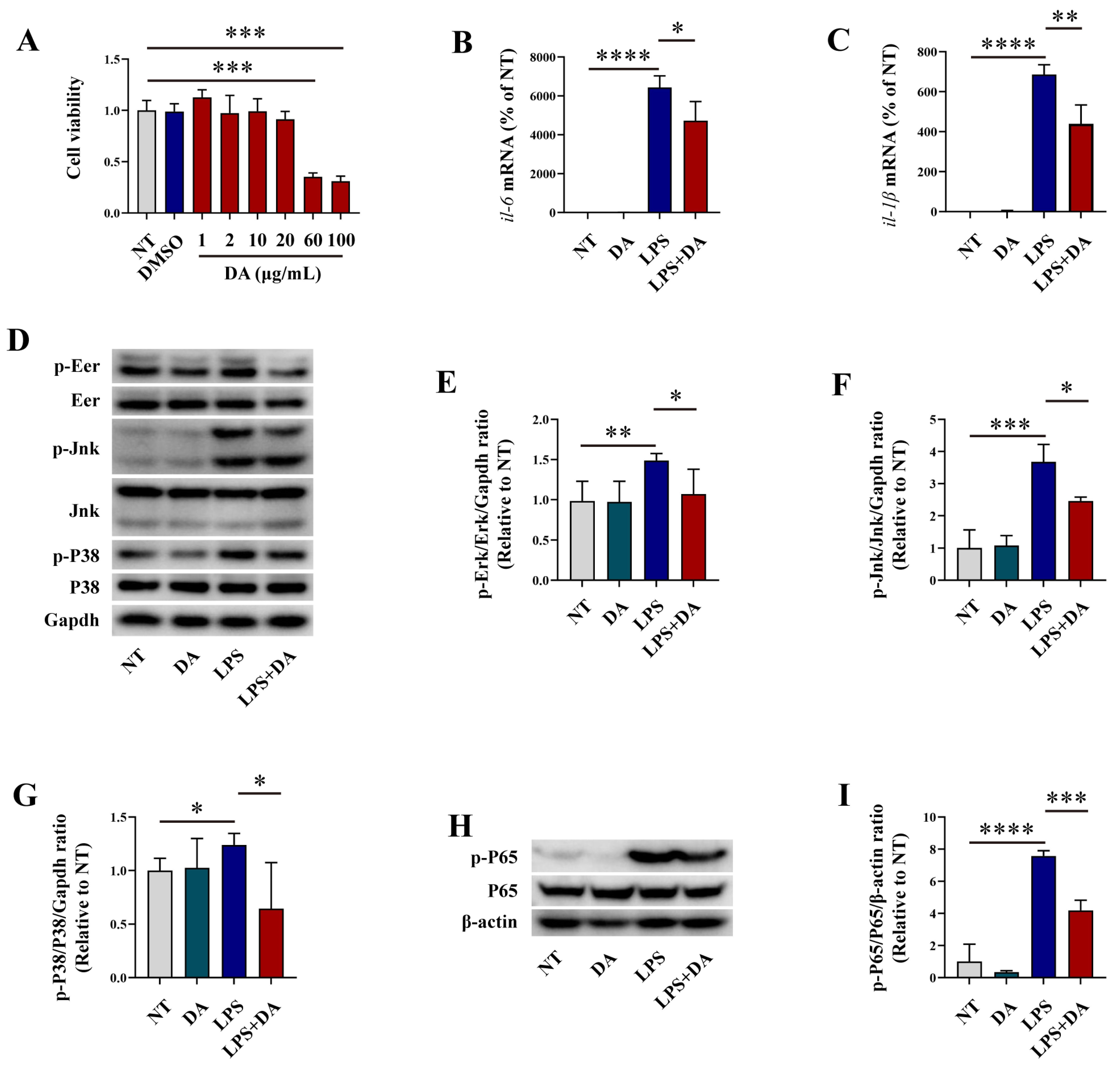
3.1. DA Inhibits Inflammatory Response in LPS-Induced Macrophages
3.2. DA Inhibits Oxidative Stress in LPS-Induced Macrophages
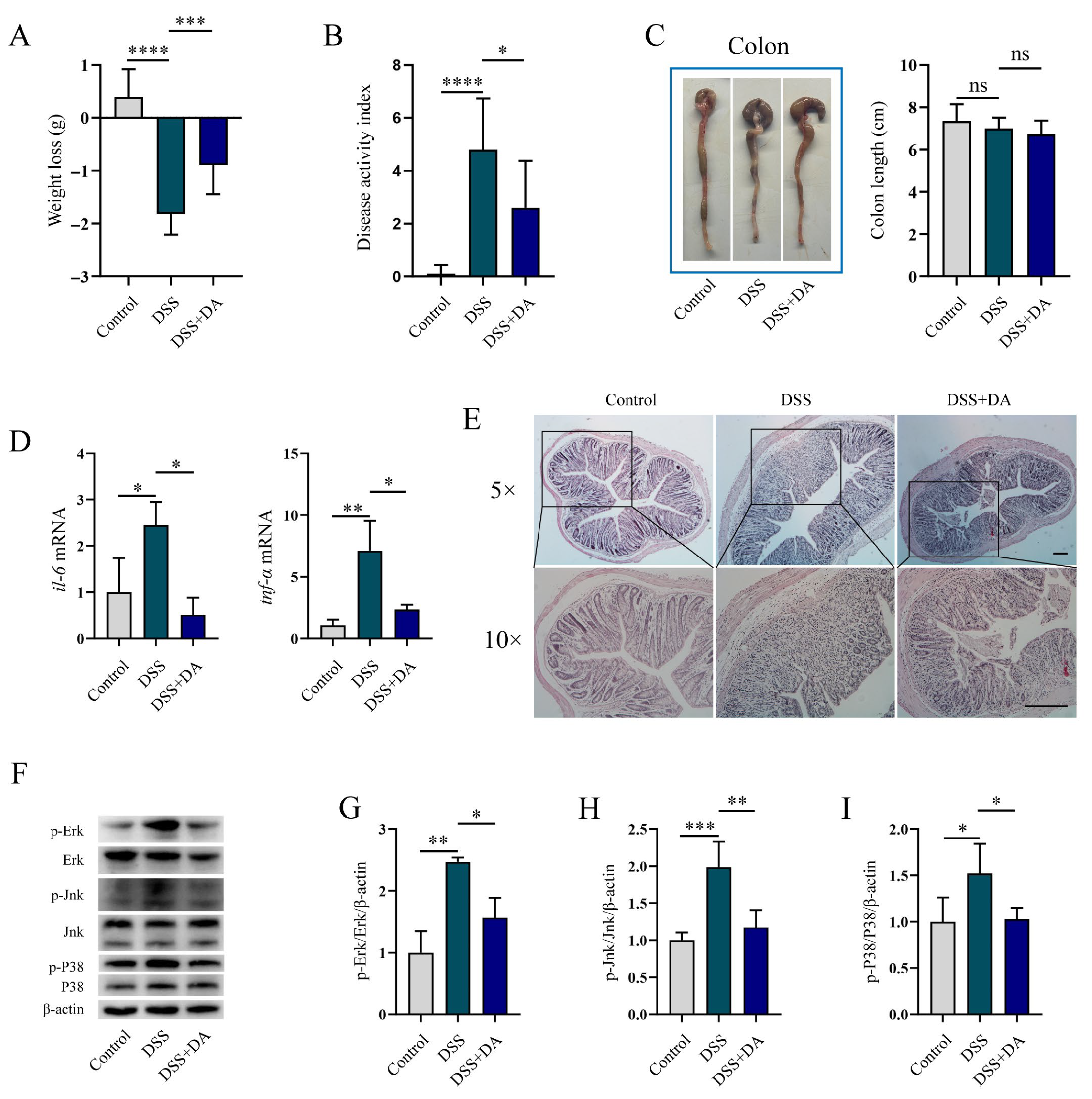
3.3. DA Mitigates Intestinal Inflammatory Injury in DSS-Induced Mice
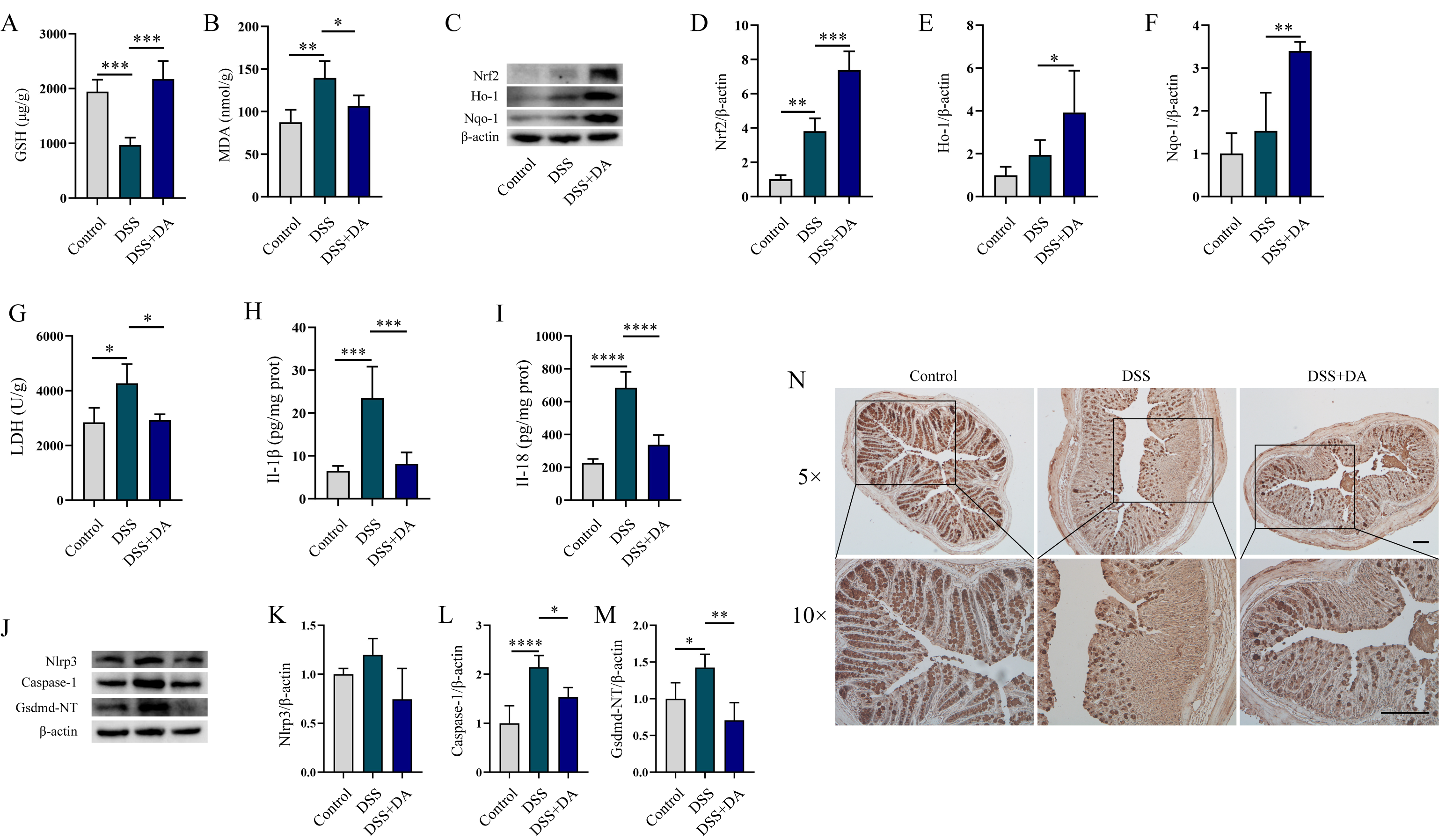
3.4. DA Suppresses Oxidative Stress and Pyroptosis in DSS-Induced Mice
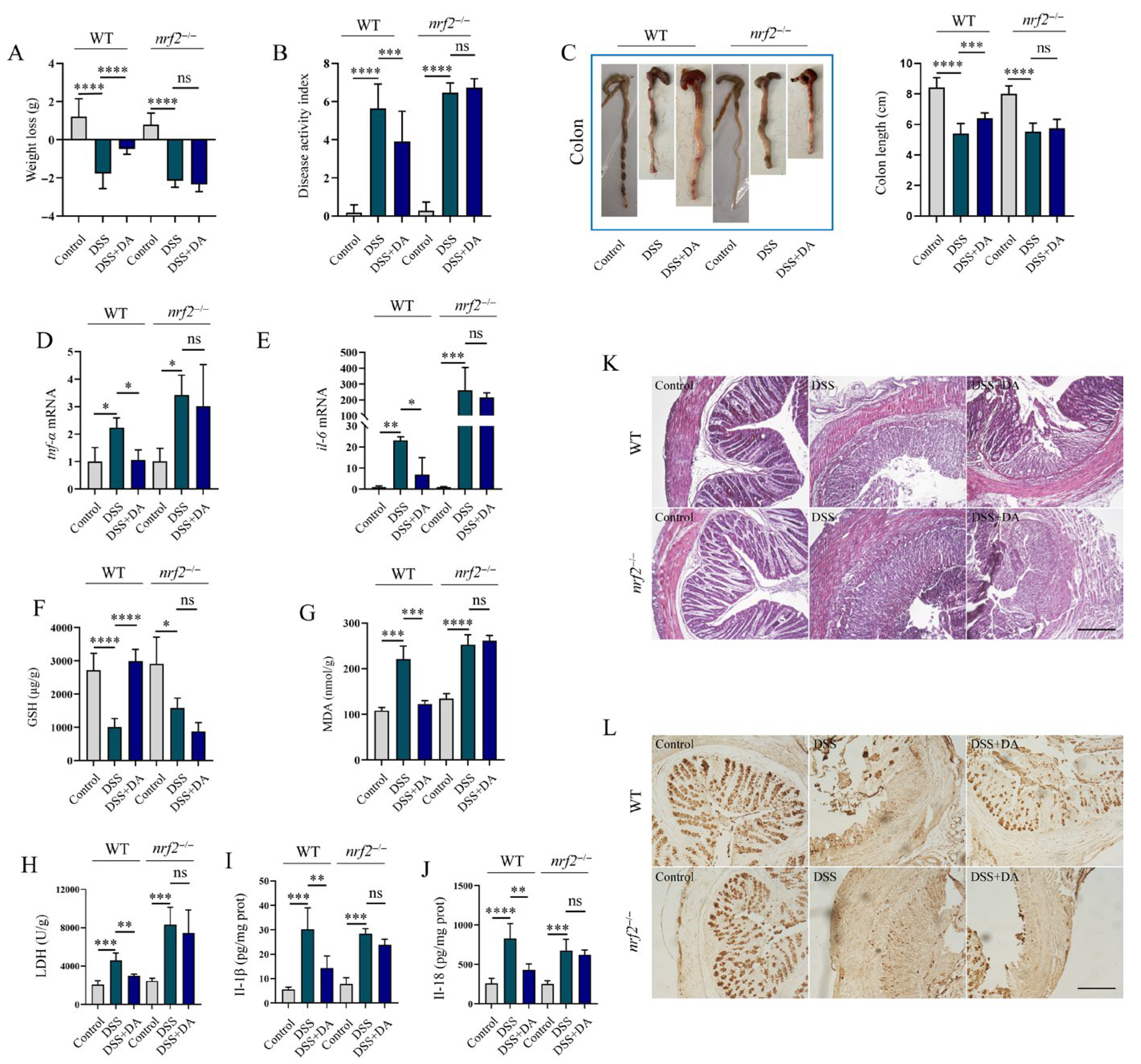
3.5. Nrf2 Mitigates DSS-Induced Intestinal Inflammatory Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DA | Dehydroandrographolide |
| LPS | Lipopolysaccharide |
| tnf-α | Tumor Necrosis Factor-α |
| il-6 | Interleukin-6 |
| il-1β | Interleukin-1β |
| Erk | Extracellular Signal-Regulated Kinase |
| Jnk | C-Jun N-Terminal Kinase |
| P38 | p38 Mitogen-Activated Pro-Tein Kinase |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| Cox-2 | Cyclooxygenase-2 |
| iNos | Inducible Nitric Oxide Synthase |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| Nqo-1 | Nad(P)H Quinone Dehydrogenase 1 |
| Ho-1 | Heme Oxygenase-1 |
| Akt | Protein Kinase B |
| Ampk-α1 | 5′-Adenosine Monophos-Phate-Activated Protein Kinase-α1 |
| WT | Wild type |
| DAI | Disease Activity Index |
| Nlrp3 | Nod-Like Receptor Family Pyrin Domain-Containing 3 |
| nrf2−/− | nrf2 Knockout |
| IBD | Inflammatory Bowel Disease |
| UC | Ulcerative Colitis |
| CD | Crohn’s Disease |
| DMSO | Dimethyl Sulfoxide |
| RIPA | Radioimmunoprecipitation Assay |
| PMSF | Phenylmethylsulfonyl Fluoride |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal Bovine Serum |
| H&E | Hematoxylin And Eosin |
| ECL | Enhanced Chemiluminescence |
| ROS | Reactive Oxygen Species |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein Diacetate |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| LDH | Lactate Dehydrogenase |
| SEM | Standard Error Of The Mean |
| ANOVA | One-Way Analysis Of Variance |
| LSD | Least Significant Difference |
| Mapk | Mitogen-Activated Protein Kinase |
| Jak | Janus Kinase |
| S1p | Sphingosine-1-Phosphate |
| TNBS | Trinitrobenzene Sulfonic Acid |
| Txnip | Thioredoxin-Interacting Protein |
| Rtks | Receptor Tyrosine Kinases |
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Brinks, R.; Landwehr, S. Age- and time-dependent model of the prevalence of non-communicable diseases and application to dementia in Germany. Theor. Popul. Biol. 2014, 92, 62–68. [Google Scholar] [CrossRef]
- Hracs, L.; Windsor, J.W.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; Quan, J.; Goddard, Q.; Caplan, L.; Markovinovic, A.; et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 2025, 642, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 1991, 325, 928–937. [Google Scholar] [CrossRef]
- Kirsner, J.B.; Shorter, R.G. Recent developments in nonspecific inflammatory bowel disease (second of two parts). N. Engl. J. Med. 1982, 306, 837–848. [Google Scholar] [CrossRef]
- Pouillon, L.; Travis, S.; Bossuyt, P.; Danese, S.; Peyrin-Biroulet, L. Head-to-head trials in inflammatory bowel disease: Past, present and future. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 365–376. [Google Scholar] [CrossRef]
- Toskas, A.; Akbar, A. IBD therapeutics: What is in the pipeline? Frontline Gastroenterol. 2022, 13, e35–e43. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, S.; Xu, B.; Liu, F.; Huo, G.; Li, B. Alleviation effects of Bifidobacterium animalis subsp. lactis XLTG11 on dextran sulfate sodium-induced colitis in mice. Microorganisms 2021, 9, 2093. [Google Scholar] [CrossRef]
- Yang, T.; Ma, X.; Wang, R.; Liu, H.; Wei, S.; Jing, M.; Li, H.; Zhao, Y. Berberine inhibits IFN-gamma signaling pathway in DSS-induced ulcerative colitis. Saudi Pharm. J. 2022, 30, 764–778. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, B.; Min, X.; Hou, Y.; Lin, L.; Wu, Q.; Shi, J.; Chen, X. Therapeutic potential of isobavachalcone, a natural flavonoid, in murine experimental colitis by inhibiting NF-kappaB p65. Phytother. Res. 2021, 35, 5861–5870. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, M.Y.; Chen, J.Y.; Xu, Z.W.; Zhang, J.W.; Li, T.; Zhang, L.L.; Wei, W. CP-25 exerts therapeutic effects in mice with dextran sodium sulfate-induced colitis by inhibiting GRK2 translocation to downregulate the TLR4-NF-kappaB-NLRP3 inflammasome signaling pathway in macrophages. IUBMB Life 2021, 73, 1406–1422. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Che, D.; Zheng, Y.; Hou, Y.; Li, T.; Du, X.; Geng, S. Dehydroandrographolide targets CD300f and negatively regulated MRGPRX2-induced pseudo-allergic reaction. Phytother. Res. PTR 2022, 36, 2173–2185. [Google Scholar] [CrossRef]
- Xiong, W.B.; Shao, Z.J.; Xiong, Y.; Chen, J.; Sun, Y.; Zhu, L.; Zhou, L.M. Dehydroandrographolide enhances innate immunity of intestinal tract through up-regulation the expression of hBD-2. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2015, 23, 37. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.B.; Huang, X.Y.; Geng, C.A.; Zhao, Y.; Wang, L.J.; Guo, R.H.; Liang, W.J.; Zhang, X.M.; Chen, J.J. Synthesis, structure-activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg. Med. Chem. Lett. 2014, 24, 2353–2359. [Google Scholar] [CrossRef]
- Ling, L.; Louis, H.; Isang, B.B.; Emori, W.; Benjamin, I.; Ahuekwe, E.F.; Cheng, C.R.; Manicum, A.E. Inflammatory studies of dehydroandrographolide: Isolation, spectroscopy, biological activity, and theoretical modeling. Appl. Biochem. Biotechnol. 2024, 196, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shen, Y.; Zhang, L.; Wang, F.; Xiang, S. Pharmacological Effects and Pharmacokinetic Profiles of Dehydroandrographolide. Mediat. Inflamm. 2025, 2025, 4123997. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Liu, X.; Hu, J.; Mu, J.; Xie, J.; Yao, C.; Li, L. Protective effect of dehydroandrographolide on obstructive cholestasis in bile duct-ligated mice. Oncotarget 2017, 8, 87903–87913. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Chen, J.C.; Yang, W.E.; Chien, S.Y.; Chen, M.K.; Lo, Y.S.; Hsi, Y.T.; Chuang, Y.C.; Lin, C.C.; Yang, S.F. Dehydroandrographolide inhibits oral cancer cell migration and invasion through NF-κB-, AP-1-, and SP-1-modulated matrix metalloproteinase-2 inhibition. Biochem. Pharmacol. 2017, 130, 10–20. [Google Scholar] [CrossRef]
- Che, D.; Hou, Y.; Zeng, Y.; Li, C.; Zhang, Y.; Wei, D.; Hu, S.; Liu, R.; An, H.; Wang, Y.; et al. Dehydroandrographolide inhibits IgE-mediated anaphylactic reactions via calcium signaling pathway. Toxicol. Appl. Pharmacol. 2019, 366, 46–53. [Google Scholar] [CrossRef]
- Kong, N.; Guan, H.; Duan, X.; Cao, R.; Li, H.; Xing, F.; Du, X.; Zheng, Y.; Zhang, L.; Li, Y.; et al. Dehydroandrographolide alleviates rheumatoid arthritis by inhibiting neutrophil activation via LMIR3 in collagen induced arthritis rats. Cell Cycle 2024, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Zhang, S.S.; Jin, S.W.; Xia, T.J.; Liao, Y.H.; Pan, R.L.; Yan, M.Z.; Chang, Q. Anti-inflammatory effect and pharmacokinetics of dehydroandrographolide, an active component of Andrographis paniculata, on Poly(I:C)-induced acute lung injury. Biomed. Pharmacother. 2024, 174, 116456. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Chi, Y.; Xie, J.; Liu, X.; Hu, J.; Yang, F.; Li, L. Anti-inflammatory activity of dehydroandrographolide by tlr4/nf-kappab signaling pathway inhibition in bile duct-ligated mice. Cell. Physiol. Biochem. 2018, 49, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Jiang, L.; Chen, L.; Wang, W.; Gu, J.; Kuai, J.; Yang, X.; Ma, Y.; Han, C.; Wei, W. Involvement of embryo-derived and monocyte-derived intestinal macrophages in the pathogenesis of inflammatory bowel disease and their prospects as therapeutic targets. Int. J. Mol. Sci. 2024, 25, 690. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Wen, D.; Guo, J.; Xiong, Q.; Li, P.; Zhao, L.; Wang, J.; Wu, C.; Dong, L. Polydatin has anti-inflammatory and antioxidant effects in LPS-induced macrophages and improves DSS-induced mice colitis. Immun. Inflamm. Dis. 2021, 9, 959–970. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Karin, M. Mitogen activated protein kinases as targets for development of novel anti-inflammatory drugs. Ann. Rheum. Dis. 2004, 63 (Suppl. 2), ii62–ii64. [Google Scholar] [CrossRef]
- Kobayashi, E.; Suzuki, T.; Yamamoto, M. Roles nrf2 plays in myeloid cells and related disorders. Oxidative Med. Cell. Longev. 2013, 2013, 529219. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing global epidemiology of inflammatory bowel diseases: Sustaining health care delivery into the 21st century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.; Panaccione, R.; Wolf, D.; Pollack, P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: The CLASSIC-I trial. Gastroenterology 2006, 130, 323–333; quiz 591. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Vieujean, S.; Jairath, V.; Peyrin-Biroulet, L.; Dubinsky, M.; Iacucci, M.; Magro, F.; Danese, S. Understanding the therapeutic toolkit for inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 371–394. [Google Scholar] [CrossRef]
- Campbell, S.; Ghosh, S. Effective maintenance of inflammatory bowel disease remission by azathioprine does not require concurrent 5-aminosalicylate therapy. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1297–1301. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, W.; Song, Y.; Chen, K.; Lv, L.; Yang, H.; Wang, W.; Xiao, Y. Anti-inflammatory effect of clostridium butyricum-derived extracellular vesicles in ulcerative colitis: Impact on host micrornas expressions and gut microbiome profiles. Mol. Nutr. Food Res. 2023, 67, e2200884. [Google Scholar] [CrossRef] [PubMed]
- Kolesnick, R.; Xing, H.R. Inflammatory bowel disease reveals the kinase activity of KSR1. J. Clin. Investig. 2004, 114, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Geertsema, S.; Bourgonje, A.R.; Fagundes, R.R.; Gacesa, R.; Weersma, R.K.; van Goor, H.; Mann, G.E.; Dijkstra, G.; Faber, K.N. The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol. Med. 2023, 29, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, R.; Wang, F.; Li, S.; Li, L.; Li, Y.; Qin, P.; Wei, M.; Yang, J.; Wu, J.; et al. Liberation of daidzein by gut microbial beta-galactosidase suppresses acetaminophen-induced hepatotoxicity in mice. Cell Host Microbe 2023, 31, 766–780.e767. [Google Scholar] [CrossRef]
- Wu, M.; Chen, W.; Lu, Y.; Zhu, G.; Hao, L.; Li, Y.P. Galpha13 negatively controls osteoclastogenesis through inhibition of the Akt-GSK3beta-NFATc1 signalling pathway. Nat. Commun. 2017, 8, 13700. [Google Scholar] [CrossRef]
- Ma, S.; Qin, J.; Zhang, Y.; Luan, J.; Sun, N.; Hou, G.; He, J.; Xiao, Y.; Zhang, W.; Gao, M. Disrupting mitochondrial dynamics attenuates ferroptosis and chemotoxicity via upregulating NRF2-mediated FSP1 expression. Cell Rep. 2025, 44, 116234. [Google Scholar] [CrossRef]
- Zheng, H.J.; Guo, J.; Jia, Q.; Huang, Y.S.; Huang, W.J.; Zhang, W.; Zhang, F.; Liu, W.J.; Wang, Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 142, 303–313. [Google Scholar] [CrossRef]
- Cao, J.; Bao, Q.; Hao, H. Indole-3-carboxaldehyde alleviates LPS-induced intestinal inflammation by inhibiting ROS production and NLRP3 inflammasome activation. Antioxidants 2024, 13, 1107. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrin, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yu, P.; Chu, C.; Wang, Z.; Xu, W.; Cheng, F.; Zhao, H.; Qiu, Z. Magnesium hydride attenuates intestinal barrier injury during hemorrhage shock by regulating neutrophil extracellular trap formation via the ROS/MAPK/PAD4 pathway. Int. Immunopharmacol. 2024, 130, 111688. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Li, P.; Liu, R.; Francis, O.B.; Song, S.; Sui, Y.; Yang, Y.; Wang, Y.; Sun, X.; Miao, R.; et al. The main active components of Prunella vulgaris L. alleviate myocardial ischemia-reperfusion injury by inhibiting oxidative stress and ferroptosis via the NRF2/GPX4 pathway. J. Ethnopharmacol. 2025, 345, 119630. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Lei, S.Y.; Yang, T.; Xu, Y.S.; Wang, H.L.; Feng, C.L.; Tang, W. PDE4 inhibitor apremilast ameliorates TNBS-induced irritable bowel syndrome in mice by activating the Nrf-2 signaling pathway in enteric glial cells. Acta Pharmacol. Sin. 2025. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, H.; Xie, W.; Meng, M.; Wang, Y.; Ma, N.; Chang, G.; Shen, X. Subacute ruminal acidosis induces pyroptosis via the mitophagy-mediated NLRP3 inflammasome activation in the livers of dairy cows fed a high-grain diet. J. Dairy Sci. 2024, 107, 4092–4107. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, N. N-lobe of TXNIP is critical in the allosteric regulation of NLRP3 via txnip binding. Front. Aging Neurosci. 2022, 14, 893919. [Google Scholar] [CrossRef]
- Youl, E.; Magous, R.; Cros, G.; Oiry, C. MAP Kinase cross talks in oxidative stress-induced impairment of insulin secretion. Involvement in the protective activity of quercetin. Fundam. Clin. Pharmacol. 2014, 28, 608–615. [Google Scholar] [CrossRef]
- Wu, J.R.; Zhang, X.M.; Zhang, B. Potassium dehydroandrographolide succinate injection for the treatment of child epidemic parotitis: A systematic review and meta-analysis. Chin. J. Integr. Med. 2015, 21, 866–873. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Liu, Y.M.; Liu, G.Y.; Zhang, M.Q.; Jia, J.Y.; Lu, C.; Yu, C. Pharmacokinetics and tolerance of dehydroandrographolide succinate injection after intravenous administration in healthy Chinese volunteers. Acta Pharmacol. Sin. 2012, 33, 1332–1336. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, Z.; Lei, X.; Yang, Z.; Yu, S.; Chen, G. Dehydroandrographolide Alleviates Oxidative Stress, Inflammatory Response, and Pyroptosis in DSS-Induced Colitis Mice by Modulating Nrf2 Signaling Pathway. Biomolecules 2025, 15, 1580. https://doi.org/10.3390/biom15111580
Wang M, Li Z, Lei X, Yang Z, Yu S, Chen G. Dehydroandrographolide Alleviates Oxidative Stress, Inflammatory Response, and Pyroptosis in DSS-Induced Colitis Mice by Modulating Nrf2 Signaling Pathway. Biomolecules. 2025; 15(11):1580. https://doi.org/10.3390/biom15111580
Chicago/Turabian StyleWang, Meifen, Zhenyu Li, Xinghua Lei, Ziyue Yang, Shuixing Yu, and Guangxin Chen. 2025. "Dehydroandrographolide Alleviates Oxidative Stress, Inflammatory Response, and Pyroptosis in DSS-Induced Colitis Mice by Modulating Nrf2 Signaling Pathway" Biomolecules 15, no. 11: 1580. https://doi.org/10.3390/biom15111580
APA StyleWang, M., Li, Z., Lei, X., Yang, Z., Yu, S., & Chen, G. (2025). Dehydroandrographolide Alleviates Oxidative Stress, Inflammatory Response, and Pyroptosis in DSS-Induced Colitis Mice by Modulating Nrf2 Signaling Pathway. Biomolecules, 15(11), 1580. https://doi.org/10.3390/biom15111580





