D-Aspartic Acid in Vertebrate Reproduction: Animal Models and Experimental Designs ‡
Abstract
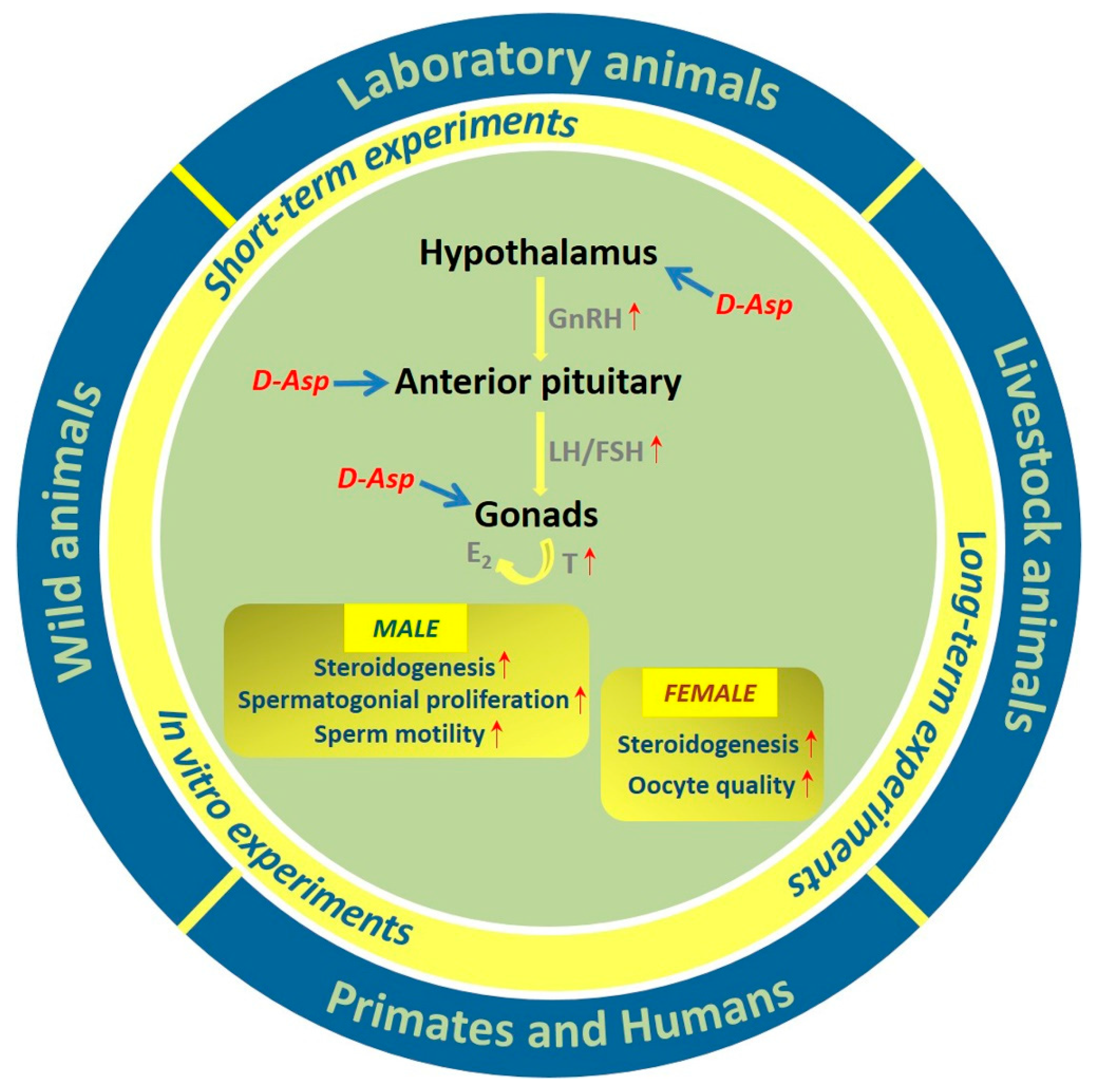
1. Introduction
2. Wild Animals
2.1. In Vivo Experiments
2.2. In Vitro Experiments
3. Laboratory Animals
3.1. In Vivo Experiments
3.2. In Vitro Experiments
4. Livestock Animals
4.1. In Vivo Experiments
4.2. In Vitro Experiments
5. Primates
6. Humans
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- D’Aniello, A.D. Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, M.M.; Santillo, A.; Chieffi Baccari, G. Current knowledge of D-aspartate in glandular tissues. Amino Acids 2014, 46, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Furuchi, T.; Homma, H. Free D-aspartate in mammals. Biol. Pharm. Bull. 2005, 28, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Homma, H. Biochemistry of D-aspartate in mammalian cells. Amino Acids 2007, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Shi, T.; Sweedler, J.V. D-Aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids 2012, 43, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Brückner, H.; Westhauser, T. Chromatographic determination of L-and D-amino acids in plants. Amino Acids 2003, 24, 43–55. [Google Scholar] [PubMed]
- Wolosker, H.; D’Aniello, A.; Snyder, S.H. D-aspartate disposition in neuronal and endocrine tissues: Ontogeny, biosynthesis and release. Neuroscience 2000, 100, 183–189. [Google Scholar] [CrossRef]
- Katane, M.; Homma, H. D-aspartate oxidase: The sole catabolic enzyme acting on free D-aspartate in mammals. Chem. Biodivers. 2010, 7, 1435–1449. [Google Scholar] [CrossRef]
- Huang, A.S.; Beigneux, A.; Weil, Z.M.; Kim, P.M.; Molliver, M.E.; Blackshaw, S.; Nelson, R.J.; Young, S.G.; Snyder, S.H. D-aspartate regulates melanocortin formation and function: Behavioral alterations in D-aspartate oxidase-deficient mice. J. Neurosci. 2006, 26, 2814–2819. [Google Scholar] [CrossRef]
- Archibald, K.; Perry, M.J.; Molnár, E.; Henley, J.M. Surface expression and metabolic half-life of AMPA receptors in cultured rat cerebellar granule cells. Neuropharmacology 1998, 37, 1345–1353. [Google Scholar] [CrossRef]
- Gill, S.S.; Mueller, R.W.; McGuire, P.F.; Pulido, O.M. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol. Pathol. 2000, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Pulido, O.M. Glutamate receptors in peripheral tissues: Current knowledge, future research and implications for toxicology. Toxicol. Pathol. 2001, 29, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Falvo, S.; Chieffi, P.; Burrone, L.; Chieffi Baccari, G.; Longobardi, S.; Di Fiore, M.M. D-aspartate affects NMDA receptor-extracellular signal—Regulated kinase pathway and upregulates androgen receptor expression in the rat testis. Theriogenology 2014, 81, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Falvo, S.; Di Fiore, M.M.; Di Giacomo Russo, F.; Chieffi, P.; Usiello, A.; Pinelli, C.; Chieffi Baccari, G. AMPA receptor expression in mouse testis and spermatogonial GC-1 cells: A study on its regulation by excitatory amino acids. J. Cell. Biochem. 2019, 120, 11044–11055. [Google Scholar] [CrossRef] [PubMed]
- Monteforte, R.; Santillo, A.; Chieffi, P.; Chieffi Baccari, G. D-Aspartate in frog harderian gland. In D-Amino Acids: A New Frontier in Amino Acid and Protein Research—Practical Methods and Protocols; Konno, R., Brückner, H., D’Aniello, A., Fisher, G.H., Fujii, N., Homma, H., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2007; pp. 215–220. [Google Scholar]
- Rastogi, R.K.; Pinelli, C.; Polese, G.; D’Aniello, B.; Chieffi Baccari, G. Hormones and Reproductive Cycles in Anuran Amphibians. In Hormones and Reproduction of Vertebrates, 1st ed.; Norris, D., Lopez, K.H., Eds.; Elsevier: Denver, CO, USA, 2011; Volume 2, pp. 171–186. [Google Scholar]
- Raucci, F.; Santillo, A.; D’Aniello, A.; Chieffi, P.; Chieffi Baccari, G. D-aspartate modulates transcriptional activity in Harderian gland of frog, Rana esculenta: Morphological and molecular evidence. J. Cell. Physiol. 2005, 204, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Rosati, L.; Prisco, M.; Di Fiore, M.M.; Santillo, A.; Sciarrillo, R.; Valiante, S.; Laforgia, V.; Coraggio, F.; Andreuccetti, P.; Agnese, M. Sex steroid hormone secretion in the wall lizard Podarcis sicula testis: The involvement of VIP. J. Exp. Zool. A Ecol. Genet. Physiol. 2015, 323, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Rosati, L.; Agnese, M.; Di Fiore, M.M.; Andreuccetti, P.; Prisco, M. P450 aromatase: A key enzyme in the spermatogenesis of the Italian wall lizard. Podarcis sicula. J. Exp Biol. 2016, 219, 2402–2408. [Google Scholar] [CrossRef] [PubMed]
- Rosati, L.; Santillo, A.; Di Fiore, M.M.; Andreuccetti, P.; Prisco, M. Testicular steroidogenic enzymes in the lizard Podarcis sicula during the spermatogenic cycle. Comptes Rendus Biol. 2017, 340, 492–498. [Google Scholar] [CrossRef]
- Santillo, A.; Monteforte, R.; Raucci, F.; D’Aniello, A.; Chieffi Baccari, G. Occurrence of D-Aspartate in the harderian gland of Podarcis s. sicula and its effect on gland secretion. J. Exp. Zool. A Comp. Exp. Biol. 2006, 305, 610–619. [Google Scholar] [CrossRef]
- Santillo, A.; Falvo, S.; Chieffi Baccari, G.; Di Fiore, M.M. Seasonal changes in gene expression of steroidogenic enzymes, androgen and estrogen receptors in frog testis. Acta Zool. 2017, 98, 221–227. [Google Scholar] [CrossRef]
- Santillo, A.; Falvo, S.; Di Fiore, M.M.; Chieffi Baccari, G. Seasonal changes and sexual dimorphism in gene expression of StAR protein, steroidogenic enzymes and sex hormone receptors in the frog brain. Gen. Comp. Endocrinol. 2017, 246, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Rosati, L.; Prisco, M.; Chieffi Baccari, G.; Andreuccetti, P.; Falvo, S.; Di Fiore, M.M. Aromatase immunolocalization and activity in the lizard’s brain: Dynamic changes during the reproductive cycle. Comptes Rendus Biol. 2019, 342, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, M.M.; Lamanna, C.; Assisi, L.; Botte, V. Opposing effects of D-aspartic acid and nitric oxide on tuning of testosterone production in mallard testis during the reproductive cycle. Reprod. Biol. Endocrinol. 2008, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; Assisi, L.; D’Aniello, S.; Spinelli, P.; Botte, V.; Di Fiore, M.M. Testicular endocrine activity is upregulated by D-aspartic acid in the green frog, Rana esculenta. J. Endocrinol. 2004, 182, 365. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; D’Aniello, S.; Di Fiore, M.M. Endocrine roles of D-aspartic acid in the testis of lizard Podarcis s. sicula. J. Endocrinol. 2005, 187, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; Di Fiore, M.M. The reproductive activity in the testis of Podarcis s. sicula involves D-aspartic acid: A study on c-kit receptor protein, tyrosine kinase activity and PCNA protein during annual sexual cycle. Gen. Comp. Endocrinol. 2009, 161, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; Di Fiore, M.M. D-Asp: A new player in reproductive endocrinology of the amphibian Rana esculenta. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- Assisi, L.; Botte, V.; D’Aniello, A.; Di Fiore, M.M. Enhancement of aromatase activity by D-aspartic acid in the ovary of the lizard Podarcis s. sicula. Reproduction 2001, 121, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, M.M.; Assisi, L.; Botte, V.; D’Aniello, A. D-Aspartic acid is implicated in the control of testosterone production by the vertebrate gonad. Studies on the female green frog. Rana esculenta. J. Endocrinol. 1998, 157, 199–208. [Google Scholar] [CrossRef]
- Raucci, F.; Di Fiore, M.M. The maturation of oocyte follicular epithelium of Podarcis s. sicula is promoted by D-aspartic acid. J. Histochem. Cytochem. 2010, 58, 157–171. [Google Scholar] [CrossRef]
- Burrone, L.; Raucci, F.; Di Fiore, M.M. Steroidogenic gene expression following D-aspartate treatment in frog testis. Gen. Comp. Endocrinol. 2012, 175, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Burrone, L.; Santillo, A.; Pinelli, C.; Chieffi Baccari, G.; Di Fiore, M.M. Induced synthesis of P450 aromatase and 17β-estradiol by d-aspartate in frog brain. J. Exp. Biol. 2012, 215, 3559–3565. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Pinelli, C.; Burrone, L.; Chieffi Baccari, G.; Di Fiore, M.M. D-Aspartic acid implication in the modulation of frog brain sex steroid levels. Gen. Comp. Endocrinol. 2013, 181, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Burrone, L.; Di Giovanni, M.; Di Fiore, M.M.; Chieffi Baccari, G.; Santillo, A. Effects of D-Aspartate Treatment on D-Aspartate Oxidase, Superoxide Dismutase, and Caspase 3 Activities in Frog (Rana esculenta) Tissues. Chem. Biodivers. 2010, 7, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, M.; Burrone, L.; Chieffi Baccari, G.; Topo, E.; Santillo, A. Distribution of free D-aspartic acid and D-aspartate oxidase in frog Rana esculenta tissues. J. Exp. Zool. A Ecol. Genet. Physiol. 2010, 313, 137–143. [Google Scholar] [PubMed]
- Di Fiore, M.M.; Burrone, L.; Santillo, A.; Chieffi Baccari, G. Endocrine Activity of D-Aspartate in Nonmammalian Animals. In D-amino Acids: Physiology, Metabolism and Application; Yoshimura, T., Nishikawa, T., Homma, H., Eds.; Springer: Tokyo, Japan, 2016; pp. 157–172. [Google Scholar]
- Santillo, A.; Chieffi Baccari, G.; Falvo, S.; Di Giacomo Russo, F.; Venditti, M.; Di Fiore, M.M. Effects of D-Aspartate on sex hormone-dependent tissues in Pelophylax esculentus. In Amphibians: Biology, Ecology and Conservation; Cannon, L., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2018; pp. 21–37. [Google Scholar]
- Raucci, F.; D’Aniello, A.; Di Fiore, M.M. Stimulation of androgen production by D-aspartate through the enhancement of StAR, P450scc and 3β-HSD mRNA levels in vivo rat testis and in culture of immature rat Leydig cells. Steroids 2014, 84, 103–110. [Google Scholar] [CrossRef]
- D’Aniello, A.; Di Fiore, M.M.; Fisher, G.H.; Milone, A.; Seleni, A.; D’Aniello, S.; Perna, A.F.; Ingrosso, D. Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. FASEB J. 2000, 14, 699–714. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, G.; Tolino, A.; D’Aniello, A.; Errico, F.; Fisher, G.H.; Di Fiore, M.M. The role of D-aspartic acid and N-methyl-D-aspartic acid in the regulation of prolactin release. Endocrinology 2000, 141, 3862–3870. [Google Scholar] [CrossRef]
- Ishio, S.; Yamada, H.; Hayashi, M.; Yatsushiro, S.; Noumi, T.; Yamaguchi, A.; Moriyama, Y. D-Aspartate modulates melatonin synthesis in rat pinealocytes. Neurosci. Lett. 1998, 249, 143–146. [Google Scholar] [CrossRef]
- Dunlop, D.S.; Neidle, A.; McHale, D.; Dunlop, D.M.; Lajtha, A. The presence of free D-aspartic acid in rodents and man. Biochem. Biophys. Res. Commun. 1986, 141, 27–32. [Google Scholar] [CrossRef]
- D’Aniello, A.; Di Fiore, M.M.; D’Aniello, G.; Colin, F.E.; Lewis, G.; Setchell, B.P. Secretion of D-aspartic acid by the rat testis and its role in endocrinology of the testis and spermatogenesis. FEBS Lett. 1998, 436, 23–27. [Google Scholar] [CrossRef]
- Sakai, K.; Homma, H.; Lee, J.A.; Fukushima, T.; Santa, T.; Tashiro, K.; Iwatsubo, T.; Imai, K. Localization of D-Aspartic Acid in Elongate Spermatids in Rat Testis. Arch. Biochem. Biophys. 1998, 351, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, M.M.; Santillo, A.; Falvo, S.; Longobardi, S.; Chieffi Baccari, G. Molecular mechanisms elicited by D-aspartate in leydig cells and spermatogonia. Int. J. Mol. Sci. 2016, 17, 1127. [Google Scholar] [CrossRef] [PubMed]
- Raspa, M.; Mahabir, E.; Paoletti, R.; Protti, M.; Mercolini, L.; Schiller, P.; Scavizzi, F. Effects of oral d-aspartate on sperm quality in B6N mice. Theriogenology 2018, 121, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, K.N. D-Aspartic acid induced oxidative stress and mitochondrial dysfunctions in testis of prepubertal rats. Amino Acids 2010, 38, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Topo, E.; Soricelli, A.; D’Aniello, A.; Ronsini, S.; D’Aniello, G. The role and molecular mechanism of D-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod. Biol. Endocrinol. 2009, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, M.M.; Santillo, A.; Falvo, S.; Chieffi Baccari, G.; Venditti, M.; Di Giacomo Russo, F.; Lispi, M.; D’Aniello, A. Sex hormone levels in the brain of d-aspartate-treated rats. Comptes Rendus Biol. 2018, 341, 9–15. [Google Scholar] [CrossRef]
- Falvo, S.; Di Fiore, M.M.; Burrone, L.; Chieffi Baccari, G.; Longobardi, S.; Santillo, A. Androgen and oestrogen modulation by D-aspartate in rat epididymis. Reprod. Fertil. Dev. 2016, 28, 1865–1872. [Google Scholar] [CrossRef]
- Xia, J.D.; Chen, J.; Sun, H.J.; Zhou, L.H.; Zhu, G.Q.; Chen, Y.; Dai, Y.T. Centrally mediated ejaculatory response via sympathetic outflow in rats: Role of N-methyl-D-aspartic acid receptors in paraventricular nucleus. Andrology 2017, 5, 153–159. [Google Scholar] [CrossRef][Green Version]
- Staudt, M.D.; de Oliveira, C.V.; Lehman, M.N.; McKenna, K.E.; Coolen, L.M. Activation of NMDA receptors in lumbar spinothalamic cells is required for ejaculation. J. Sex. Med. 2011, 8, 1015–1026. [Google Scholar] [CrossRef]
- Pinilla, L.; Tena-Sempere, M.; Aguilar, E. Role of excitatory amino acid pathways in control of gonadotrophin secretion in adult female rats sterilized by neonatal administration of oestradiol or testosterone. J. Reprod. Fertil. 1998, 113, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Tanaka, H.; Kageyama, S.; Nagasawa, M.; Wada, A.; Murai, R.; Kobayashi, K.; Hanada, E.; Agata, Y.; Kawauchi, A. The effect of D-Aspartate on spermatogenesis in mouse testis. Biol. Reprod. 2016, 94, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Falvo, S.; Chieffi, P.; Di Fiore, M.M.; Senese, R.; Chieffi Baccari, G. D-Aspartate induces proliferative pathways in spermatogonial GC-1 cells. J. Cell. Physiol. 2016, 231, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, A.; De Toni, L.; Ferigo, M.; Rocca, M.; Speltra, E.; Ferlin, A.; Foresta, C. D-Aspartic acid stimulates steroidogenesis through the delay of LH receptor internalization in a mammalian Leydig cell line. J. Endocrinol. Invest. 2016, 39, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, K.N. Oxidative alterations induced by d-aspartic acid in prepubertal rat testis in vitro: A mechanistic study. Theriogenology 2008, 70, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Homma, H.; Lee, J.A.; Imai, K. D-Aspartate stimulation of testosterone synthesis in rat Leydig cells. FEBS Lett. 1999, 444, 160–164. [Google Scholar] [CrossRef]
- Nagata, Y.; Homma, H.; Matsumoto, M.; Imai, K. Stimulation of steroidogenic acute regulatory protein (StAR) gene expression by D-aspartate in rat Leydig cells. FEBS Lett. 1999, 454, 317–320. [Google Scholar]
- Pampillo, M.; del Carmen Díaz, M.; Duvilanski, B.H.; Rettori, V.; Seilicovich, A.; Lasaga, M. Differential effects of glutamate agonists and D-aspartate on oxytocin release from hypothalamus and posterior pituitary of male rats. Endocrine 2001, 15, 309–315. [Google Scholar] [CrossRef]
- Pampillo, M.; Theas, S.; Duvilanski, B.; Seilicovich, A.; Lasaga, M. Effect of ionotropic and metabotropic glutamate agonists and D-aspartate on prolactin release from anterior pituitary cells. Exp. Clin. Endocrinol. Diabetes 2002, 110, 138–144. [Google Scholar] [CrossRef]
- Pampillo, M.; Scimonelli, T.; Bottino, M.C.; Duvilanski, B.H.; Rettori, V.; Seilicovich, A.; Lasaga, M. The effect of D-aspartate on luteinizing hormone-releasing hormone, α-melanocyte-stimulating hormone, GABA and dopamine release. Neuroreport 2002, 13, 2341–2344. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cadavid, N.F.; Ryndin, I.; Vernet, D.; Magee, T.R.; Rajfer, J. Presence of NMDA receptor subunits in the male lower urogenital tract. J. Androl. 2000, 21, 566–578. [Google Scholar] [PubMed]
- Sheffield, J.; Roman, C.; Roper, B.; Poole, R.; Pickworth, C. Flushing and Synchronization Protocol Impacts on out of Season Breeding in Ewes. J. Anim. Sci. 2018, 96, 76. [Google Scholar] [CrossRef]
- Boni, R.; Santillo, R.; Macchia, G.; Spinelli, P.; Ferrandino, G.; D’Aniello, A. D-Aspartate and reproductive activity in sheep. Theriogenology 2006, 65, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, C.; Assisi, L.; Botte, V.; Di Fiore, M.M. Endogenous testicular D-aspartic acid regulates gonadal aromatase activity in boar. J. Endocrinol. Invest. 2006, 29, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, C.; Assisi, L.; Botte, V.; Di Fiore, M.M. Involvement of D-Asp in P450 aromatase activity and estrogen receptors in boar testis. Amino Acids 2007, 32, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, C.; Assisi, L.; Vittoria, A.; Botte, V.; Di Fiore, M.M. D-Aspartic acid and nitric oxide as regulators of androgen production in boar testis. Theriogenology 2007, 67, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Ikuta, T.; Hemmi, H.; Takahashi, S.; Kera, Y.; Yoshimura, T. Enzymatic assay for d-aspartic acid using d-aspartate oxidase and oxaloacetate decarboxylase. Biosci. Biotechnol. Biochem. 2012, 76, 2150–2152. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, A.; Tanaka, H.; Ishida, T.; Horiike, K. D-aspartate oxidase localisation in pituitary and pineal glands of the female pig. J. Neuroendocrinol. 2010, 22, 1165–1172. [Google Scholar] [CrossRef]
- Yamamoto, A.; Tanaka, H.; Ishida, T.; Horiike, K. Immunohistochemical localization of D-aspartate oxidase in porcine peripheral tissues. Amino Acids 2011, 41, 529–536. [Google Scholar] [CrossRef]
- Errico, F.; Pirro, M.T.; Affuso, A.; Spinelli, P.; De Felice, M.; D’Aniello, A.; Di Lauro, R. A physiological mechanism to regulate D-aspartic acid and NMDA levels in mammals revealed by D-aspartate oxidase deficient mice. Gene 2006, 374, 50–57. [Google Scholar] [CrossRef]
- Errico, F.; Rossi, S.; Napolitano, F.; Catuogno, V.; Topo, E.; Fisone, G.; D’Aniello, A.; Centonze, D.; Usiello, A. D-aspartate prevents corticostriatal long-term depression and attenuates schizophrenia-like symptoms induced by amphetamine and MK-801. J. Neurosci. 2008, 28, 10404–10414. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Zhandi, M.; Kohram, H.; Zaghari, M.; Sadeghi, M.; Gholami, M.; Deldar, H.; Di Fiore, M.M.; Benson, A.P. D-Aspartate amends reproductive performance of aged roosters by changing gene expression and testicular histology. Reprod. Fertil. Dev. 2018, 30, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Zhandi, M.; Kohram, H.; Zaghari, M.; Sadeghi, M.; Sharafi, M. Improvement of post-thawed sperm quality and fertility of Arian rooster by oral administration of D-aspartic acid. Theriogenology 2017, 92, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.; Joss, J.; Scaramuzzi, R. The effects of N-methyl-D, L-aspartic acid and aspartic acid on the plasma concentration of gonadotrophins, GH and prolactin in the ewe. J. Endocrinol. 1996, 149, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Macchia, G.; Topo, E.; Mangano, N.; D’Aniello, E.; Boni, R. DL-Aspartic acid administration improves semen quality in rabbit bucks. Anim. Reprod. Sci. 2010, 118, 337–343. [Google Scholar] [CrossRef]
- Estienne, M.J.; Schillo, K.K.; Green, M.A.; Hileman, S.M.; Boling, J.A. N-methyl-D, L-aspartate stimulates growth hormone but not luteinizing hormone secretion in the sheep. Life Sci. 1989, 44, 1527–1533. [Google Scholar] [CrossRef]
- Estienne, M.; Harter-Dennis, J.; Barb, C.; Hartsock, T.; Campbell, R.; Armstrong, J. N-methyl-D, L-aspartate-induced growth hormone secretion in barrows: Possible mechanisms of action. J. Anim. Sci. 1996, 74, 597–602. [Google Scholar] [CrossRef]
- Estienne, M.; Broughton, D.; Barb, C. Serum concentrations of luteinizing hormone, growth hormone, testosterone, estradiol and leptin in boars treated with n-methyl-d, l-aspartate. J. Anim. Sci. 2000, 78, 365–370. [Google Scholar] [CrossRef]
- Barb, C.; Campbell, R.; Armstrong, J.D.; Cox, N. Aspartate and glutamate modulation of growth hormone secretion in the pig: Possible site of action. Domest. Anim. Endocrinol. 1996, 13, 81. [Google Scholar] [CrossRef]
- Estienne, M.; Harter-Dennis, J.; Barb, C.; Hartsock, T. Luteinizing hormone and growth hormone concentrations in serum of prepubertal gilts treated with N-methyl-D, L-aspartate. Domest. Anim. Endocrinol. 1995, 12, 207–213. [Google Scholar] [CrossRef]
- Chang, W.; Barb, C.; Kraeling, R.; Rampacek, G.; Asanovich, K. N-methyl-d, l-aspartate modulation of pituitary hormone secretion in the pig: Role of opioid peptides. Domest. Anim. Endocrinol. 1993, 10, 305–313. [Google Scholar] [CrossRef]
- Estienne, M.; Hurlock, W.; Barb, C. Serum concentrations of luteinizing hormone, growth hormone, and cortisol in gilts treated with N-methyl-D, L-aspartate during the estrous cycle or after ovariectomy. J. Anim. Sci. 1998, 76, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, B.P.; Davison, L.A. Comparison of the effects of N-methyl-DL-aspartic acid on gonadotropin and prolactin secretion in anestrous mares and mares exhibiting estrous cycles during anestrus. Biol. Reprod. 1997, 57, 36–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sticker, L.; Thompson, D.; Gentry, L. Pituitary hormone and insulin responses to infusion of amino acids and N-methyl-D, L-aspartate in horses. J. Anim. Sci. 2001, 79, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Rizos, D.; Longobardi, S.; Talevi, R. Treatment with zinc, d-aspartate, and coenzyme Q10 protects bull sperm against damage and improves their ability to support embryo development. Theriogenology 2014, 82, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Barbato, V.; Talevi, R.; Braun, S.; Merolla, A.; Sudhakaran, S.; Longobardi, S.; Gualtieri, R. Supplementation of sperm media with zinc, D-aspartate and co-enzyme Q10 protects bull sperm against exogenous oxidative stress and improves their ability to support embryo development. Zygote 2017, 25, 168–175. [Google Scholar] [CrossRef]
- Boni, R.; Gallo, A.; Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 2017, 5, 133–145. [Google Scholar] [CrossRef]
- Wilson, R.; Knobil, E. Acute effects of N-methyl-DL-aspartate on the release of pituitary gonadotropins and prolactin in the adult female rhesus monkey. Brain Res. 1982, 248, 177–179. [Google Scholar] [CrossRef]
- Gay, V.M.; Plant, T.L. N-methyl-D, L-aspartate elicits hypothalamic gonadotropin-releasing hormone release in prepubertal male rhesus monkeys (Macaca mulatto). Endocrinology 1987, 120, 2289–2296. [Google Scholar] [CrossRef]
- Melville, G.W.; Siegler, J.C.; Marshall, P.W. Three and six grams supplementation of d-aspartic acid in resistance trained men. J. Int. Soc. Sports Nutr. 2015, 12, 15. [Google Scholar] [CrossRef]
- Melville, G.W.; Siegler, J.C.; Marshall, P.W. The effects of d-aspartic acid supplementation in resistance-trained men over a three month training period: A randomised controlled trial. PLoS ONE 2017, 12, e0182630. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, G.; Ronsini, S.; Notari, T.; Grieco, N.; Infante, V.; D’Angel, N.; Mascia, F.; Di Fiore, M.M.; Fisher, G.; D’Aniello, A. D-Aspartate, a key element for the improvement of sperm quality. Adv. Sex. Med. 2012, 2, 45. [Google Scholar] [CrossRef]
- Willoughby, D.S.; Leutholtz, B. D-Aspartic acid supplementation combined with 28 days of heavy resistance training has no effect on body composition, muscle strength and serum hormones associated with the hypothalamo-pituitary-gonadal axis in resistance-trained men. Nutr. Res. 2013, 33, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Spillane, M.; Schwarz, N. Heavy resistance training and supplementation with the alleged testosterone booster NMDA has no effect on body composition, muscle performance, and serum hormones associated with the hypothalamo-pituitary-gonadal axis in resistance-trained males. J. Sports Sci. Med. 2014, 13, 192. [Google Scholar] [PubMed]
- D’Aniello, G.; Ronsini, S.; Guida, F.; Spinelli, P.; D’Aniello, A. Occurrence of D-aspartic acid in human seminal plasma and spermatozoa: Possible role in reproduction. Fertil. Steril. 2005, 84, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Longobardi, S.; Gualtieri, R. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Reprod. Biol. Endocrinol. 2013, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Giacone, F.; Condorelli, R.A.; Mongioì, L.M.; Bullara, V.; La Vignera, S.; Calogero, A.E. In vitro effects of zinc, D-aspartic acid, and coenzyme-Q10 on sperm function. Endocrine 2017, 56, 408–415. [Google Scholar] [CrossRef]
- D’Aniello, G.; Grieco, N.; Di Filippo, M.; Cappiello, F.; Topo, E.; D’Aniello, E.; Ronsini, S. Reproductive implication of D-aspartic acid in human pre-ovulatory follicular fluid. Hum. Reprod. 2007, 22, 3178–3183. [Google Scholar] [CrossRef]
| BREEDING SEASON | OUT-OF-BREEDING SEASON | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| a) Long-term experiments | |||||||||
| Injections of 2 µmol/g b.w./d D-Asp for 10–15 days | D-Asp uptake | Increase/Decrease | D-Asp uptake | Increase/Decrease | References | ||||
| P. esculentus ♂ | |||||||||
| Testis | +96% | >1000% E2 +19% StAR mRNA +80% P450 aro mRNA | +100% | +67% T −90% E2 +500% [D-AspO] +43% StAR mRNA +54% 5α-Red2 mRNA | Burrone et al. [33] | ||||
| Brain | +450% | −60% T +100% E2 +43% P-CREB +40% P450 aro mRNA +175% P450 aro protein −25 % AR mRNA +25% ERα mRNA | +400% | +140% D-AspO activity +120% caspase 3 | Burrone et al. [36] Burrone et al. [33] Santillo et al. [35] | ||||
| b) Short-term experiments | |||||||||
| One Injection of 2 µmol/g b.w./ D-Asp | D-Asp uptake | Increase/Decrease | D-Asp uptake | Increase/Decrease | References | ||||
| 3 h | 6 h | 3 h | 6 h | 3 h | 6 h | 3 h | 6 h | ||
| P. esculentus ♂ | |||||||||
| Testis | +500% | +800% | +475% T | +25% T | Raucci et al. [40] | ||||
| Serum | +255% T | +33% T | Raucci and Di Fiore [29] | ||||||
| ♀ | |||||||||
| Ovary | +900% | +775% | −52% T | −21% T | Di Fiore et al. [31] | ||||
| Serum | −82% T | −47% T | −50% T | −83% T | Raucci and Di Fiore [29] | ||||
| P. s. sicula ♂ | |||||||||
| Testis | +140% | +100% | +32% T −50% E2 | +20% T −37% E2 | +800% | +500% | +96% T −71% E2 +23% c-Kit −50% PK | +78% T −42% E2 +130% PTK +29% PCNA | Raucci et al. [27] Raucci and Di Fiore [28] |
| Serum | +5% T −25% E2 | +23% T −75% E2 | +450% T −61% E2 | +1150% T −84% E2 | |||||
| ♀ | |||||||||
| Ovary | >1000% | >1000% | −37% T +200% E2 | −12% T +80% E2 | Assisi et al. [30] | ||||
| Serum | −42% T +300% E2 | −28% T +100% E2 | Raucci and Di Fiore [32] | ||||||
| Incubation | Increase/Decrease | References | |
|---|---|---|---|
| P. esculentus (breeding season) | |||
| Ovarian follicles | +2 μmol/mL D-Asp (3 h) | −62%T | Di Fiore et al. [31] |
| Ovarian follicles + pituitary | +2 μmol/mL D-Asp (3 h) | −56%T | |
| P. s. sicula (out-of-breeding season) | |||
| Ovarian tissue | +2 μmol/mL D-Asp (3 h) | +700% Aromatase activity | Assisi et al. [30] |
| Acetonic powder of ovarian follicles | +2 μmol/mL D-Asp (3 h) | +566% Aromatase activity | |
| A. platyrhyncos (out-of-breeding season) | |||
| Testis slices | +1–2 mM D-Asp (3 h) | +33–150% T | Di Fiore et al. [25] |
| D-Asp Uptake | Increase/Decrease | References | |
|---|---|---|---|
| Sexually immature B6N Mice | +14–20% IVF | Raspa et al. [48] | |
| 20 mM D-Asp drinking solution for 1–6 weeks | |||
| ♂ | |||
| Testis | +71% T +300–360% Epitestosterone +170% LH | ||
| Serum | +25–46% T +36–81% Epitestosterone +36–46% LH | ||
| Wistar Rats Prepubertal Injections of 100–500 mg/kg b.w./d D-Asp for 7 days | |||
| ♂ | |||
| Testis | + 75% mitochondrial ROS +30% cytosol ROS +20–50% MDA, hydroperoxide levels, LPO, GSH, catalase activity, GPX, GST, LDH, 3β-HSD, NO, D-AspO −15% MDH | Chandrashakar and Muralidhara [49] | |
| Sexually mature 20 mM D-Asp drinking solution | |||
| ♂ | |||
| Testis (treatment for 12–15 days) | +460–720% | +70% T +80% Androstenedione +100–120% P-ERK1/2 +37% StAR (mRNA-protein) +33% P450scc mRNA +38% 3β-HSD mRNA −25% P450 aro protein +55% AR protein −47% ERα protein +130% NR1-NR2A mRNAs | Topo et al. [50] Raucci et al. [40] Santillo et al. [13] |
| Pituitary (treatment for 12 days) | +580% | Topo et al. [50] | |
| Serum (treatment for 12–15 days) | +100% | +100–120% T +51–128% LH +40% Androstenedione | Topo et al. [50] Raucci et al. [40] |
| Brain (treatment for 30 days) | +100% | +40% P +110% T +35% E2 | Di Fiore et al. [51] |
| D-Asp Uptake | Increase/Decrease | References | |||||
|---|---|---|---|---|---|---|---|
| 30 min | 1–2 h | 5–8 h | 30 min | 1–2 h | 5–8 h | ||
| Wistar Rats One injection of 2 µmol/g b.w. D-Asp | |||||||
| ♂ | |||||||
| Testis | +133% | +311% | +150% NMDA | D’Aniello et al. [41,42] | |||
| Adenohypophysis | >1000% | >1000% | +125–166% NMDA | ||||
| Hypothalamus | +152% | +267% | +200–860% NMDA | ||||
| Epididymis | +500% | +110% | +70% | + 900% T +62% DHT +100% E2 +111 % AR mRNA +233% P450 aro mRNA +166% ERα mRNA | +600% T +62% DHT +80% E2 +200 % 5αRed1 mRNA +150% P450 aro mRNA +366% ERα mRNA | + 450% T +112% DHT +74% E2 +466 % 5αRed1 mRNA +78% % 5αRed2 mRNA +100% P450 aro mRNA +166% ERα | Falvo et al. [52] |
| Serum | >1000% | >1000% | +145% PRL | +36% T +34% P +110% LH +200% PRL +97% GH | +236% T +172% P +145% LH +161% GH | D’Aniello et al. [41,42] | |
| Brain | +288% | +288% | +211% | +26% T +28% E2 | +80% P +66% T +42% E2 | +90% P +93% T +85% E2 | Di Fiore et al. [51] |
| Incubation | Increase/Decrease | References | |
|---|---|---|---|
| Mouse | |||
| Cultured ICR testis tissue | +1–10 mM D-Asp (4 wks) | −21–71% Acr-GFP | Tomita et al [56] |
| GC-1 spermatogonia | +200 µM D-Asp (0.5-2 h) | +60–100% GluA1 +157% GluA2/3 +83% P-ERK1/2 +183% P-Akt +29% PCNA +84% AuroraB +266% P450 aro mRNA +62% P450 aro protein +112% ERβ | Santillo et al. [14,57] |
| +50 µM NMDA (0.5–4 h) | +66–116% GluA1 +63–75% GluA2/3 +66–133% P-ERK1/2 +33–133% P-ERK2 +40–50% PCNA +40% AuroraB | Santillo et al. [14] | |
| Leydig cells | +0.1 nM D-Asp+10 ng/mL hCG (48 h) | +25% T + 83% StAR protein | Di Nisio et al. [58] |
| Wistar Rat | |||
| Prepubertal testis | +0–1 mM D-Asp (2 h) | +50–280% MDA (cytosol) +83–183% MDA (mitochondria) +143% ROS +332% LPO +82% HP | Chandrashakar and Muralidhara [49,59] |
| Immature Leydig cells | +0.2 mM D-Asp (2–24 h) | +112% StAR mRNA +14% P450scc mRNA +33% 3β-HSD mRNA +36% StAR protein +100% Androstenedione release +185% T release | Raucci et al. [40] |
| Mature Leydig cells | +200 µM D-Asp (16 h) +200 µM D-Asp (16 h)+ 5 mIU hCG/ml (2 h) | +50% T +250% StAR mRNA +90% StAR protein | Nagata et al. [60,61] |
| +0.1 or 1.0 mM D-Asp (1h) | +142–200% T +200–325% cAMP | Topo et al. [50] | |
| Hypothalamus | +1 mM D-Asp (0.5 h) | +105% oxytocin | Pampillo et al. [62] |
| Adenohypophysis | +0.1–1 mM D-Asp (1 h) | +150–300% PRL +16% LH +166% GH + 150–200% cGMP | D’Aniello et al. [41,42] Topo et al. [50] |
| +0.1 mM NMDA (1 h) | +185% GH +83% LH | ||
| +0.1–1 mM D-Asp (4 h) (anterior and posterior hypophysis cell coltures) | +11–13% PRL | Pampillo et al. [63] | |
| +0.1–1 mM D-Asp (4 h) (anterior hypophysis cell coltures) | +15–25 % PRL | ||
| + 0.01–0.1 mM NMDA (4 h) (anterior hypophysis cell coltures) | +71–110 % PRL | ||
| Neurohypophysis | +0.1 mM D-Asp (0.5 h) +0.1 mM NMDA (0.5 h) | −35% oxytocin −45% oxytocin | Pampillo et al. [62] |
| Adenohypophysis+ hypothalamus | +1 mM D-Asp (0.5–4 h) | +450% PRL +72% LH +33% GH | D’Aniello et al. [41,42] |
| +0.1 mM NMDA (1 h) | +202% LH +87% GH | ||
| a) Long-term experiments | D-Asp uptake | Increase/Decrease | References |
| G. g. domesticus | |||
| Oral administration of 100–200 mg D-Asp/kg for 12 wks | |||
| Testis | +120–680% StAR mRNA +100–1000% P450scc mRNA +100–337% 3β-HSD mRNA +100–766% AR mRNA +120% LHR mRNA +100% Grin1 mRNA +300–1150% Grin2b mRNA +250–1800% PCNA mRNA | Ansari et al. [76] | |
| Serum | +10–24% T | ||
| Sperm | +sperm motility +Fertility +Hatchability +Plasma membrane integrity +Mitochondrial activity | Ansari et al. [77] | |
| O. aries | |||
| S.C. administration of 22.2 mg D-Asp/kg b.w. every 3 or 6 days for 1 month | |||
| Serum | +40% LH | Boni et al. [67] | |
| b) Short-term experiments | D-Asp uptake | Increase/Decrease | References |
| O. aries | |||
| S.C. injection of 44.4 mg D-Asp/kg b.w. | |||
| Ovary | +650% (24 h) | Boni et al. [67] | |
| Pituitary | >1000% (12–24 h) | +200% NMDA (12 h) | |
| Brain | +125% (12–24 h) | +250% NMDA (12 h) | |
| Serum | >1000% (3 h) | >1000% NMDA (5–12 h) +40% LH (2 h) |
| Increase/Decrease | References | |
|---|---|---|
| Oral administration of: | ||
| DADAVit® for 12 days | +33% LH +42% T | Topo et al. [50] |
| 6 g D-Asp for 14 days | −15% T in resistance trained men | Melville et al. [94] |
| 6 g D-Asp for 3 months | −95% E2 in resistance trained men No positively affecting training outcomes | Melville et al. [95] |
| DADAVit® or GENADIS® for 3 months | +sperm concentration and motility in asthenozoospermic and oligoasthenozoospermic men +pregnancy rate | D’Aniello et al. [96] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fiore, M.M.; Boni, R.; Santillo, A.; Falvo, S.; Gallo, A.; Esposito, S.; Chieffi Baccari, G. D-Aspartic Acid in Vertebrate Reproduction: Animal Models and Experimental Designs ‡. Biomolecules 2019, 9, 445. https://doi.org/10.3390/biom9090445
Di Fiore MM, Boni R, Santillo A, Falvo S, Gallo A, Esposito S, Chieffi Baccari G. D-Aspartic Acid in Vertebrate Reproduction: Animal Models and Experimental Designs ‡. Biomolecules. 2019; 9(9):445. https://doi.org/10.3390/biom9090445
Chicago/Turabian StyleDi Fiore, Maria Maddalena, Raffaele Boni, Alessandra Santillo, Sara Falvo, Alessandra Gallo, Sabrina Esposito, and Gabriella Chieffi Baccari. 2019. "D-Aspartic Acid in Vertebrate Reproduction: Animal Models and Experimental Designs ‡" Biomolecules 9, no. 9: 445. https://doi.org/10.3390/biom9090445
APA StyleDi Fiore, M. M., Boni, R., Santillo, A., Falvo, S., Gallo, A., Esposito, S., & Chieffi Baccari, G. (2019). D-Aspartic Acid in Vertebrate Reproduction: Animal Models and Experimental Designs ‡. Biomolecules, 9(9), 445. https://doi.org/10.3390/biom9090445









