Effects of Fludioxonil on the Cell Growth and Apoptosis in T and B Lymphocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Cultures
2.3. Treatment of Fludioxonil
2.4. Water Soluble Tetrazolium Salt (WST) Assay
2.5. FACS Analysis of Cell Cycle Arrest by PI Staining
2.6. FACS Analysis of Apoptosis by FITC, AF488-Annexin V/PI Staining
2.7. Analysis of Mitochondrial Membrane Potential
2.8. Western Blot Assay
2.9. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3. Results
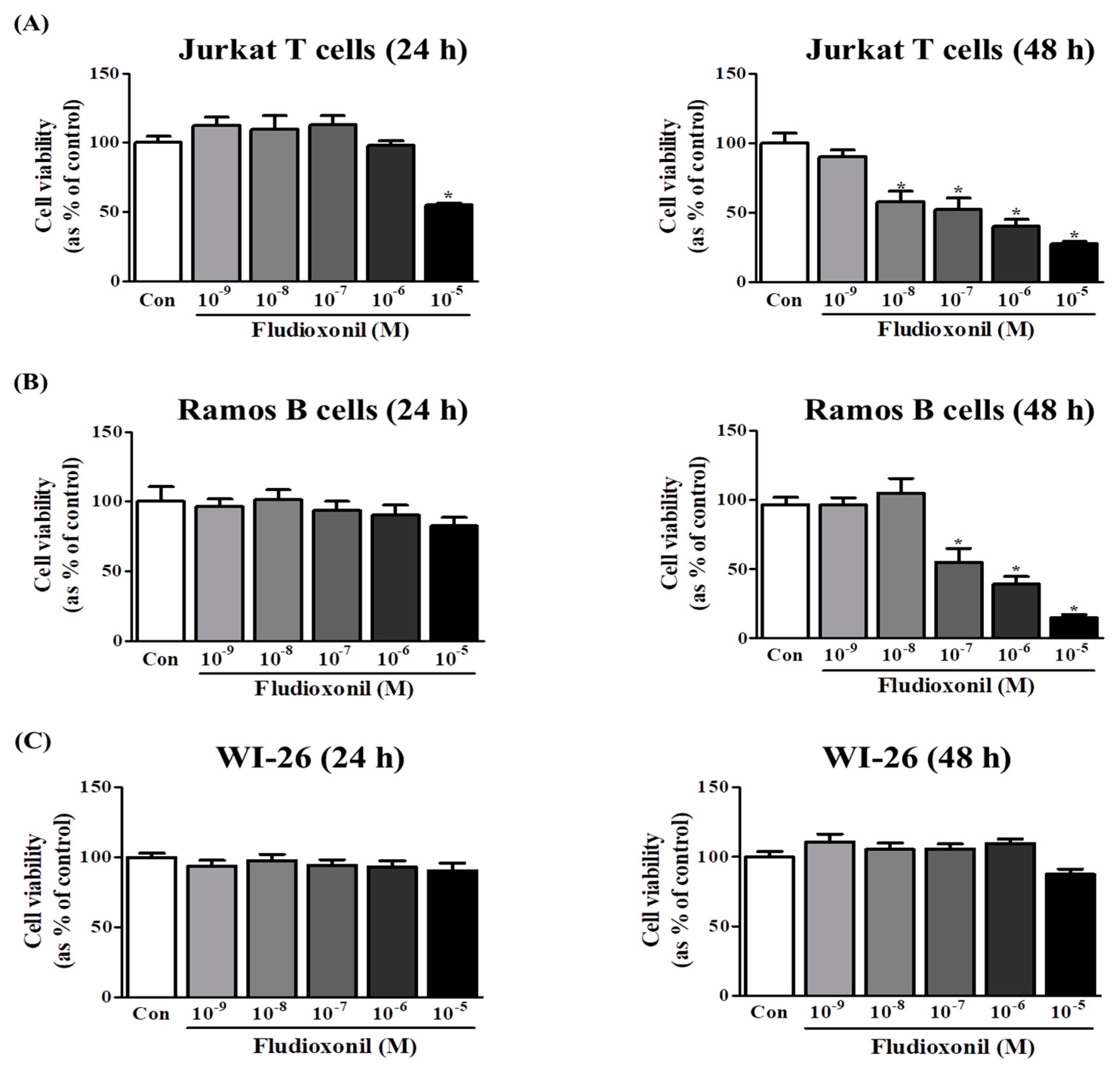
3.1. Fludioxonil Exposure Supressed Cell Viability
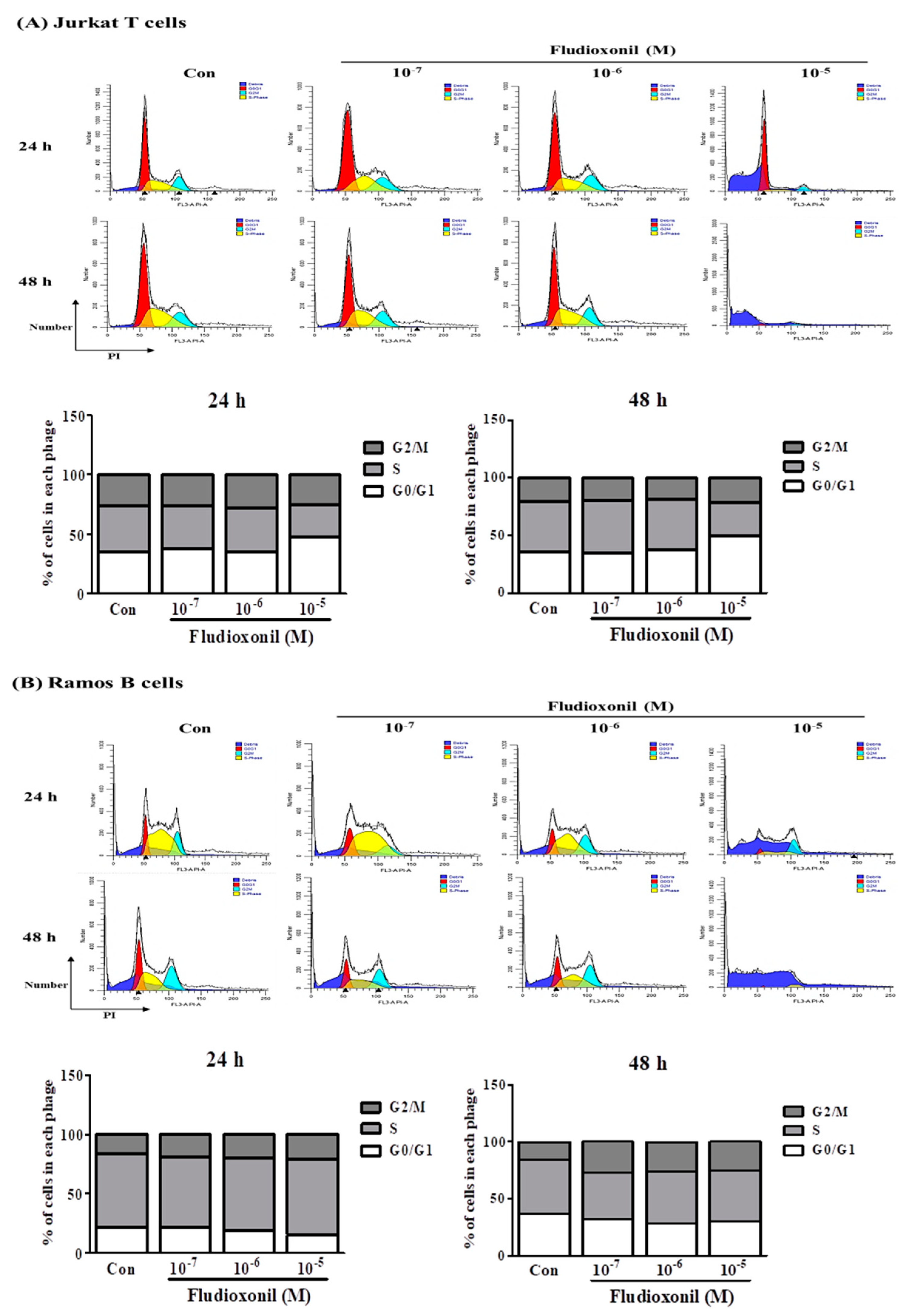
3.2. Fludioxonil Exposure Induced Cell Cycle Arrest
3.3. Alteration of Cell Cycle-Related Genes Expression by Fludioxonil
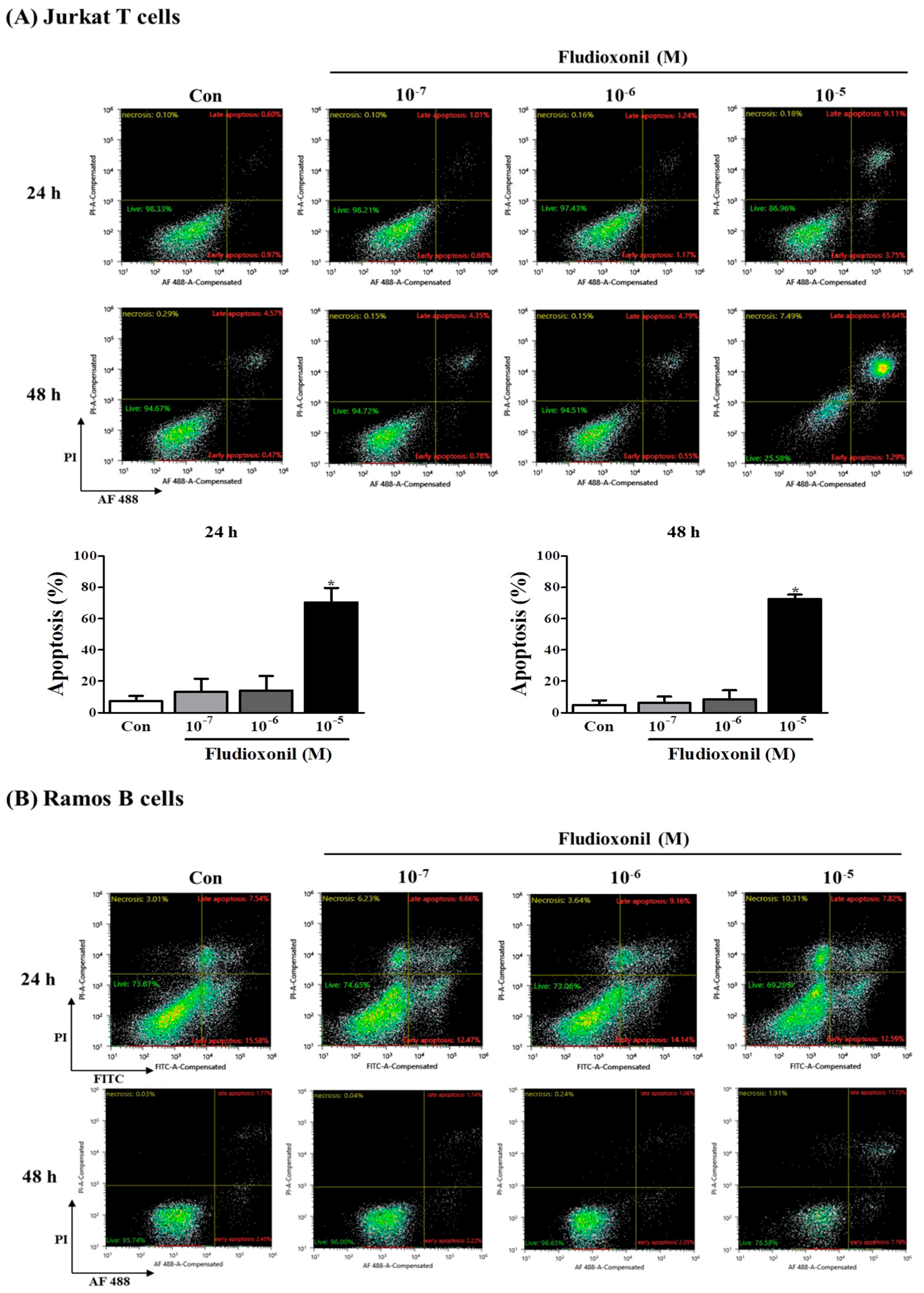
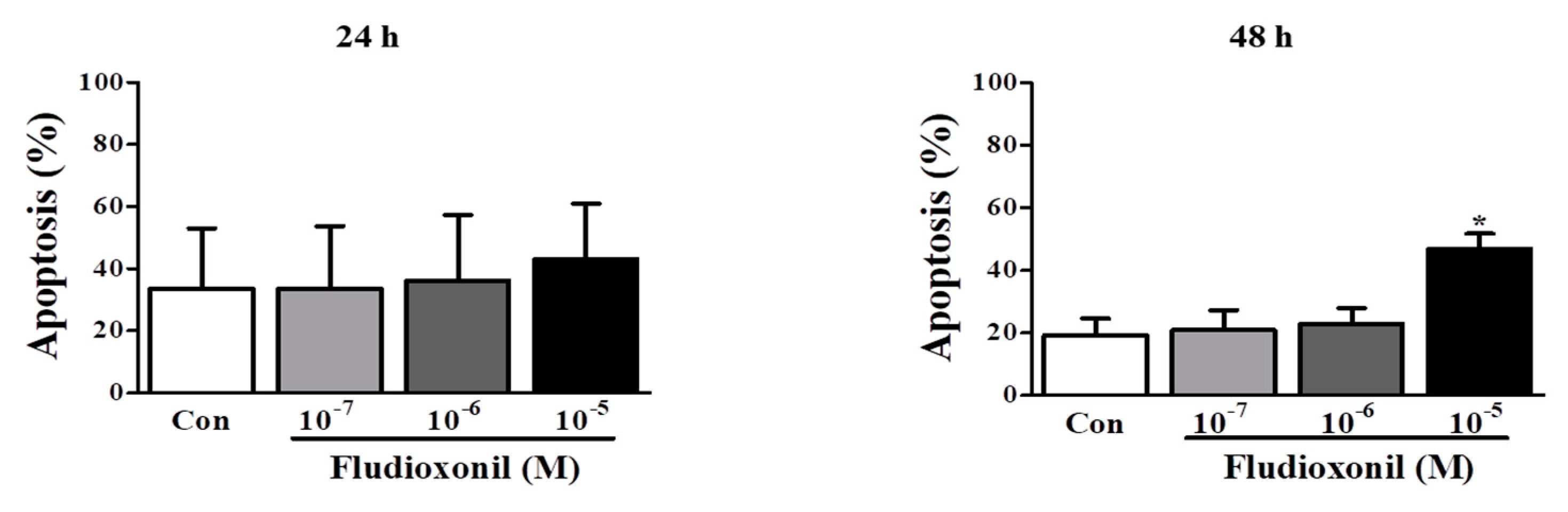
3.4. Apoptosis of Immune Cells by Fludioxonil Exposure
3.5. Decrease of Mitochondrial Membrane Potential by Fludioxonil
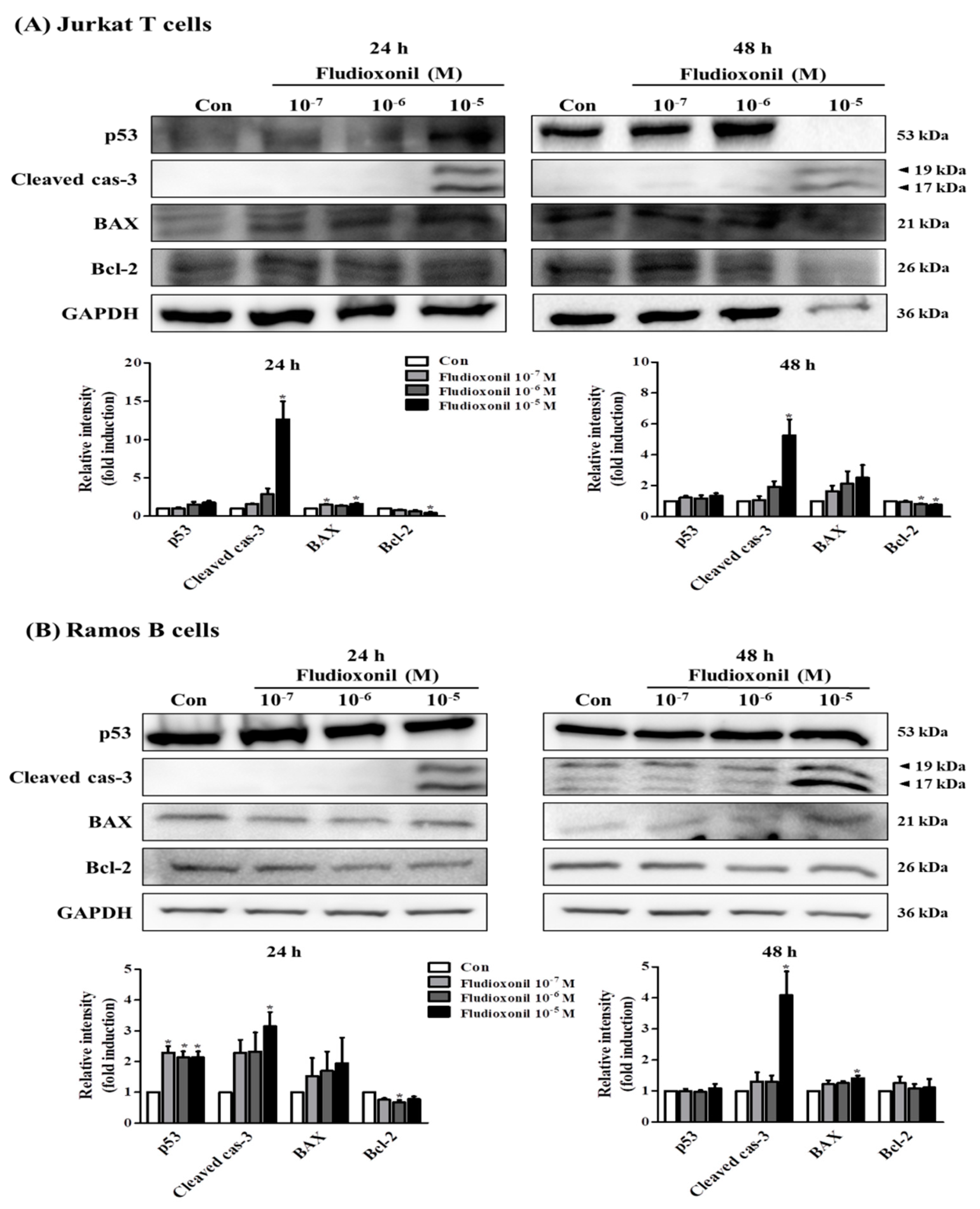
3.6. Alteration of Apoptosis-Related Genes Expression by Fludioxonil
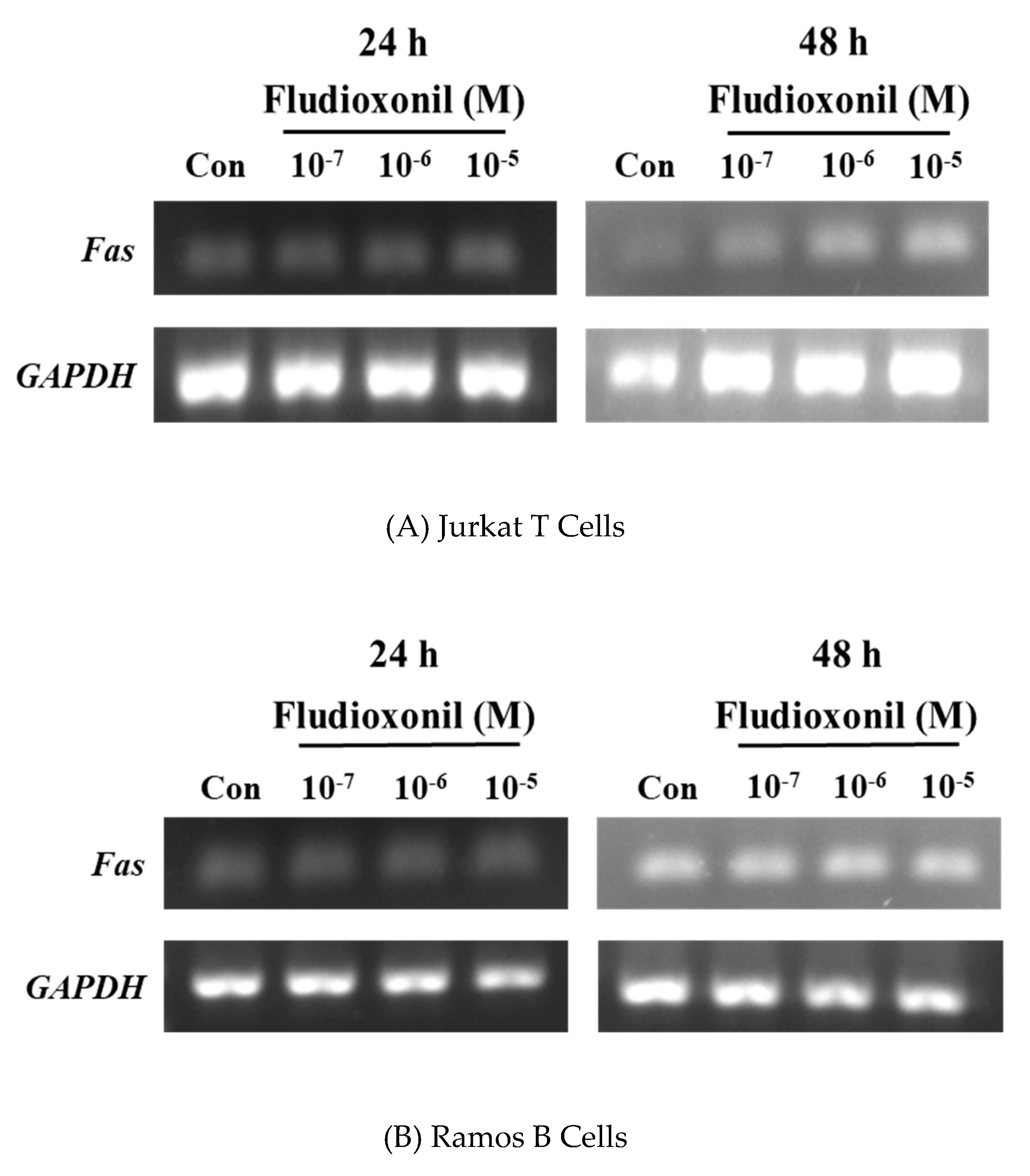
3.7. Increase of Fas Gene Expression by Fludioxonil
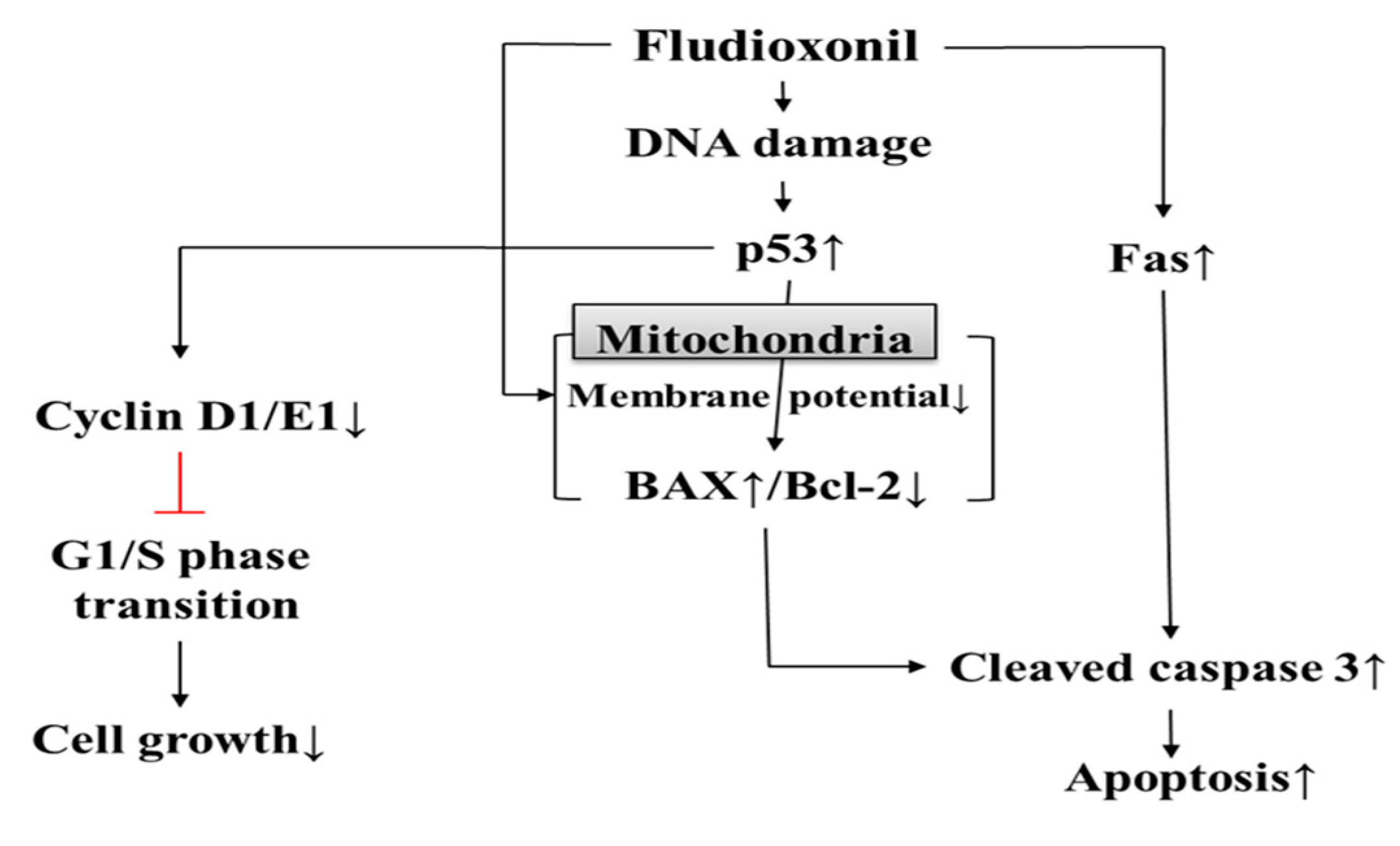
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Jennings, A. Worldwide Regulations of Standard Values of Pesticides for Human Health Risk Control: A Review. Int. J. Environ. Res. Public Heal. 2017, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Mokarizadeh, A.; Faryabi, M.R.; Rezvanfar, M.A.; Abdollahi, M. A comprehensive review of pesticides and the immune dysregulation: Mechanisms, evidence and consequences. Toxicol. Mech. Methods 2015, 25, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Sokooti, M.; Galli, C.L.; Moretto, A.; Colosio, C. Pesticide induced immunotoxicity in humans: A comprehensive review of the existing evidence. Toxicology 2013, 307, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Pesticide Chemical Research in Toxicology: Lessons from Nature. Chem. Res. Toxicol. 2017, 30, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Matsubara, T.; Watanabe, N. Pyrrolnitrin, a new antifungal antibiotic. Microbiological and toxicological observations. J. Antibiot. 1965, 18, 211–219. [Google Scholar] [PubMed]
- Raaijmakers, J.M.; Vlami, M.; De Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, K.; Gowariker, V.; Krishnamurthy, V.N.; Gowariker, S. Alphabetical Entries. The Pesticide Encyclopedia; Paranjape, K., Gowariker, V., Krishnamurthy, V.N., Eds.; CABI: Wolin Ford, UK, 2014. [Google Scholar]
- Teng, Y.; Manavalan, T.T.; Hu, C.; Medjakovic, S.; Jungbauer, A.; Klinge, C.M. Endocrine disruptors fludioxonil and fenhexamid stimulate miR-21 expression in breast cancer cells. Toxicol. Sci. 2013, 131, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Graillot, V.; Takakura, N.; Audebert, M.; Cravedi, J.-P.; Le Hegarat, L.; Fessard, V.; Cravedi, J. Genotoxicity of pesticide mixtures present in the diet of the French population. Environ. Mol. Mutagen. 2012, 53, 173–184. [Google Scholar] [CrossRef]
- Beck, G.; Habicht, G.S. Immunity and the invertebrates. Sci. Am. 1996, 275, 60–66. [Google Scholar] [CrossRef]
- Matzinger, P. The Danger Model: A Renewed Sense of Self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Litman, G.W.; Cannon, J.P.; Dishaw, L.J. Reconstructing Immune Phylogeny: New Perspectives. Nat. Rev. Immunol. 2005, 5, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Pancer, Z. Cooper MD: The evolution of adaptive immunity. Annu. Rev. Immunol. 2006, 24, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.-L. Adaptive Immunity By Convergent Evolution. Nat. Rev. Immunol. 2018, 18, 294. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Weaver, C. Basic Concepts in immunology. In Janeway’s Immunobiology; Murphy, K.M., Weaver, C., Eds.; Garland Science, Taylor & Francis Group, LLC: Thames, UK, 2016. [Google Scholar]
- King, K.L.; Cidlowski, J.A. Cell cycle and apoptosis: Common pathways to life and death. J. Cell Biochem. 1995, 58, 175–180. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, T.K.; Sang, N.; Giordano, A. Cyclins, Cyclin-Dependent Kinases and Cdk Inhibitors: Implications in Cell Cycle Control and Cancer. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 127–156. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Introduction. In Means to an End: Apoptosis and Other Cell Death Mechanisms; Green, D.R., Ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011. [Google Scholar]
- Zakeri, Z.; Bursch, W.; Tenniswood, M.; A Lockshin, R. Cell death: programmed, apoptosis, necrosis, or other? Cell Death Differ. 1995, 2, 87–96. [Google Scholar] [PubMed]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Lu, M.; Petersen, S.; Ashkenazi, A. Apoptosis initiation through the cell-extrinsic pathway. Methods Enzymol. 2014, 544, 99–128. [Google Scholar]
- Mohan, S.; Abdul, A.B.; Abdelwahab, S.I.; Al-Zubairi, A.S.; Sukari, M.A.; Abdullah, R.; Taha, M.M.E.; Ibrahim, M.Y.; Syam, S. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: Its activation coupled with G0/G1 phase cell cycle arrest. J. Ethnopharmacol. 2010, 131, 592–600. [Google Scholar] [CrossRef]
- Saelens, X.; Festjens, N.; Walle, L.V.; Van Gurp, M.; Van Loo, G.; Vandenabeele, P. Toxic proteins released from mitochondria in cell death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef] [Green Version]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.W.; Peterson, K.L.; Dai, H.; Schneider, P.; Lee, S.-H.; Zhang, J.-S.; Koenig, A.; Bronk, S.; Billadeau, D.D.; Gores, G.J.; et al. High Cell Surface Death Receptor Expression Determines Type I Versus Type II Signaling*. J. Boil. Chem. 2011, 286, 35823–35833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, R.E.; Hwang, K.A.; Kim, C.W.; Byun, Y.S.; Nam, K.H.; Choi, K.C. Effect of dioxin and 17beta-estradiol on the expression of cytochrome P450 1A1 gene via an estrogen receptor dependent pathway in cellular and xenografted models. Environ. Toxicol. 2017, 32, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Bocher, M.; Boldicke, T.; Sasse, F. Cytotoxic effect of atrazine on murine B-lymphocytes in vitro. Sci. Total Environ. 1993, 132, 429–433. [Google Scholar] [CrossRef]
- Bissonnette, S.L.; Teague, J.E.; Sherr, D.H.; Schlezinger, J.J. An endogenous prostaglandin enhances environmental phthalate-induced apoptosis in bone marrow B cells: Activation of distinct but overlapping pathways. J. Immunol. 2008, 181, 1728–1736. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Kawada, T. Carbamate Pesticide-Induced Apoptosis in Human T Lymphocytes. Int. J. Environ. Res. Public Heal. 2015, 12, 3633–3645. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Jang, Y.; Kang, K.; Song, D.H.; Kim, R.; Chang, H.W.; Lee, D.E.; Song, C.K.; Choi, B.; Kang, M.J.; et al. Atrazine induces endoplasmic reticulum stress-mediated apoptosis of T lymphocytes via the caspase-8-dependent pathway. Environ. Toxicol. 2016, 31, 998–1008. [Google Scholar] [CrossRef]
- Go, R.E.; Kim, C.W.; Jeon, S.Y.; Byun, Y.S.; Jeung, E.B.; Nam, K.H.; Choi, K.C. Fludioxonil induced the cancer growth and metastasis via altering epithelial-mesenchymal transition via an estrogen receptor-dependent pathway in cellular and xenografted breast cancer models. Environ. Toxicol. 2017, 32, 1439–1454. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Roberts, K.; Raff, M.; Walter, P. The adaptive Immune System. In Molecular Biology of the Cell; Robertson, M., Ed.; Garland Publishing Incorporated: Princeton, NJ, USA, 2002. [Google Scholar]
- Male, D.K. Introduction to the immune system. In Immunology; Ozols, I., Cook, L., Eds.; Mosby Elsevier: Maryland Heights, MI, USA, 2006. [Google Scholar]
- Scully, C.; A Georgakopoulou, E.; Hassona, Y. The immune system: basis of so much health and disease: 3. adaptive immunity. Dent. Updat. 2017, 44, 322–327. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; De Bruin, R.A.M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Boil. 2013, 14, 518–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Caldon, C.E.; Musgrove, E.A. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 2010, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, Q.; Xie, S.; Xu, J.; Huang, B.; Wu, Y.; Xia, D. Cypermethrin Induces Macrophages Death through Cell Cycle Arrest and Oxidative Stress-Mediated JNK/ERK Signaling Regulated Apoptosis. Int. J. Mol. Sci. 2016, 17, 885. [Google Scholar] [CrossRef] [PubMed]
- Shounan, Y.; Feng, X.; O’Connell, P.J. Apoptosis detection by annexin V binding: A novel method for the quantitation of cell-mediated cytotoxicity. J. Immunol Methods 1998, 217, 61–70. [Google Scholar] [CrossRef]
- Salvioli, S.; Ardizzoni, A.; Franceschi, C.; Cossarizza, A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997, 411, 77–82. [Google Scholar] [CrossRef]
- Chazotte, B. Labeling mitochondria with JC-1. Cold Spring Harb. Protoc. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H. p53: Death star. Cell 2000, 103, 691–694. [Google Scholar] [CrossRef]
- Martin, D.A.; Elkon, K.B. Mechanisms of apoptosis. Rheum Dis Clin. North. Am. 2004, 30, 441–454. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis*. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Proteins | Company | Cat No. | Description | Western Blot |
|---|---|---|---|---|
| p53 | Santa Cruz | SC-126 | Mouse mAb | 1:1000 |
| BAX | Online | ABIN 135027 | Mouse mAb | 1:1000 |
| Bcl-2 | Bio Legend | 658702 | Mouse mAB | 1:500 |
| Cleaved caspase-3 | Cell signaling | D175 | Rabbit pAb | 1:2000 |
| Cyclin D1 | Abcam | Ab187364 | Mouse mAb | 1:2000 |
| Cyclin E1 | Abcam | Ab3927 | Mouse mAb | 1:2000 |
| GAPDH | Abcam | Ab8245 | Mouse mAb | 1:10000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-H.; Hwang, K.-A.; Choi, K.-C. Effects of Fludioxonil on the Cell Growth and Apoptosis in T and B Lymphocytes. Biomolecules 2019, 9, 500. https://doi.org/10.3390/biom9090500
Lee G-H, Hwang K-A, Choi K-C. Effects of Fludioxonil on the Cell Growth and Apoptosis in T and B Lymphocytes. Biomolecules. 2019; 9(9):500. https://doi.org/10.3390/biom9090500
Chicago/Turabian StyleLee, Gun-Hwi, Kyung-A Hwang, and Kyung-Chul Choi. 2019. "Effects of Fludioxonil on the Cell Growth and Apoptosis in T and B Lymphocytes" Biomolecules 9, no. 9: 500. https://doi.org/10.3390/biom9090500





