Curcumin Attenuates Lead-Induced Cerebellar Toxicity in Rats via Chelating Activity and Inhibition of Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
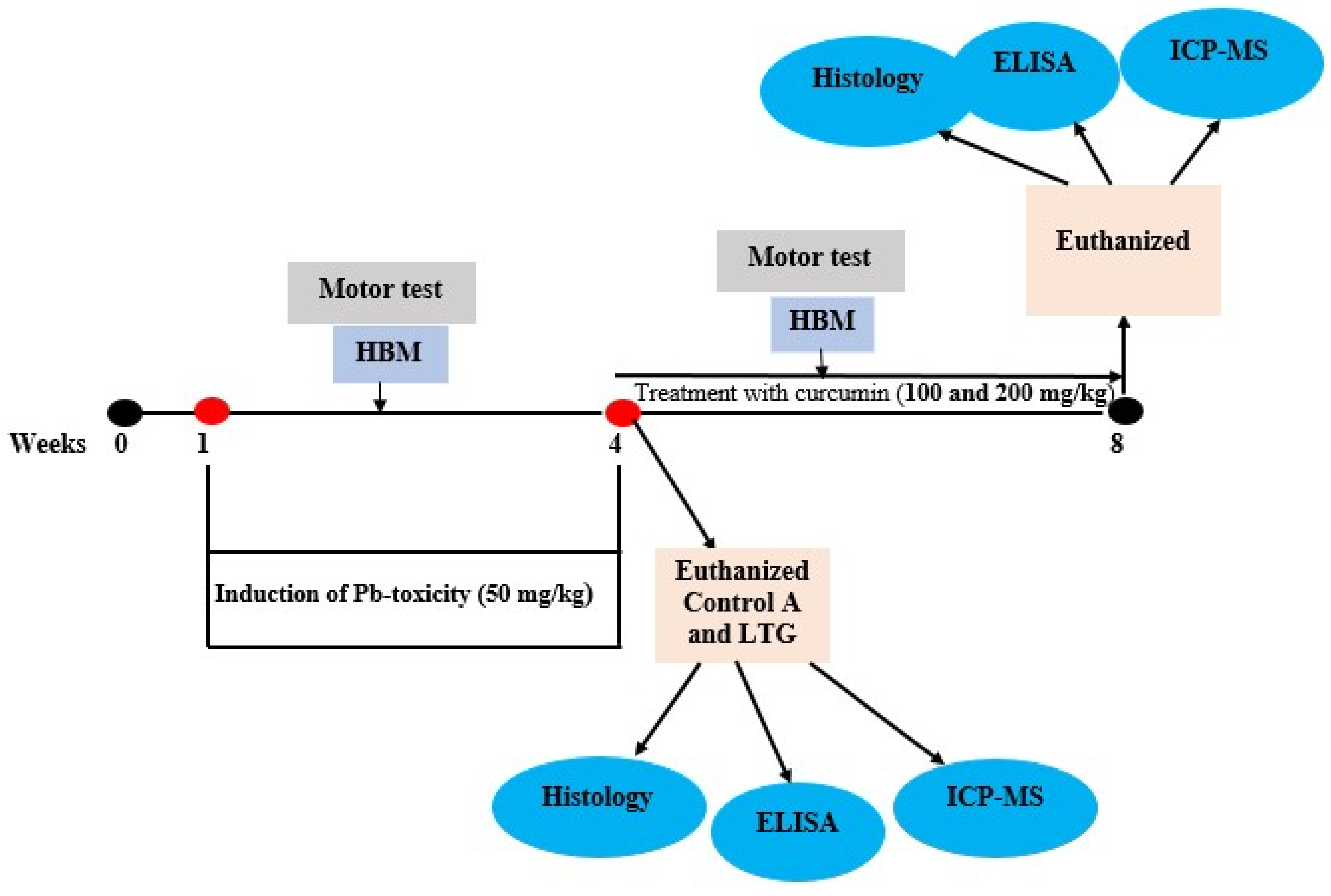
2.3. Experimental Design
2.4. Motor Activity
Horizontal Bar Method
2.5. Oxidative Stress Biomarker Analysis
2.5.1. Determination of Protein Concentration
2.5.2. Antioxidant Enzyme Activity Analysis
2.5.3. Malondialdehyde (MDA) Analysis
2.6. Inductive Coupled Plasma Mass Spectrometry (ICP-MS)
2.6.1. Sample Preparation
2.6.2. Sample analysis with ICP-MS
2.7. Histopathological Examination and Scoring
2.8. Statistical Analysis
3. Results
3.1. Induction of Pb Acetate Toxicity in Rats
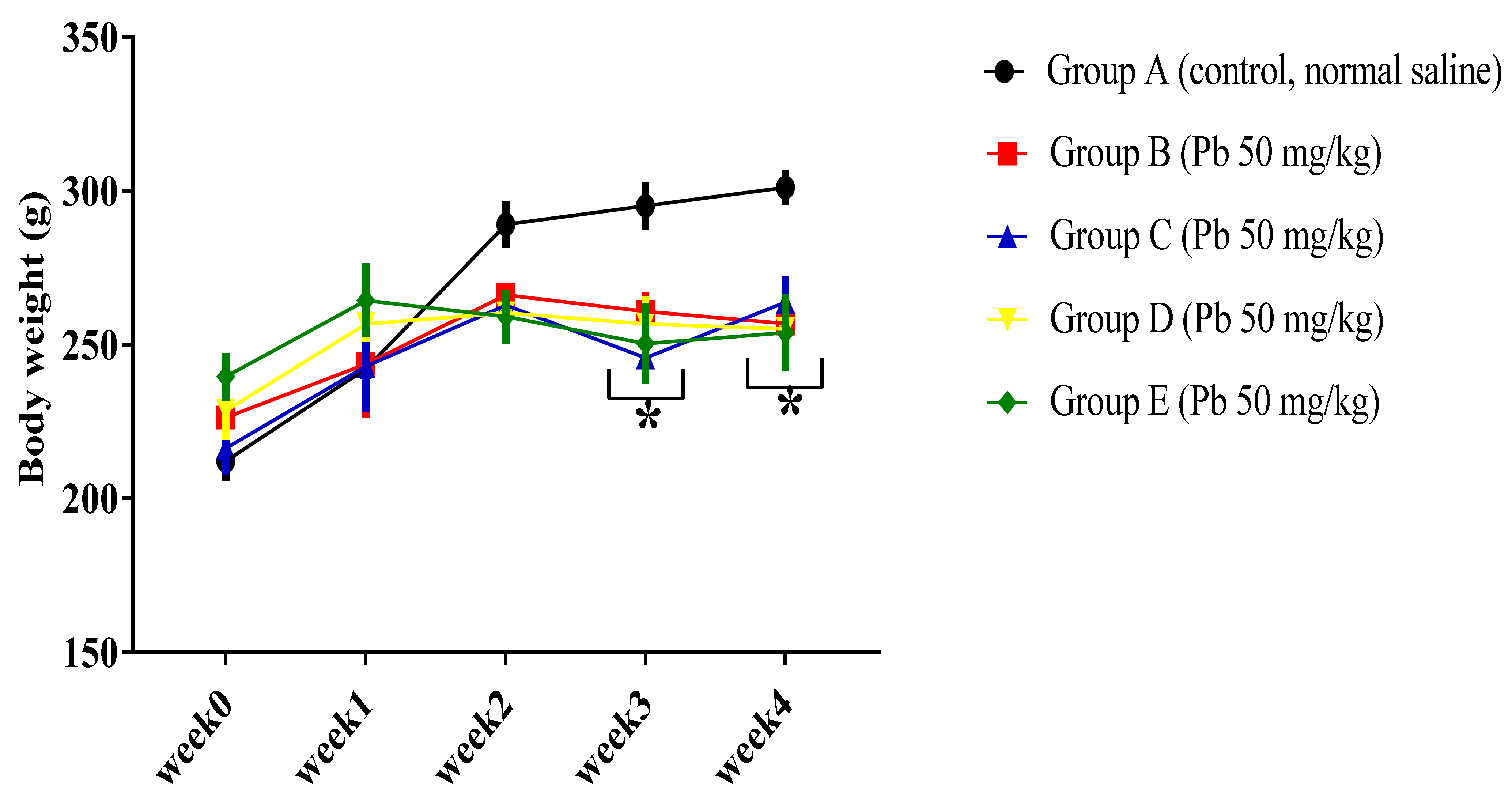
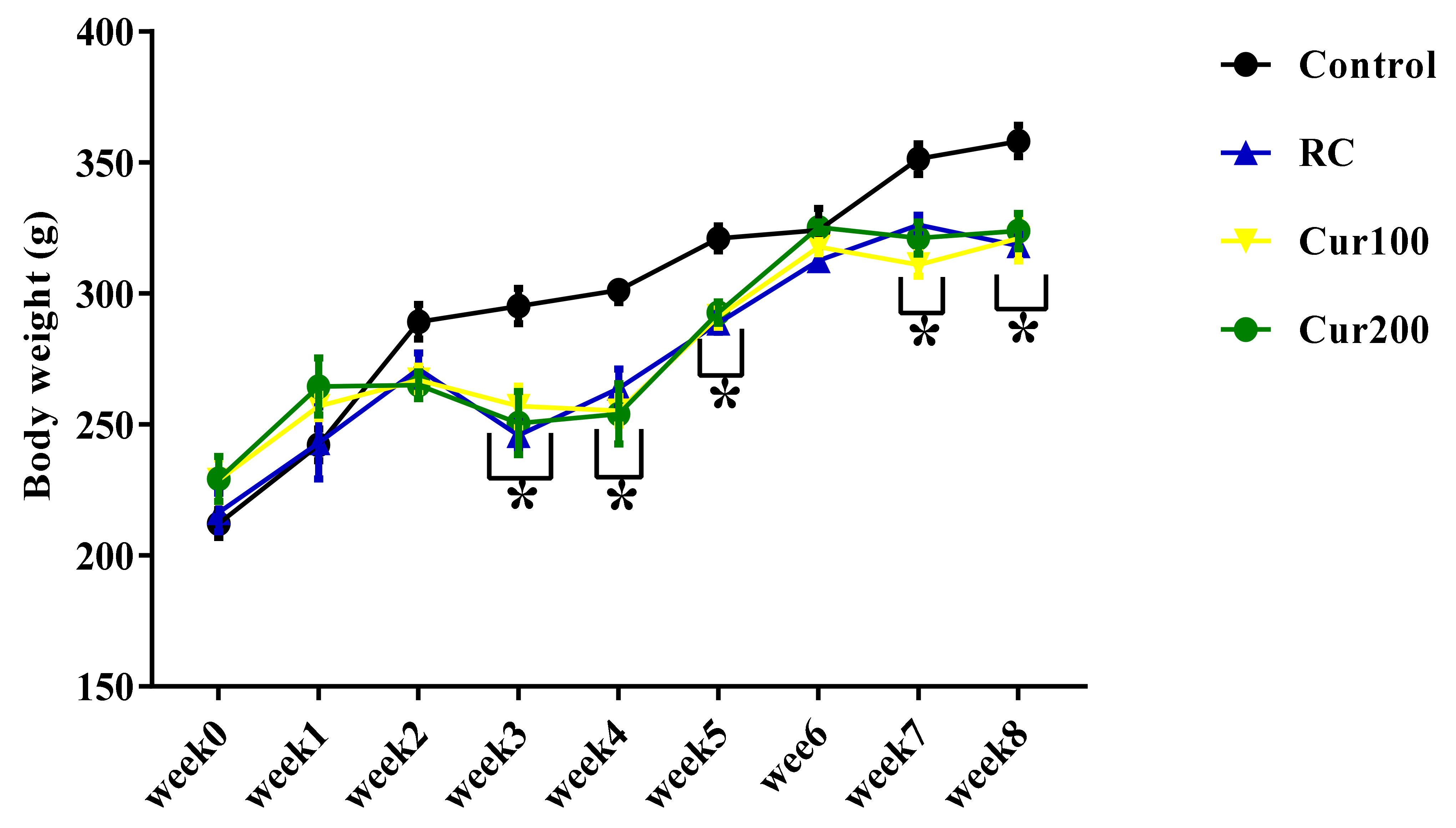
3.1.1. Effect of Pb Acetate on Body Weight of Rats during Pb Toxicity Induction
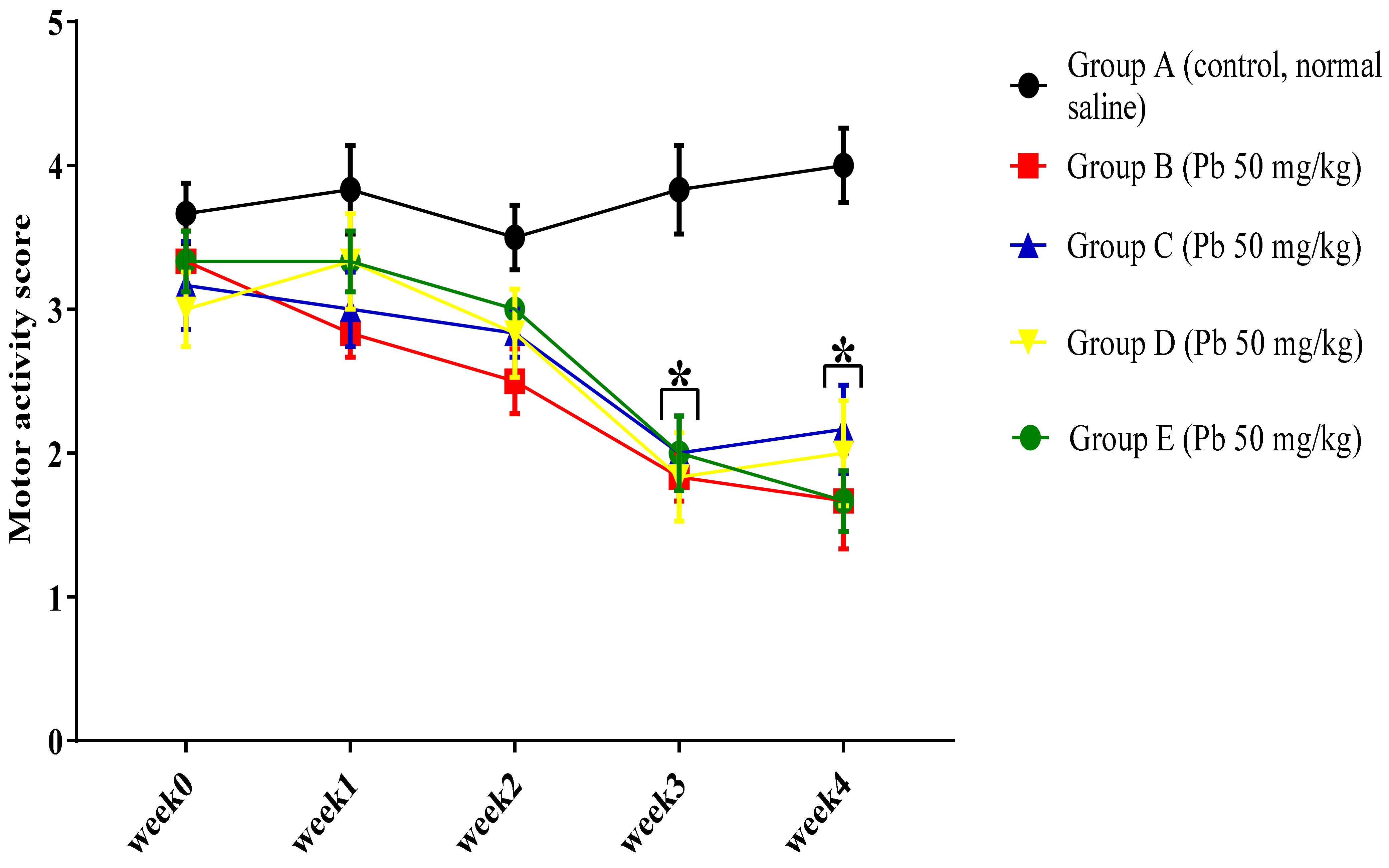
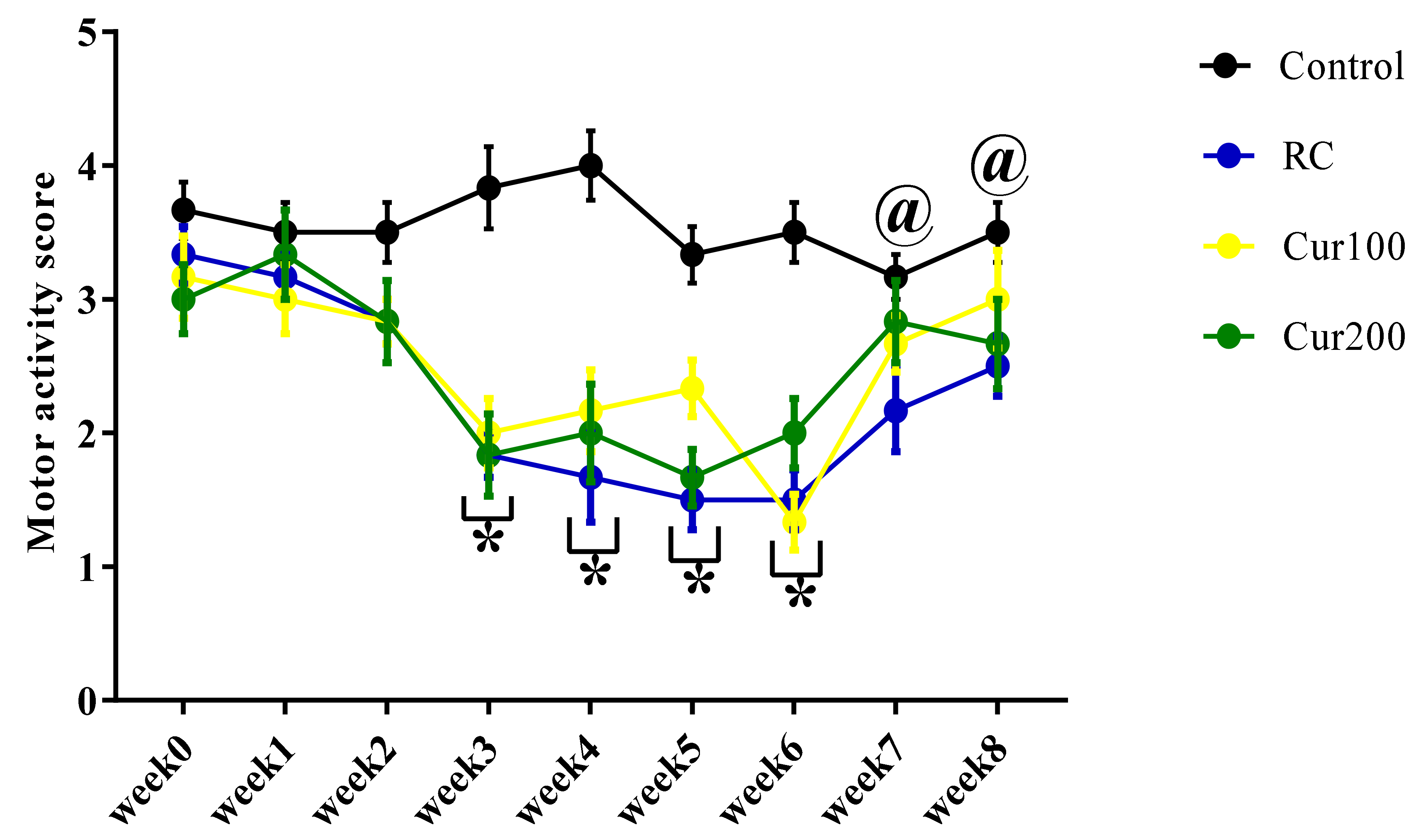
3.1.2. Effect of Pb Acetate on Motor Score and Coordination in Rats during Pb Toxicity Induction
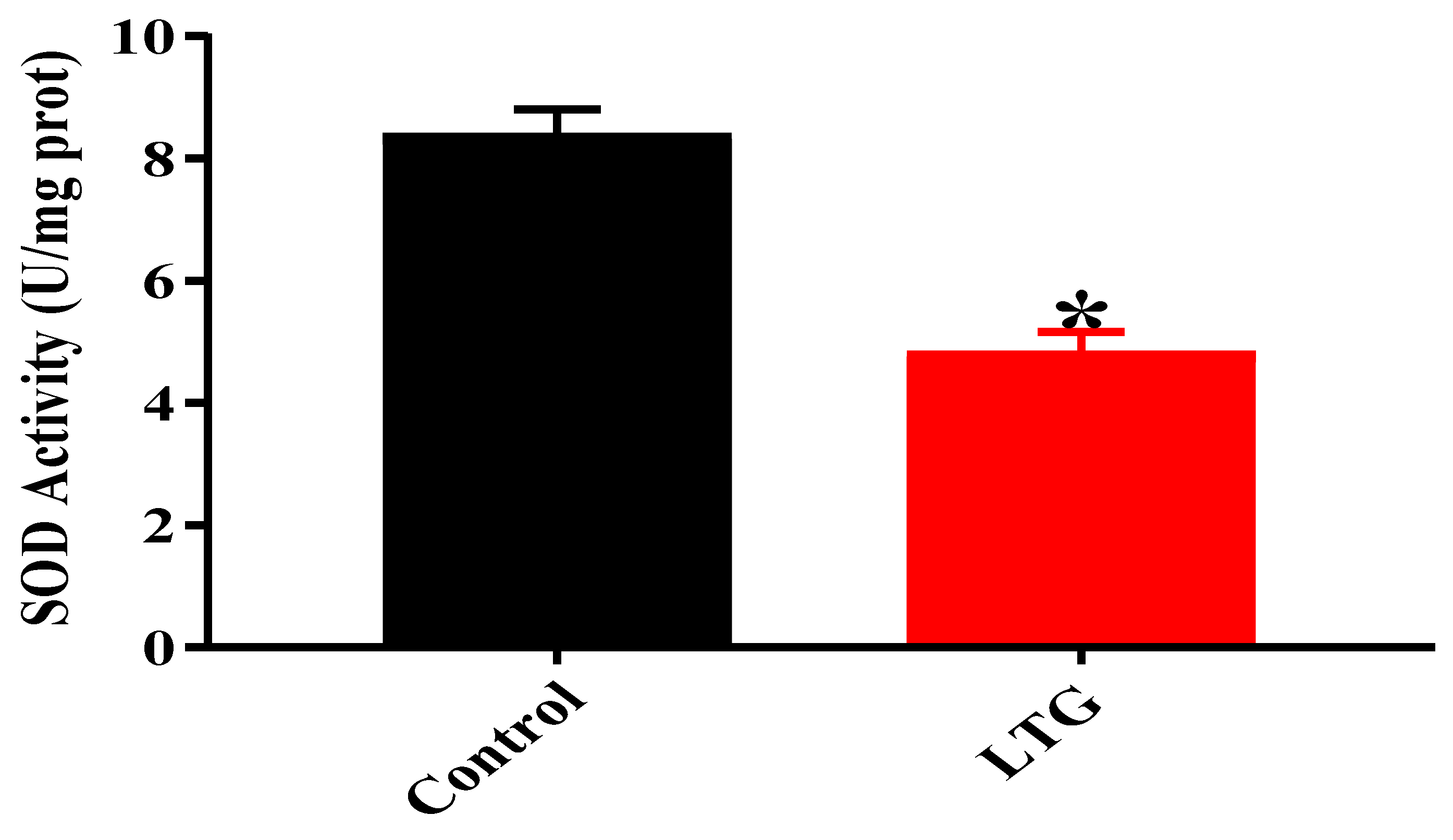
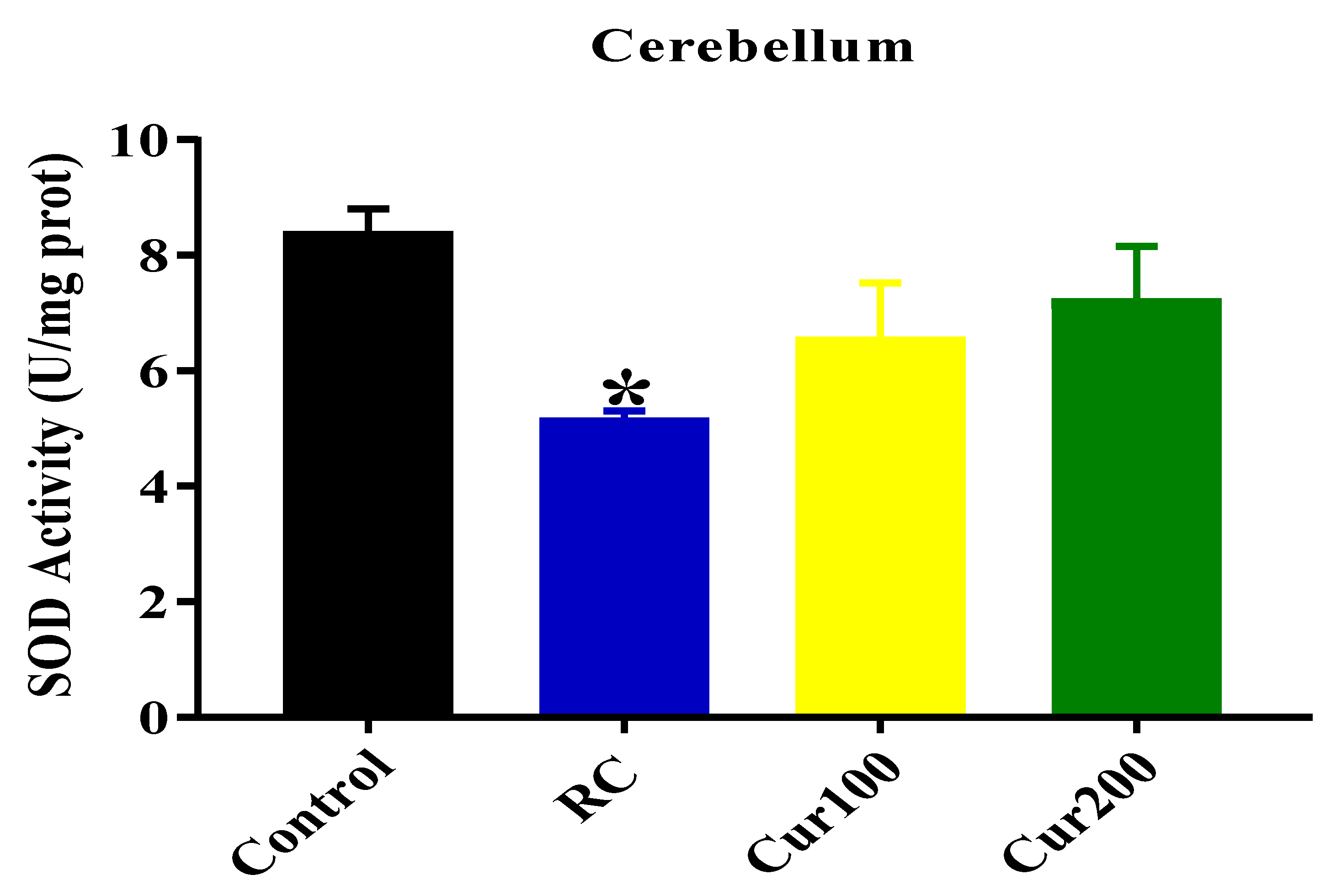
3.1.3. Effect of Pb Acetate on Oxidative Stress Status of Cerebellum in Rats of Control and LTGs during Pb Toxicity Induction
SOD Activity
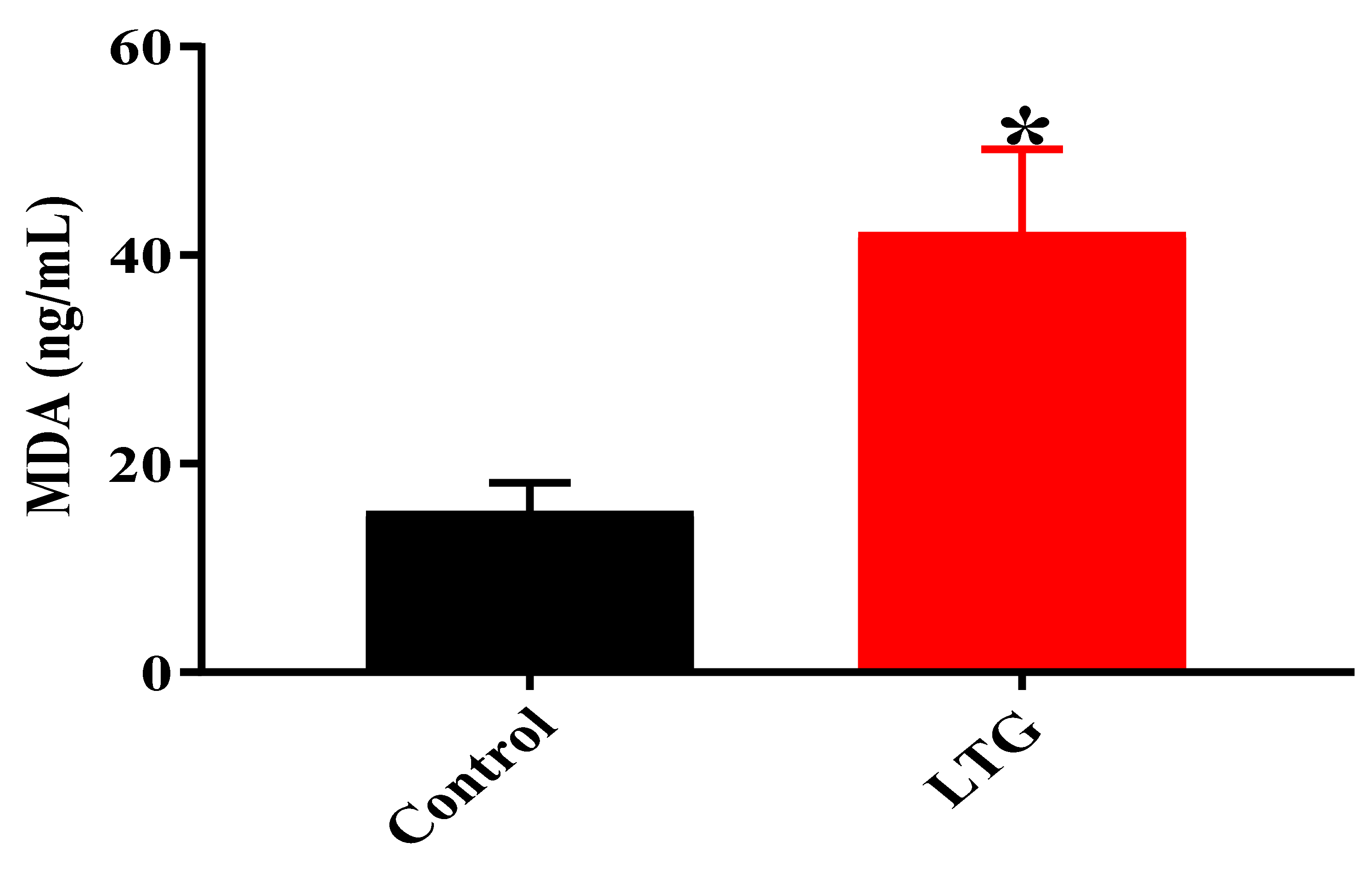
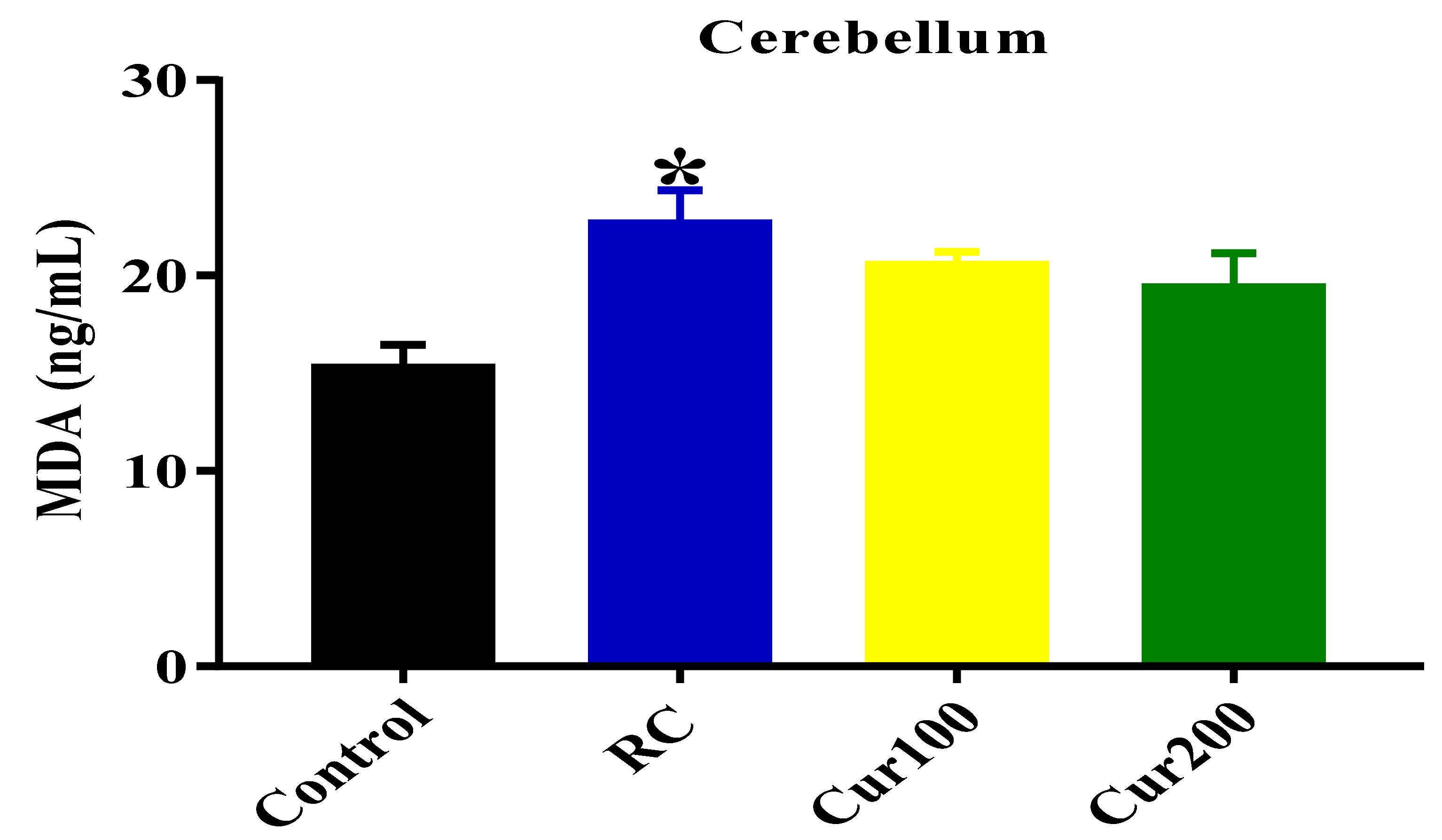
MDA Level
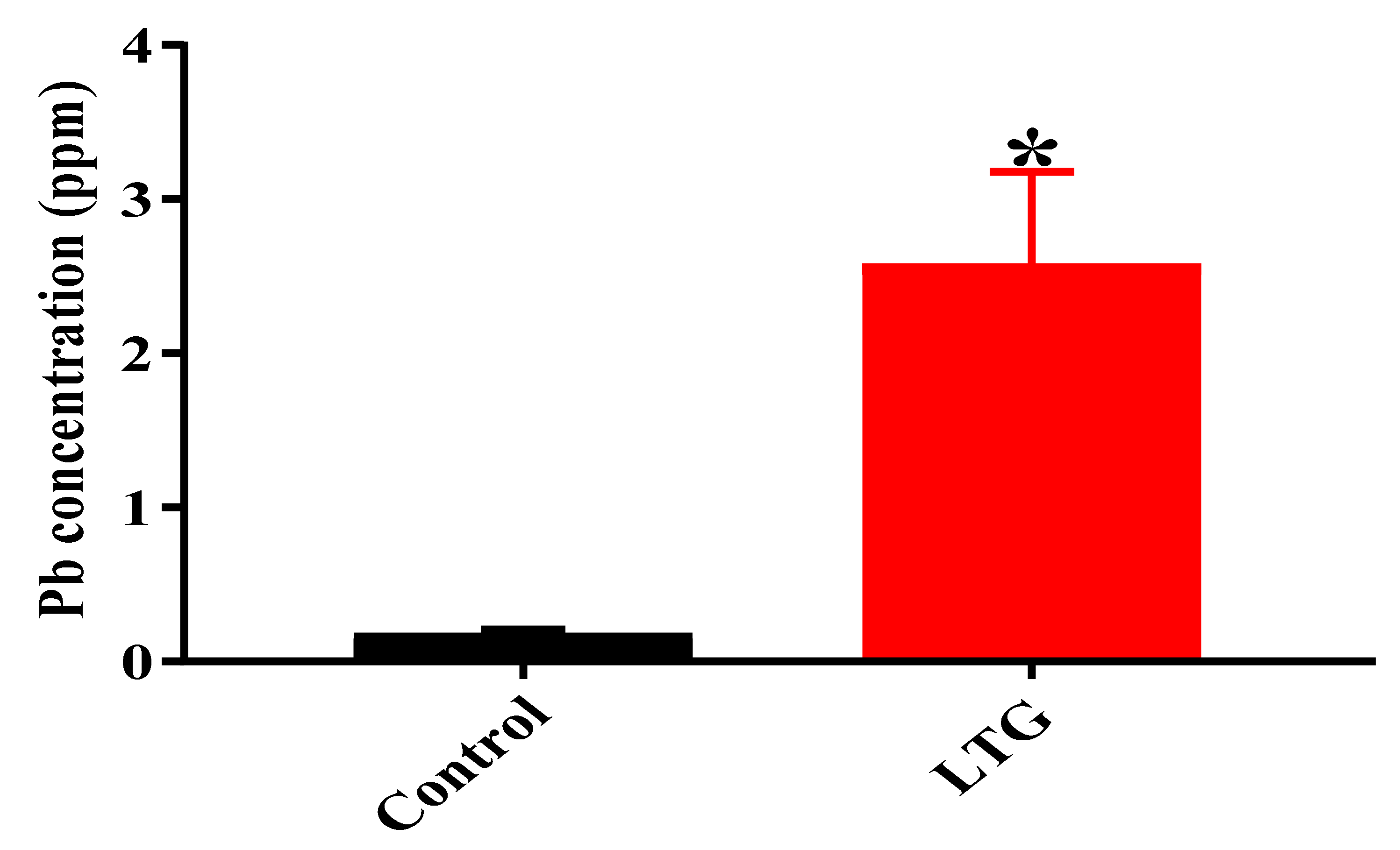
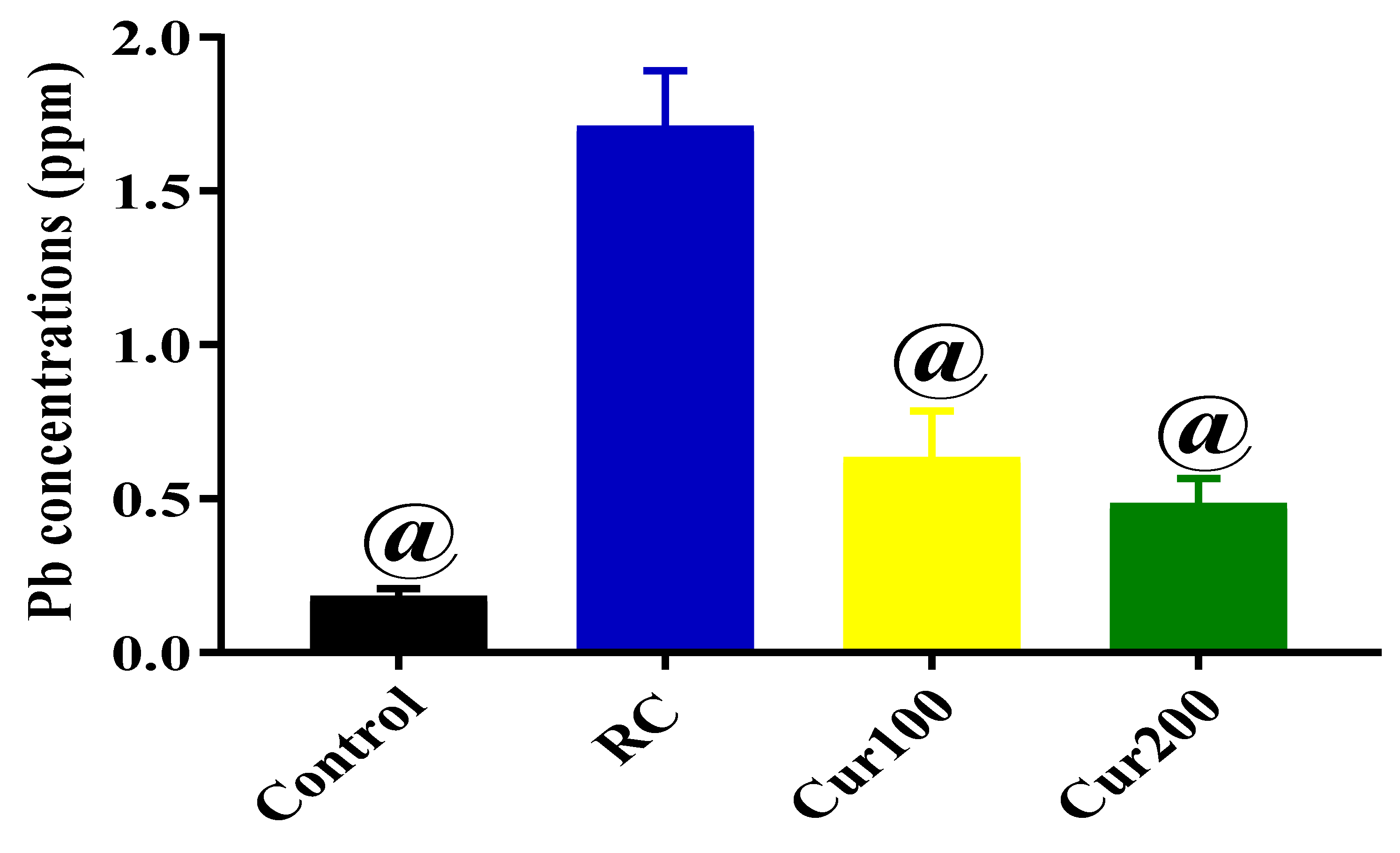
3.1.4. Determination of Pb Concentration in the Cerebellums of Rats in the Control Group and LTG after Pb-Toxicity Induction
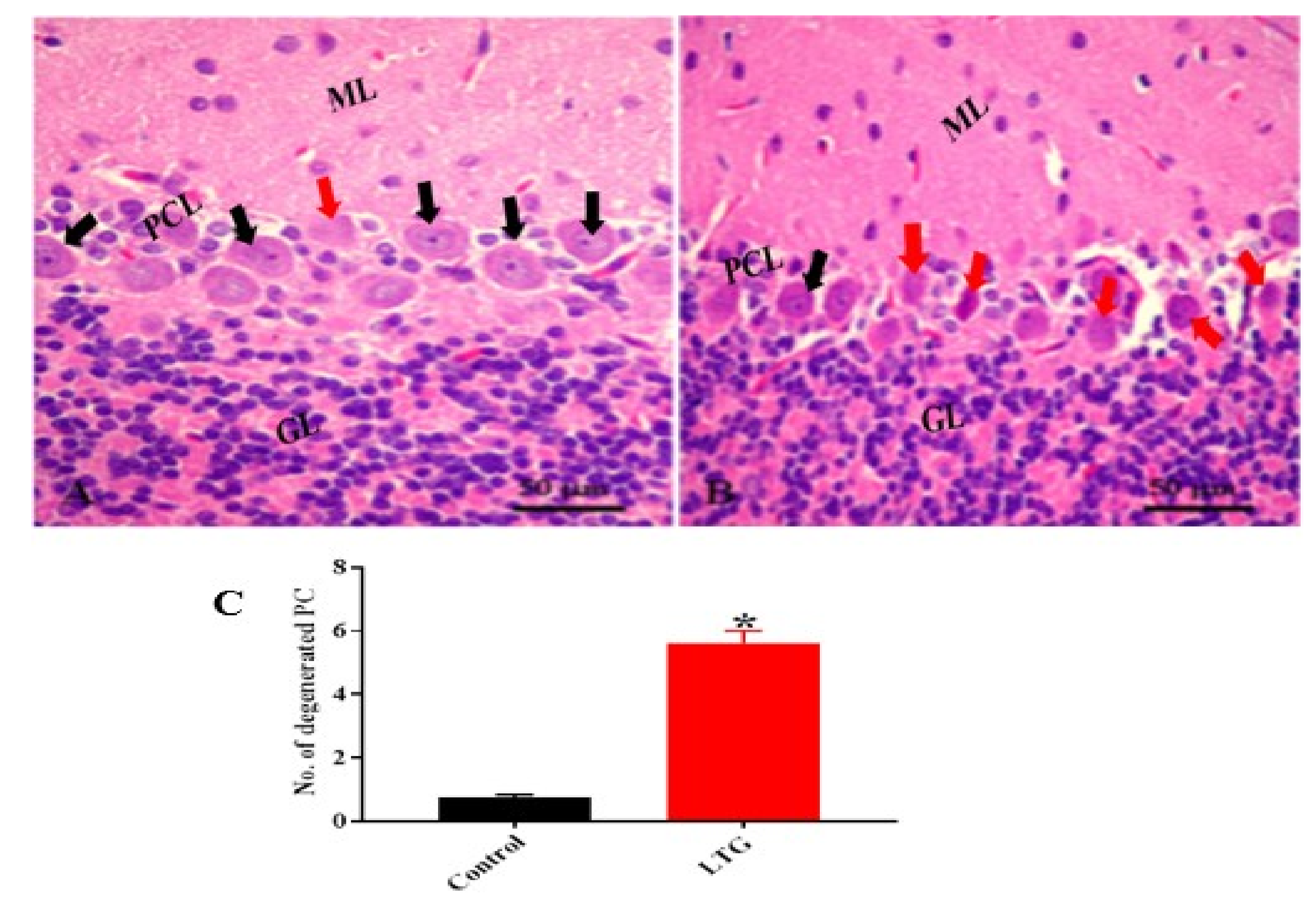
3.1.5. Effect of Pb Acetate on Histology of Cerebellum of Rats in the Control Group and LTG after the Induction of Pb Toxicity
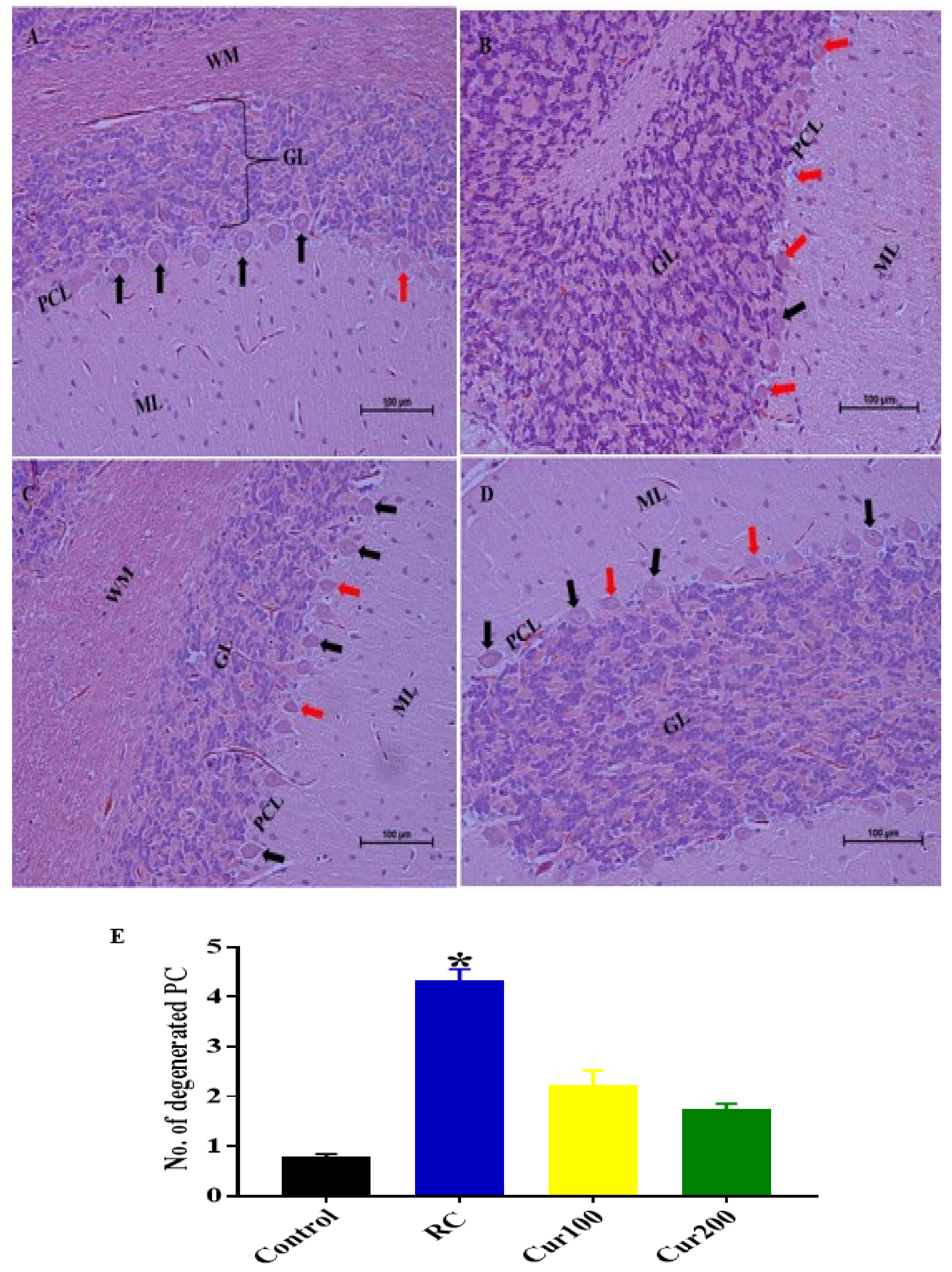
Cerebellum Stained with Hematoxylin and Eosin (H and E)
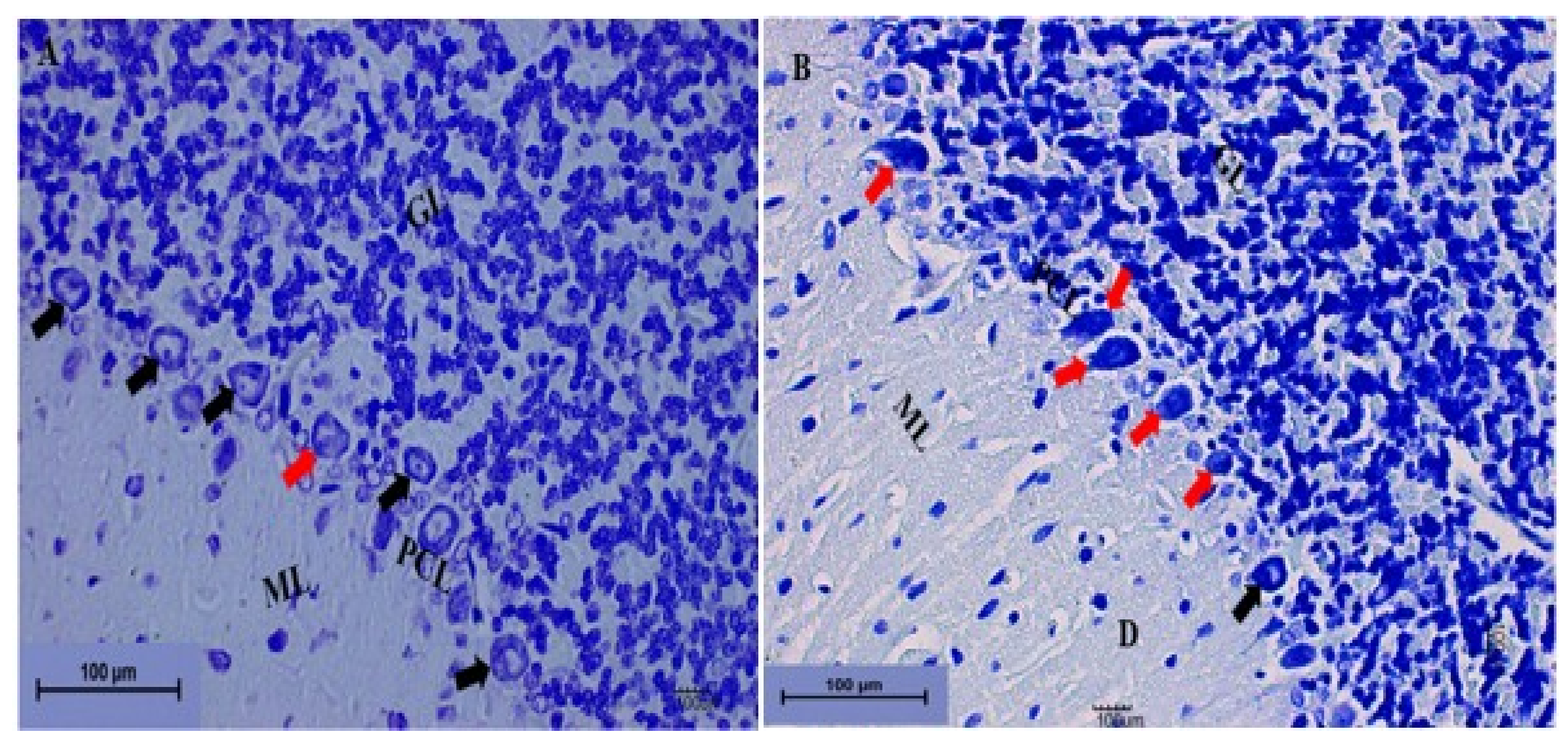
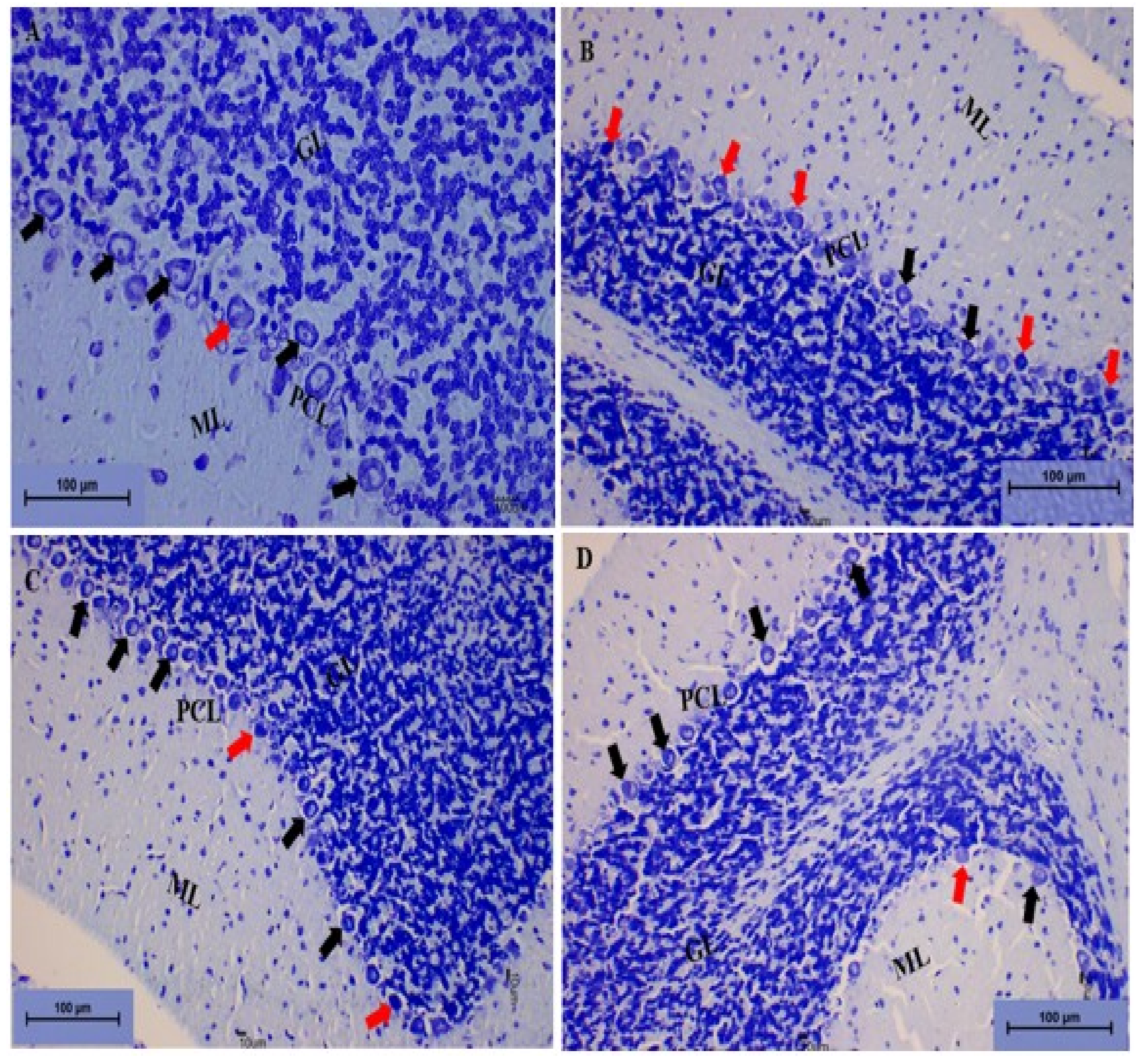
Cerebellum Stained with Toluidine Blue
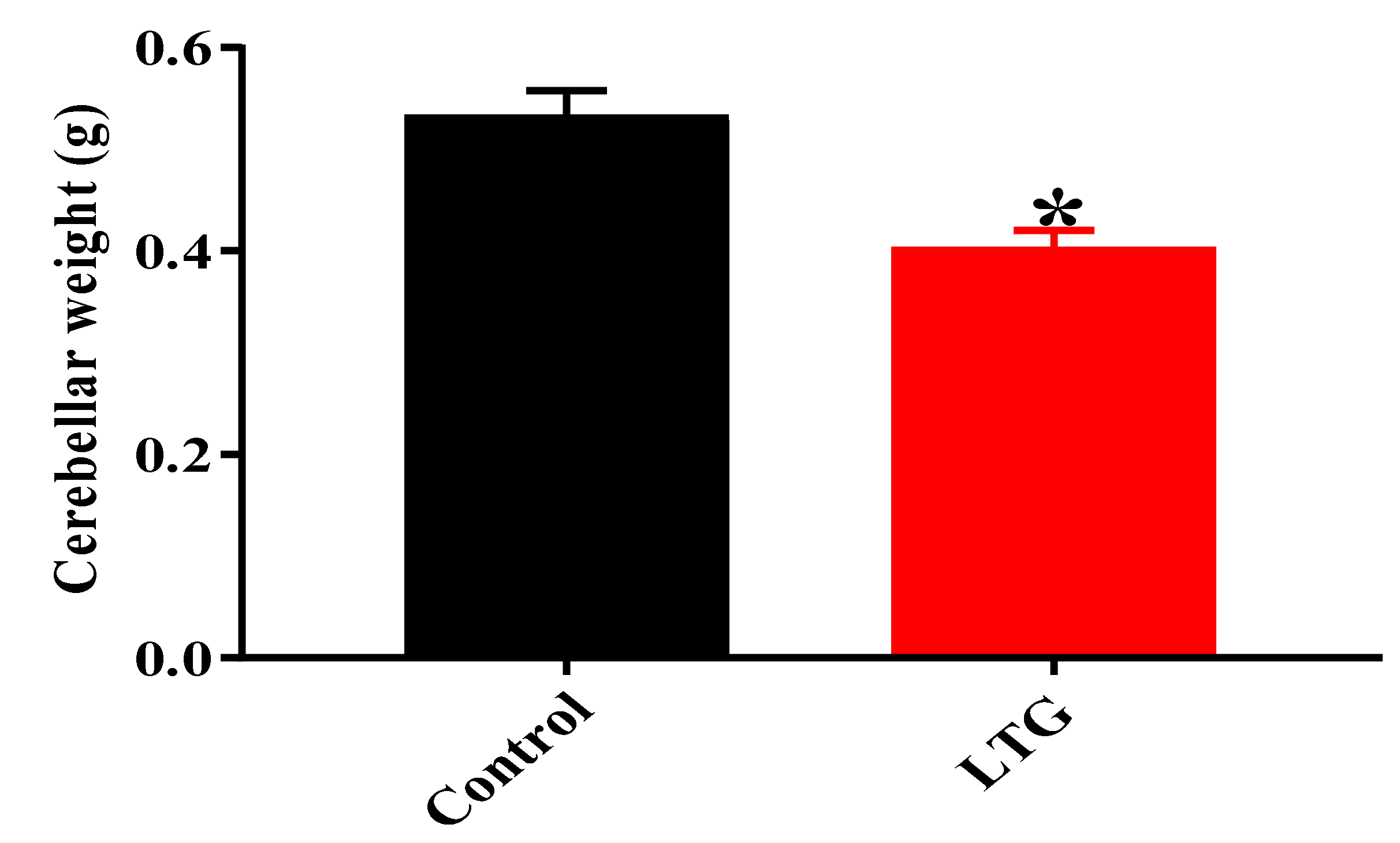
Effect of Pb Acetate on the Weight of the Cerebellums of Control Group and LTG Rats after Pb-Toxicity Induction
3.2. Treatments of Pb Acetate-Induced Rats with Curcumin
3.2.1. Effect of Curcumin on the Body Weight of Pb-Induced Rats
3.2.2. Curcumin Ameliorates Pb-Induced Alteration of Motor Coordination of Rats in the Horizontal Bar Test
3.2.3. Curcumin Reverse Pb-Induced Oxidative Stress in Rats’ Cerebellums
SOD Activity
MDA Level
3.2.4. Chelating Potentials of Curcumin on Pb-Induced Toxicity in Rats
3.2.5. Curcumin Attenuates Pb-Induced Cerebellar Damage in Rat’s Cerebellum
H and E Staining
Toluidine Blue Staining
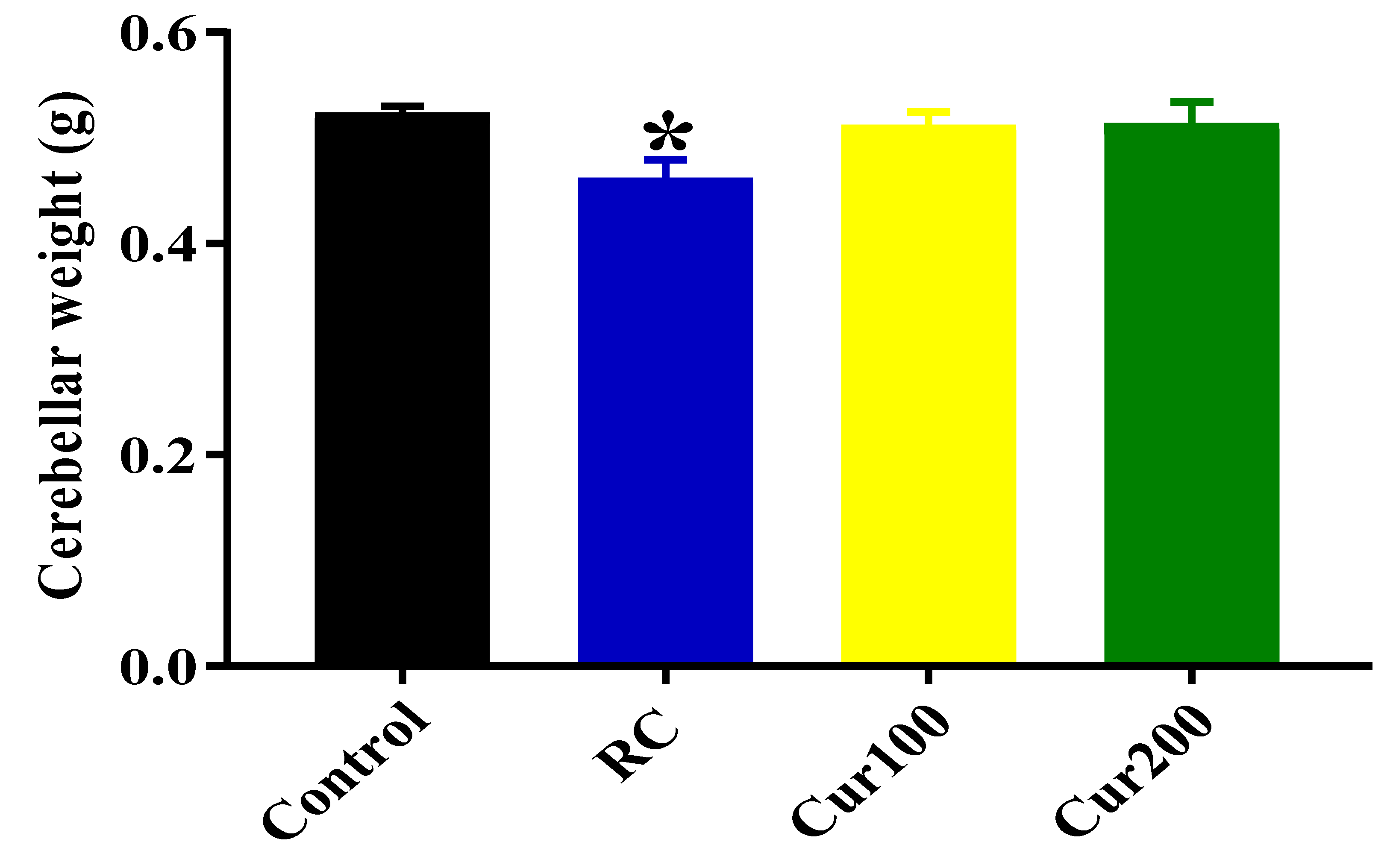
Effects of Curcumin Administration on Cerebellar Weight
4. Discussion
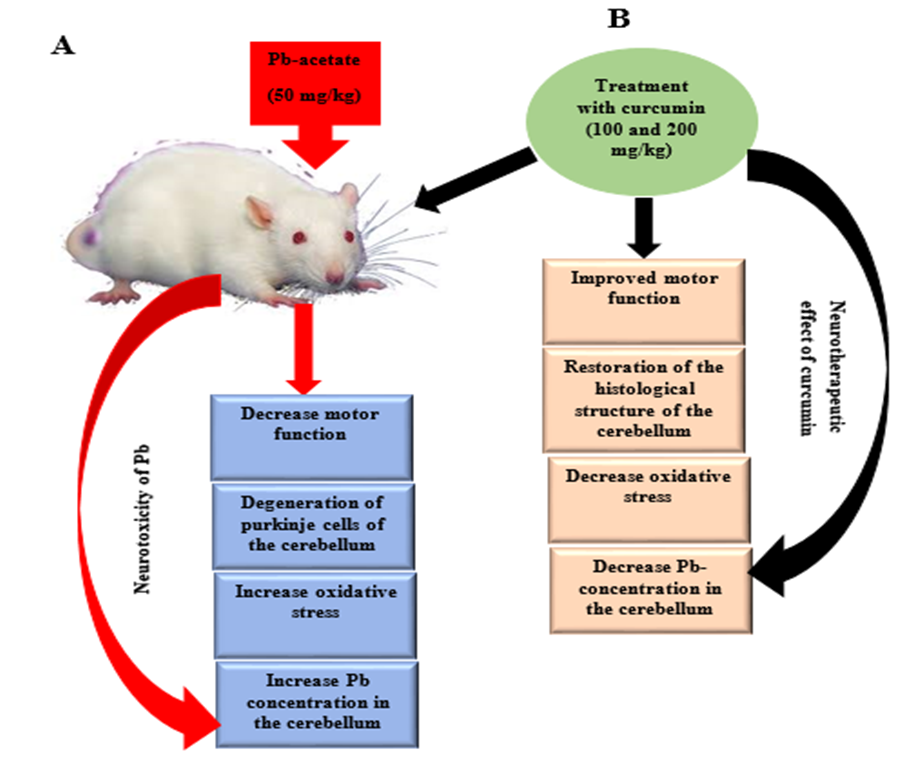
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- El-Tantawy, W.H. Antioxidant effects of Spirulina supplement against lead acetate-induced hepatic injury in rats. J. Tradit. Complement. Med. 2016, 6, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Zaher, A.O.; Abd-Ellatief, R.B.; Aboulhagag, N.A.; Farghaly, H.S.M.; Al-Wasei, F.M.M. The interrelationship between gasotransmitters and lead-induced renal toxicity in rats. Toxicol. Lett. 2019, 310, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.M.; Gilbert, S.G. Reducing occupational lead exposures: Strengthened standards for a healthy workforce. Neurotoxicology 2017, 69, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kosnett, M.J.; Wedeen, R.P.; Rothenberg, S.J.; Hipkins, K.L.; Materna, B.L.; Schwartz, B.S.; Hu, H.; Woolf, A. Recommendations for medical management of adult lead exposure. Environ. Health Perspect. 2007, 115, 463–471. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ebelt Sarnat, S.; Darrow, L.; McClellan, W.; Steenland, K. Mortality among participants in a lead surveillance program. Environ. Res. 2014, 132, 100–104. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Mason, L.H.; Harp, J.P.; Han, D.Y.; Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Chiodo, L.M.; Jacobson, S.W.; Jacobson, J.L. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol. Teratol. 2004, 26, 359–371. [Google Scholar] [CrossRef]
- Radad, K.; Hassanein, K.; Al-Shraim, M.; Moldzio, R.; Rausch, W.D. Thymoquinone ameliorates lead-induced brain damage in Sprague Dawley rats. Exp. Toxicol. Pathol. 2014, 66, 13–17. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Djordjevic, A.B.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Ishii, C.; Nakayama, S.M.M.; Kataba, A.; Ikenaka, Y.; Saito, K.; Watanabe, Y.; Makino, Y.; Matsukawa, T.; Kubota, A.; Yokoyama, K.; et al. Characterization and imaging of lead distribution in bones of lead-exposed birds by ICP-MS and LA-ICP-MS. Chemosphere 2018, 212, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.S.; Moreira, C.Z.; Cária, N.Z.; Victoriano, G.; Silva, W.F., Jr.; Magalhães, J.C. Phytotherapy: An introduction to its history, use and application. Rev. Bras. Plantas Med. 2014, 16, 290–298. [Google Scholar] [CrossRef]
- Farid, R.M. A focus on curcumin local application in oral diseases management: Mini review. IOSR J. Pharm. 2016, 6, 30–40. [Google Scholar]
- Mahmoudian-Sani, M.R.; Asadi-Samani, M.; Luther, T.; Saeedi-Boroujeni, A.; Gholamian, N. A new approach for treatment of type 1 diabetes: Phytotherapy and phytopharmacology of regulatory T cells. J. Ren. Inj. Prev. 2017, 6, 158–163. [Google Scholar] [CrossRef][Green Version]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Preventive efficacy of bulk and nanocurcumin against lead-induced oxidative stress in mice. Biol. Trace Elem. Res. 2013, 152, 31–40. [Google Scholar] [CrossRef]
- Kabeer, A.; Mailafiya, M.M.; Danmaigoro, A.; Rahim, E.A.; Bakar, Z.A. Therapeutic potential of curcumin against lead-induced toxicity: A review. Biomed. Res. Ther. 2019, 6, 3053–3066. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Gehr, T.W.B.; Ghosh, S. Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef]
- Hwang, E.S.; Lim, S.M.; Woo, E.J.; Kim, H.B.; Lee, S.; Choi, B.K.; Kwon, O.I.; Kim, H.J.; Kim, J.W.; Kyung, E.J. Evaluation of hepatoprotective effect of curcumin on liver cirrhosis using a combination of biochemical analysis and magnetic resonance-based electrical conductivity imaging. Mediat. Inflamm. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-Y.; Zhang, L.; Li, H.; Liu, T.-L.; Lai, J.-C.; Wu, Z.-B.; Qin, J. Protective effects of Curcumin on acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Pharmacol. Rep. 2018, 70, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, W.; Zhu, H.; Zhang, X.; Feng, Y.; Chen, Y.; Feng, H.; Lin, J. Curcumin attenuates blood-brain barrier disruption after subarachnoid hemorrhage in mice. J. Surg. Res. 2017, 207, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes—A DFT approach. J. Mol. Graph. Model. 2017, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jo, O. Healing and prophylactic effects of moringa oleifera leaf extract on lead induced damage to haematological and bone marrow elements in adult wistar rat models. J. Aquac. Res. Dev. 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Ekanem, A.U.; Kwari, H.D.; Garba, S.H.; Salami, H.A. Effect of lead acetate on spleen and blood parameters in albino rats. IOSR J. Dent. Med. Sci. Ver. I 2015, 14, 43–49. [Google Scholar]
- Zhang, Y.; Fang, M.; Sun, Y.; Zhang, T.; Shi, N.; Li, J.; Jin, L.; Liu, K.; Fu, J. Curcumin attenuates cerebral ischemia injury in Sprague-Dawley rats and PC12 cells by suppressing overactivated autophagy. J. Photochem. Photobiol. B Biol. 2018, 184, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Yang, M. Environmental exposure to lead (Pb) and variations in its susceptibility. J. Environ. Sci. Heal. Part. C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 159–185. [Google Scholar] [CrossRef]
- Brown, M.J.; Margolis, S. Lead in drinking water and human blood lead levels in the United States. MMWR Surveill. Summ. 2012, 61, 1–9. [Google Scholar]
- Mushak, P. Lead Exposure in Human Populations: Lead Toxicokinetics and Biomarkers of Lead Exposure; Elsevier: Amsterdam, The Netherlands, 2011; Volume 10, pp. 243–316. ISBN 9780444515544. [Google Scholar]
- Sarada, S.K.S.; Titto, M.; Himadri, P.; Saumya, S.; Vijayalakshmi, V. Curcumin prophylaxis mitigates the incidence of hypobaric hypoxia-induced altered ion channels expression and impaired tight junction proteins integrity in rat brain. J. Neuroinflamm. 2015, 12, 1–18. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Yu, H.; Wang, Q.; Chen, Y.; Xiang, L. Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury. Neurosci. Lett. 2013, 547, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.J. Measuring motor coordination in mice. J. Vis. Exp. 2013, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rattanachongkiat, S.; Millward, G.E.; Foulkes, M.E. Determination of arsenic species in fish, crustacean and sediment samples from Thailand using high performance liquid chromatography (HPLC) coupled with inductively coupled plasma mass spectrometry (ICP-MS). J. Environ. Monit. 2004, 6, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Simsek, N.; Akinci, L.; Alan, H.; Gecör, O.; Özan, Ü. Determination of trace elements in kidneys, livers and brains of rats with sealer implants by ICP-MS. Biotechnol. Biotechnol. Equip. 2017, 31, 397–402. [Google Scholar] [CrossRef]
- Costa, P.M. The handbook of histopathological practices in aquatic environment: Guide to histology for environmental toxiccology; Academic Press: London, UK, 2018; p. 292. ISBN 9780128120323. [Google Scholar]
- Mohammed Raouf, G.A.; Vaibhav, K.; Khan, A.; Tabassum, R.; Ahmed, M.E.; Javed, H.; Chander, K.; Islam, F.; Siddiqui, M.S. Terminalia arjuna bark extract inhibits histological alterations by mitigating oxidative stress in lead intoxicated mice. Orient. Pharm. Exp. Med. 2013, 13, 253–265. [Google Scholar] [CrossRef]
- Aldahmash, B.A.; El-Nagar, D.M. Antioxidant effects of captopril against lead acetate-induced hepatic and splenic tissue toxicity in Swiss albino mice. Saudi J. Biol. Sci. 2016, 23, 667–673. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- Brochin, R.; Leone, S.; Phillips, D.; Shepard, N.; Zisa, D.; Angerio, A. The cellular effect of lead poisoning and its clinical picture. Georg. Undergrad. J. Health Sci. 2008, 5, 1–8. [Google Scholar]
- Garza, A.; Vega, R.; Soto, E. Cellular mechanisms of lead neurotoxicity. Med. Sci Monit. 2006, 12, RA57–RA65. [Google Scholar]
- Offor, S.J.; Mbagwu, H.O.C.; Orisakwe, O.E. Lead induced hepato-renal damage in male albino rats and effects of activated charcoal. Front. Pharmacol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Chiroma, S.; Baharuldin, M.; Mat Taib, C.; Amom, Z.; Jagadeesan, S.; Ilham Adenan, M.; Mahdi, O.; Moklas, M. Protective effects of centella asiatica on cognitive deficits induced by D-gal/AlCl3 via inhibition of oxidative stress and attenuation of acetylcholinesterase level. Toxics 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Llaneza, D. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of OVX rats. J. Pharmacol. Exp. Ther. 2009, 93, 337–342. [Google Scholar]
- Frye, C.A.; Llaneza, D.C.; Walf, A.A. Progesterone can enhance consolidation and/or performance in spatial, object and working memory tasks in Long-Evans rats. Anim. Behav. 2009, 78, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Adolph, K.E.; Franchak, J.M. The development of motor behavior. Wiley Interdiscip. Rev. Cogn. Sci. 2017, 8, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Manto, M. Toxic Agents Causing Cerebellar Ataxias; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- El-Eraky El-Azab, N.; El-Mahalaway, A.M.; Sabry, D. Effect of methyl mercury on the cerebellar cortex of rats and the possible neuroprotective role of mesenchymal stem cells conditioned medium. histological and immunohistochemical study. J. Stem Cell. Res. Ther. 2018, 8, 2. [Google Scholar] [CrossRef]
- Nehru, B.; Sidhu, P. Neurotoxic effects of differential doses of lead on rat brain followed by recovery. J. Trace Elem. Exp. Med. 2002, 15, 131–140. [Google Scholar] [CrossRef]
- Barkur, R.R.; Bairy, L.K. Histological study on hippocampus, amygdala and cerebellum following low lead exposure during prenatal and postnatal brain development in rats. Toxicol. Ind. Health 2014, 32, 1052–1063. [Google Scholar] [CrossRef]
- Sabbar, M.; Delaville, C.; De Deurwaerdère, P.; Lakhdar-Ghazal, N.; Benazzouz, A. Lead-induced atypical Parkinsonism in rats: Behavioral, electrophysiological, and neurochemical evidence for a role of noradrenaline depletion. Front. Neurosci. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- Moore, T.L.; Bowley, B.G.E.; Shultz, P.L.; Calderazzo, S.M.; Shobin, E.J.; Uprety, A.R.; Rosene, D.L.; Moss, M.B. Oral curcumin supplementation improves fine motor function in the middle-aged rhesus monkey. Somatosens. Mot. Res. 2018, 35, 1–10. [Google Scholar] [CrossRef]
- Chongtham, A.; Agrawal, N. Curcumin modulates cell death and is protective in Huntington’s disease model. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Santos-Parker, J.R.; Lubieniecki, K.L.; Rossman, M.J.; Van Ark, H.J.; Bassett, C.J.; Strahler, T.R.; Chonchol, M.B.; Justice, J.N.; Seals, D.R. Curcumin supplementation and motor-cognitive function in healthy middle-aged and older adults. Nutr. Heal. Aging 2018, 4, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, M. Oxidative stress and neurodegeneration: The yeast model system. Front. Biosci. 2013, 18, 1174. [Google Scholar] [CrossRef]
- Patrick, L. Lead Toxicity, a review of the literature part l’ exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar] [PubMed]
- Swomley, A.M.; Butterfield, D.A. Oxidative stress in Alzheimer disease and mild cognitive impairment: Evidence from human data provided by redox proteomics. Arch. Toxicol. 2015, 89, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T.; et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Alisi, I.O.; Uzairu, A.; Abechi, S.E.; Idris, S.O. Evaluation of the antioxidant properties of curcumin derivatives by genetic function algorithm. J. Adv. Res. 2018, 12, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Ammerman, J.; Huang, C.; Sailstad, J.; Wieling, J.; Whitmire, M.L.; Wright, D.; De Lisio, P.; Keenan, F.; McCurdy, E.; Woods, B.; et al. Technical aspects of inductively coupled plasma bioanalysis techniques. Bioanalysis 2013, 5, 1831–1841. [Google Scholar] [CrossRef]
- Renner, R. Exposure on tap: Drinking water as an overlooked source of lead. Environ. Health Perspect. 2010, 118, A68–A74. [Google Scholar] [CrossRef]
- De Sousa, R.A.; Sabarense, C.M.; Prado, G.L.P.; Metze, K.; Cadore, S. Lead biomonitoring in different organs of lead intoxicated rats employing GF AAS and different sample preparations. Talanta 2013, 104, 90–96. [Google Scholar] [CrossRef]
- Daniel, S.; Limson, J.L.; Dairam, A.; Watkins, G.M.; Daya, S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J. Inorg. Biochem. 2004, 98, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, H.Y.; Ji, H.F. A theoretical study on Cu (II)-chelating properties of curcumin and its implications for curcumin as a multipotent agent to combat Alzheimer’s disease. J. Mol. Struct. THEOCHEM 2005, 757, 199–202. [Google Scholar] [CrossRef]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimer Dis. 2004, 6, 367–377. [Google Scholar] [CrossRef]
- Borsari, M.; Ferrari, E.; Grandi, R.; Saladini, M. Curcuminoids as potential new iron-chelating agents: Spectroscopic, polarographic and potentiometric study on their Fe (III) complexing ability. Inorg. Chim. Acta 2002, 328, 61–68. [Google Scholar] [CrossRef]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Matsumoto, K.; Watanabe, H. Evaluation of the nitric oxide radical scavenging activity of manganese complexes of curcumin and its derivative. Biol. Pharm. Bull. 2004, 27, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Surhio, M.M.; Ye, H.; Gao, X.; Ye, Z.; Li, J.; Ye, M. Protective effects of a Lachnum polysaccharide against liver and kidney injury induced by lead exposure in mice. Int. J. Biol. Macromol. 2019, 124, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Lomash, V.; Samim, M.; Flora, S.J.S. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chem. Biol. Interact. 2012, 199, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Goel, S.K.; Behari, J.R. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. J. Appl. Toxicol. 2010, 30, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.; Nehru, B. Lead intoxication: Histological and oxidative damage in rat cerebrum and cerebellum. J. Trace Elem. Exp. Med. 2004, 17, 45–53. [Google Scholar] [CrossRef]
- Nam, S.M.; Seo, J.S.; Nahm, S.S.; Chang, B.J. Effects of ascorbic acid on osteopontin expression and axonal myelination in the developing cerebellum of lead-exposed rat pups. Int. J. Environ. Res. Public Health 2019, 16, 983. [Google Scholar] [CrossRef] [PubMed]
- NourEddine, D.; Miloud, S.; Abdelkader, A. Effect of lead exposure on dopaminergic transmission in the rat brain. Toxicology 2005, 207, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Adekomi, D.A.; Adewole, O.S.; Tijani, A.A.; Adeniyi, T.D. Lead induces inflammation and neurodegenerative changes in the rat medial prefrontal cortex. Anatomy 2018, 11, 79–86. [Google Scholar] [CrossRef]
- Goulart, E.C.; Pereira, C.A.T.; Garcia, R.C.; Giacomelli, M.B.O.; Rodrigues, A.L.S. Effects of lead and/or zinc exposure during the second stage of rapid postnatal brain growth on delta-aminolevulinate dehydratase and negative geotaxis of suckling rats. Braz. J. Med. Biol. Res. 2001, 34, 785–790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shukla, P.K.; Khanna, V.K.; Khan, M.Y.; Srimal, R.C. Protective effect of curcumin against lead neurotoxicity in rat. Hum. Exp. Toxicol. 2003, 22, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Tüzmen, M.N.; Yücel, N.C.; Kalburcu, T.; Demiryas, N. Effects of curcumin and tannic acid on the aluminum-and lead-induced oxidative neurotoxicity and alterations in NMDA receptors. Toxicol. Mech. Methods 2015, 25, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dairam, A.; Limson, J.L.; Watkins, G.M.; Antunes, E.; Daya, S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male wistar rats. J. Agric. Food Chem. 2007, 55, 1039–1044. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.H.; Xu, Y.; Li, Y.B.; Wu, H.L.; Guo, H.; Zhang, J.Z.; Zhang, J.J.; Pan, X.Y.; Li, X.J. Curcumin produces neuroprotective effects via activating brain-derived neurotrophic factor/TrkB-dependent MAPK and PI-3K cascades in rodent cortical neurons. Prog. Neuro Psychopharm. Biol. Psychiatr. 2010, 34, 147–153. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-nezhad, M.; Farkhondeh, T. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Barbosa, F.; Tanus-Santos, J.E.; Gerlach, R.F.; Parsons, P.J. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations, and future needs. Environ. Health Perspect. 2005, 113, 1669–1674. [Google Scholar] [CrossRef]
- Omobowale, T.O.; Oyagbemi, A.A.; Akinrinde, A.S.; Saba, A.B.; Daramola, O.T.; Ogunpolu, B.S.; Olopade, J.O. Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in Wistar rats. Environ. Toxicol. Pharmacol. 2014, 37, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.A.; Moselhy, W.A.; Abdel-Hamed, M.I. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology 2012, 33, 280–289. [Google Scholar] [CrossRef] [PubMed]
| Serial No. | Duration of Rat on Horizontal Bar | Score |
|---|---|---|
| 1. | Falling time between 1 and 5 s | 1 |
| 2. | Falling time between 6 and 10 s | 2 |
| 3. | Falling time between 11 and 20 s | 3 |
| 4. | Falling time between 21 and 30 s | 4 |
| 5. | Falling time after 30 s | 5 |
| 6. | The maximum score (5) was allotted if the rat placed one forepaw on the bar support without falling. | 5 |
| Parameters | Values | Units |
|---|---|---|
| Radio Frequency Power | 1550 | W |
| Radio Frequency Matching | 1.6 | V |
| Sample depth | 9.5 | mm |
| Torch | −0.1 | mm |
| Torch-V | 0 | mm |
| Argon Gas Flow Rate | 15 | L/min |
| Carrier Gas Flow | 1.01 | L/min |
| Make up Gas Flow | 0.15 | L/min |
| Auxiliary Gas Flow Rate | 0.58 | L/min |
| Sample Uptake Rate | 0.3 | revolutions per seconds (rps) |
| Sample Uptake Rate | 100 | µL/min |
| Sampling Depth | 6–7.6 | mm |
| Spray chamber temperature | 2 | °C |
| Nebulizer Pump | 0.1 | revolution per seconds (rps) |
| Integration Time | 3 | s |
| Internal Standard (103Rh,208Bi) | 200 | part per billion (ppb) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abubakar, K.; Muhammad Mailafiya, M.; Danmaigoro, A.; Musa Chiroma, S.; Abdul Rahim, E.B.; Abu Bakar @ Zakaria, M.Z. Curcumin Attenuates Lead-Induced Cerebellar Toxicity in Rats via Chelating Activity and Inhibition of Oxidative Stress. Biomolecules 2019, 9, 453. https://doi.org/10.3390/biom9090453
Abubakar K, Muhammad Mailafiya M, Danmaigoro A, Musa Chiroma S, Abdul Rahim EB, Abu Bakar @ Zakaria MZ. Curcumin Attenuates Lead-Induced Cerebellar Toxicity in Rats via Chelating Activity and Inhibition of Oxidative Stress. Biomolecules. 2019; 9(9):453. https://doi.org/10.3390/biom9090453
Chicago/Turabian StyleAbubakar, Kabeer, Maryam Muhammad Mailafiya, Abubakar Danmaigoro, Samaila Musa Chiroma, Ezamin Bin Abdul Rahim, and Md Zuki Abu Bakar @ Zakaria. 2019. "Curcumin Attenuates Lead-Induced Cerebellar Toxicity in Rats via Chelating Activity and Inhibition of Oxidative Stress" Biomolecules 9, no. 9: 453. https://doi.org/10.3390/biom9090453
APA StyleAbubakar, K., Muhammad Mailafiya, M., Danmaigoro, A., Musa Chiroma, S., Abdul Rahim, E. B., & Abu Bakar @ Zakaria, M. Z. (2019). Curcumin Attenuates Lead-Induced Cerebellar Toxicity in Rats via Chelating Activity and Inhibition of Oxidative Stress. Biomolecules, 9(9), 453. https://doi.org/10.3390/biom9090453






