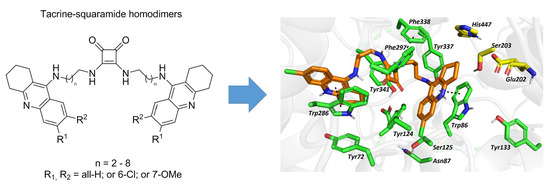
Exploring Structure-Activity Relationship in Tacrine-Squaramide Derivatives as Potent Cholinesterase Inhibitors
Abstract
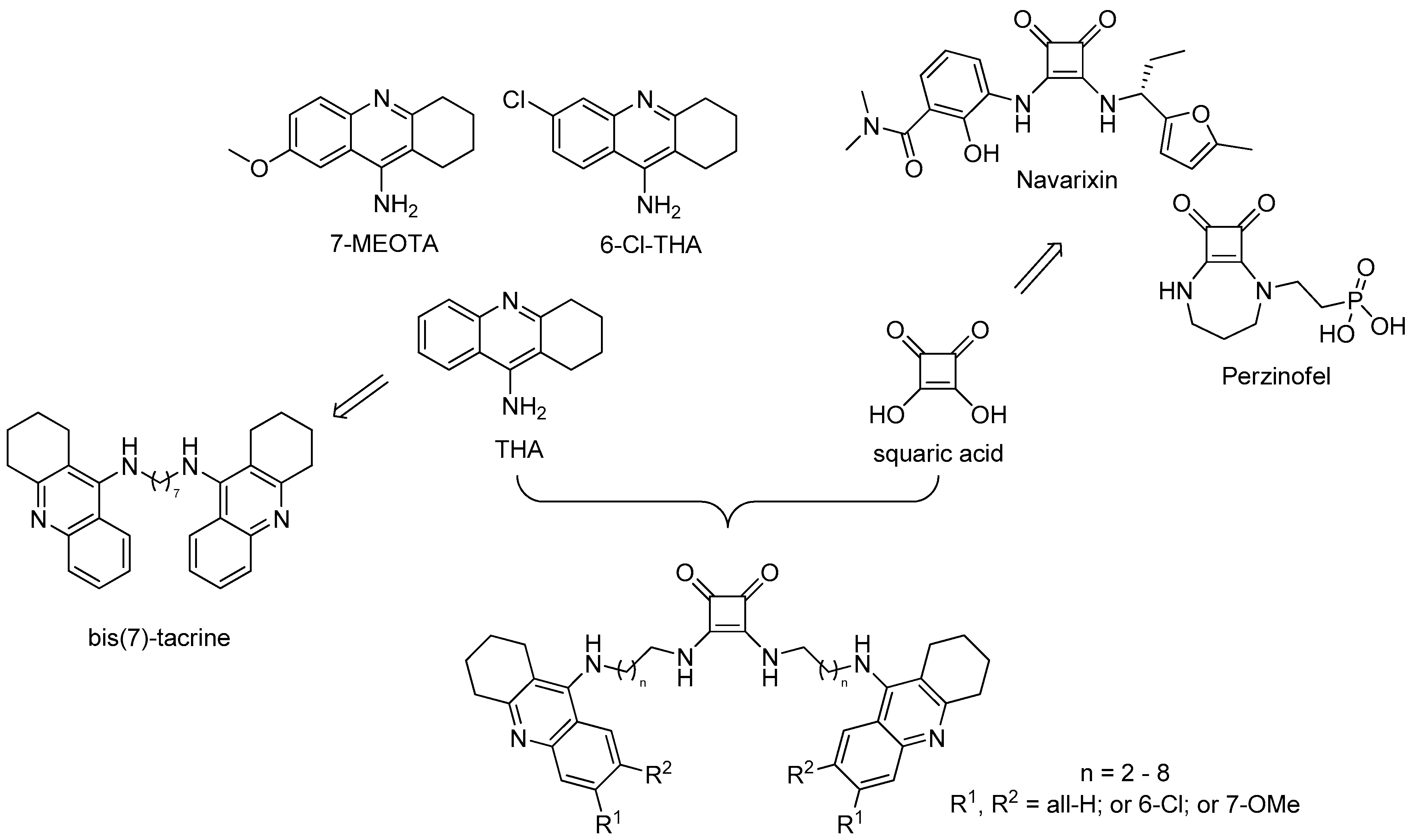
1. Introduction
2. Results and Discussion
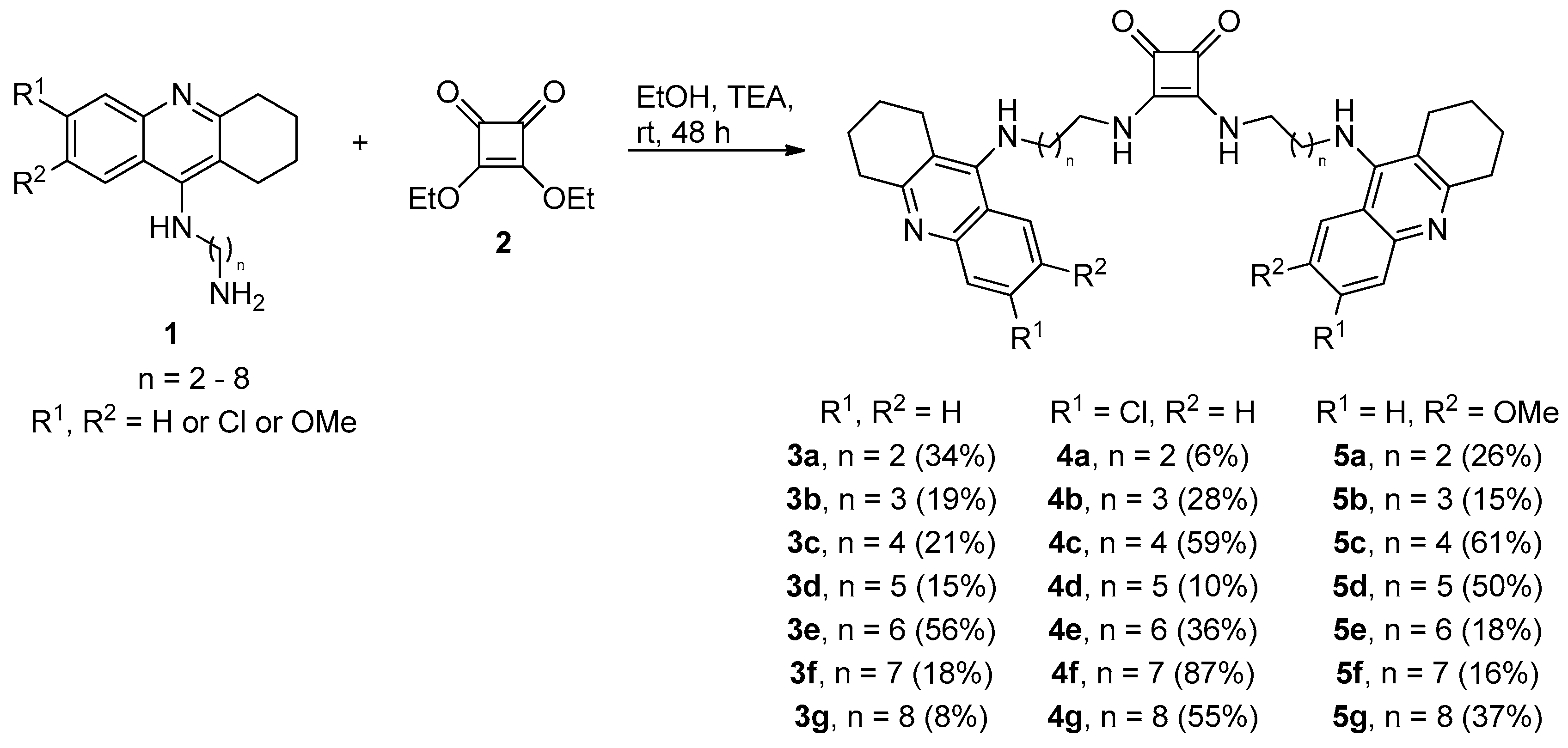
2.1. Chemistry
2.2. Evaluation of Cholinesterase Inhibitory Activity
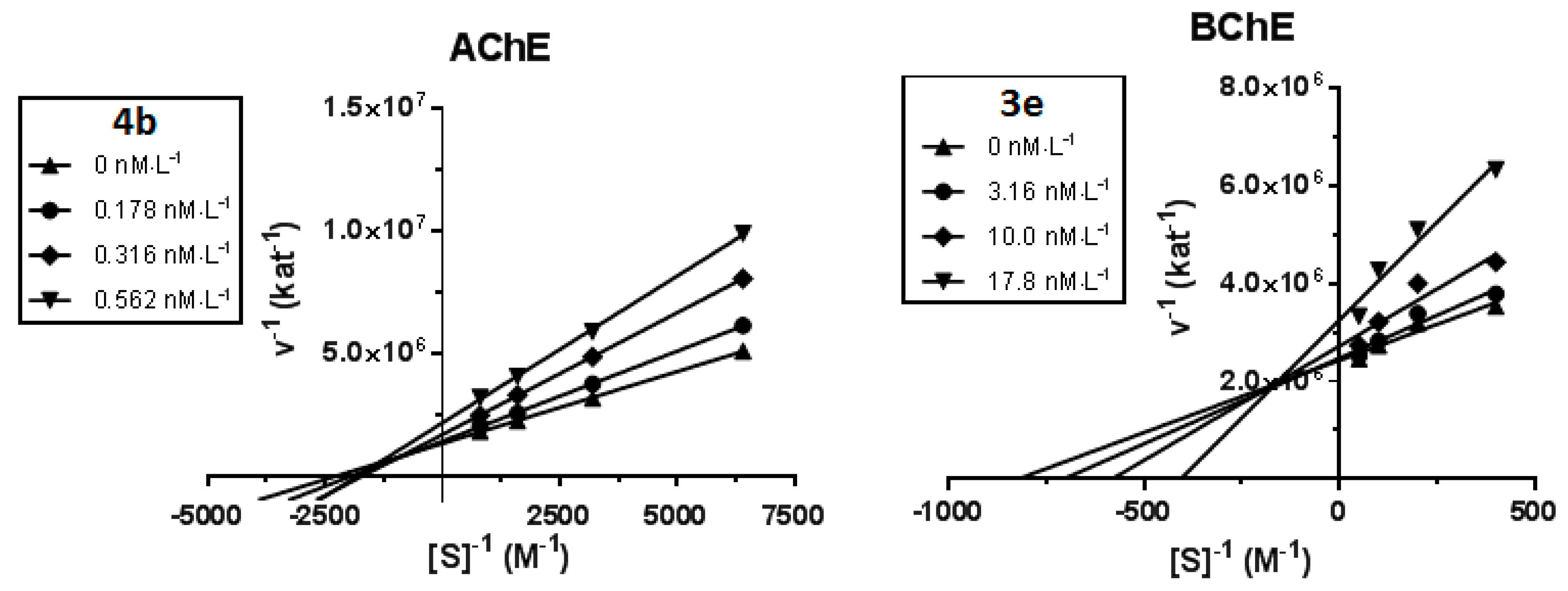
2.3. Kinetic Study of AChE and BChE Inhibition
2.4. Cytotoxicity
2.5. In Vitro BBB Permeation
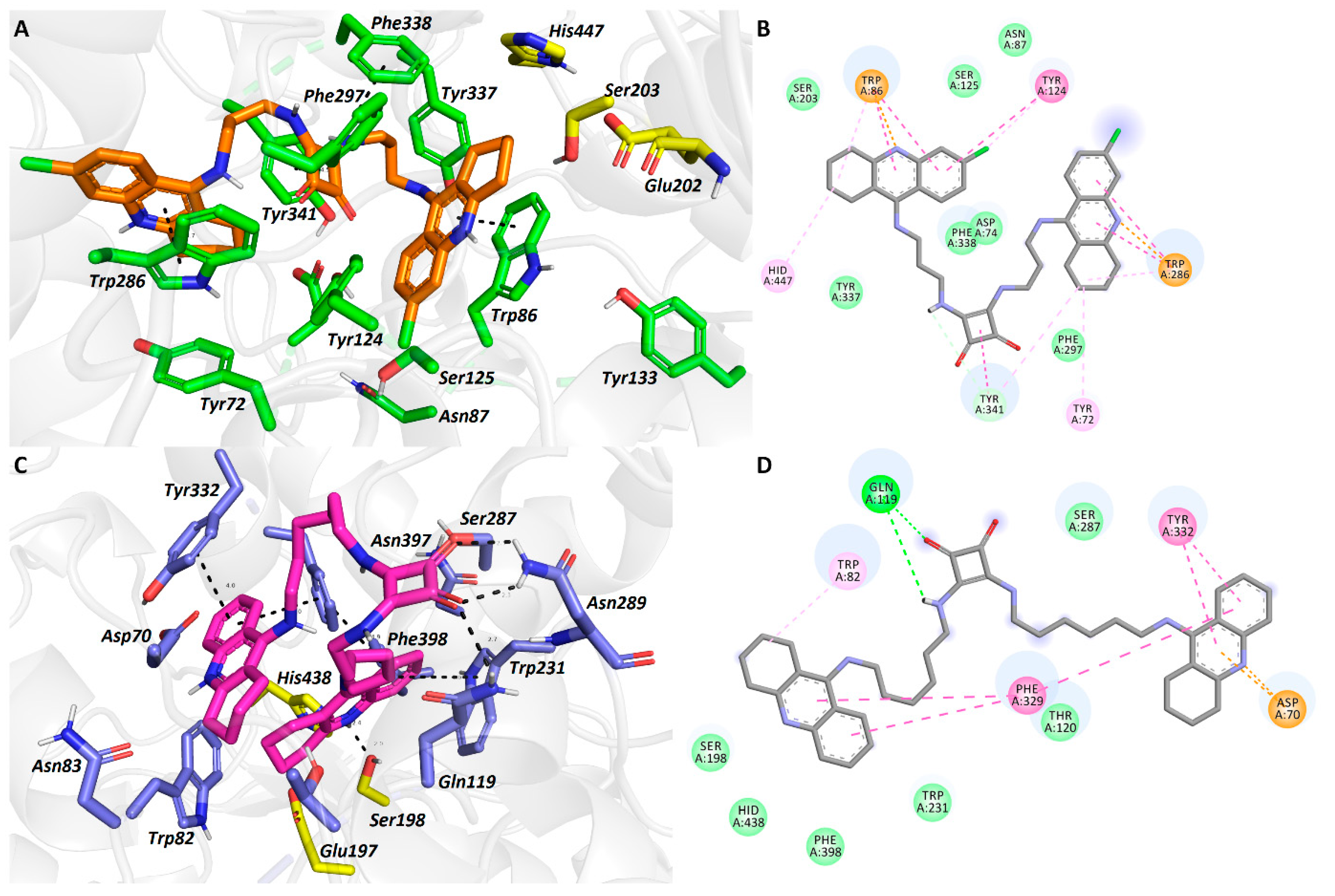
2.6. In Silico Studies
3. Conclusions
4. Experimental Section
4.1. General Chemistry Methods
General Procedure for the Preparation of Tacrine-Squaramides (3a–3g; 4a–4g and 5a–5g)
4.2. In Vitro Anti-ChE Assay
4.3. Kinetic Study of AChE and BChE Inhibition
4.4. Evaluation of Cytotoxicity by MTT Assay
4.5. Determination of in Vitro BBB Permeation
4.6. Molecular Modeling Studies
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Long, J.Z.; Cravatt, B.F. The Metabolic Serine Hydrolases and Their Functions in Mammalian Physiology and Disease. Chem. Rev. 2011, 111, 6022–6063. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Zemek, F.; Drtinova, L.; Nepovimova, E.; Sepsova, V.; Korabecny, J.; Klimes, J.; Kuca, K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014, 13, 759–774. [Google Scholar]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, CD005593. [Google Scholar]
- Vickers, J.C.; Mitew, S.; Woodhouse, A.; Fernandez-Martos, C.M.; Kirkcaldie, M.T.; Canty, A.J.; McCormack, G.H.; King, A.E. Defining the earliest pathological changes of Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. JAD 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Budimir, A. Metal ions, Alzheimer’s disease and chelation therapy. Acta Pharm. Zagreb Croat. 2011, 61, 1–14. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Sonkusare, S.K.; Kaul, C.L.; Ramarao, P. Dementia of Alzheimer’s disease and other neurodegenerative disorders--memantine, a new hope. Pharmacol. Res. 2005, 51, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Crismon, M.L. Tacrine: first drug approved for Alzheimer’s disease. Ann. Pharmacother. 1994, 28, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Holubova, K.; Nepovimova, E.; Krusek, J.; Kaniakova, M.; Korabecny, J.; Vyklicky, L.; Kuca, K.; Stuchlik, A.; Ricny, J.; et al. The pharmacology of tacrine at N-methyl-d-aspartate receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Soukup, O.; Jun, D.; Zdarova-Karasova, J.; Patocka, J.; Musilek, K.; Korabecny, J.; Krusek, J.; Kaniakova, M.; Sepsova, V.; Mandikova, J.; et al. A resurrection of 7-MEOTA: a comparison with tacrine. Curr. Alzheimer Res. 2013, 10, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; Lewis, S.; Farlow, M.R. Tacrine alters the secretion of the beta-amyloid precursor protein in cell lines. J. Neurosci. Res. 1994, 37, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; Farlow, M.R.; Sambamurti, K. The secretion of amyloid beta-peptides is inhibited in the tacrine-treated human neuroblastoma cells. Brain Res. Mol. Brain Res. 1998, 62, 131–140. [Google Scholar] [CrossRef]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994, 271, 992–998. [Google Scholar] [CrossRef]
- Zeiger, E.; Erexson, G.; Mortelmans, K.; Thilagar, A. Genetic toxicity studies of 1,2,3,4-tetrahydro-9-acridinamine (tacrine). Mutat. Res. 1997, 393, 189–197. [Google Scholar] [CrossRef]
- Misik, J.; Nepovimova, E.; Pejchal, J.; Kassa, J.; Korabecny, J.; Soukup, O. Cholinesterase Inhibitor 6-Chlorotacrine—In Vivo Toxicological Profile and Behavioural Effects. Available online: http://www.eurekaselect.com/158225/article (accessed on 2 August 2019).
- Pang, Y.P.; Quiram, P.; Jelacic, T.; Hong, F.; Brimijoin, S. Highly potent, selective, and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase. Steps toward novel drugs for treating Alzheimer’s disease. J. Biol. Chem. 1996, 271, 23646–23649. [Google Scholar] [CrossRef]
- Recanatini, M.; Cavalli, A.; Belluti, F.; Piazzi, L.; Rampa, A.; Bisi, A.; Gobbi, S.; Valenti, P.; Andrisano, V.; Bartolini, M.; et al. SAR of 9-amino-1,2,3,4-tetrahydroacridine-based acetylcholinesterase inhibitors: synthesis, enzyme inhibitory activity, QSAR, and structure-based CoMFA of tacrine analogues. J. Med. Chem. 2000, 43, 2007–2018. [Google Scholar] [CrossRef]
- Carlier, P.R.; Han, Y.F.; Chow, E.S.; Li, C.P.; Wang, H.; Lieu, T.X.; Wong, H.S.; Pang, Y.P. Evaluation of short-tether bis-THA AChE inhibitors. A further test of the dual binding site hypothesis. Bioorg. Med. Chem. 1999, 7, 351–357. [Google Scholar] [CrossRef]
- Korábečný, J. Prokognitivní Potenciál Bis(7)-takrinu Jako Zvažovaného Terapeutika Neurodegenerativních Onemocnění. MMSL 2018, 87, 34–44. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional Amine-Squaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Zhao, B.-L.; Li, J.-H.; Du, D.-M. Squaramide-Catalyzed Asymmetric Reactions. Chem. Rec. 2017, 17, 994–1018. [Google Scholar] [CrossRef]
- Karahan, S.; Tanyeli, C. Squaramide catalyzed α-chiral amine synthesis. Tetrahedron Lett. 2018, 59, 3725–3737. [Google Scholar] [CrossRef]
- Brandão, P.; Burke, A.J. Recent advances in the asymmetric catalytic synthesis of chiral 3-hydroxy and 3-aminooxindoles and derivatives: Medicinally relevant compounds. Tetrahedron 2018, 74, 4927–4957. [Google Scholar] [CrossRef]
- Kinney, W.A.; Abou-Gharbia, M.; Garrison, D.T.; Schmid, J.; Kowal, D.M.; Bramlett, D.R.; Miller, T.L.; Tasse, R.P.; Zaleska, M.M.; Moyer, J.A. Design and Synthesis of [2-(8,9-Dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)- ethyl]phosphonic Acid (EAA-090), a Potent N-Methyl-d-aspartate Antagonist, via the Use of 3-Cyclobutene-1,2-dione as an Achiral α-Amino Acid Bioisostere. J. Med. Chem. 1998, 41, 236–246. [Google Scholar] [CrossRef]
- Dwyer, M.P.; Yu, Y.; Chao, J.; Aki, C.; Chao, J.; Biju, P.; Girijavallabhan, V.; Rindgen, D.; Bond, R.; Mayer-Ezel, R.; et al. Discovery of 2-Hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5- methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): A Potent, Orally Bioavailable CXCR2/CXCR1 Receptor Antagonist. J. Med. Chem. 2006, 49, 7603–7606. [Google Scholar] [CrossRef]
- Storer, R.I.; Aciro, C.; Jones, L.H. Squaramides: physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef]
- Marín, C.; Ximenis, M.; Ramirez-Macías, I.; Rotger, C.; Urbanova, K.; Olmo, F.; Martín-Escolano, R.; Rosales, M.J.; Cañas, R.; Gutierrez-Sánchez, R.; et al. Effective anti-leishmanial activity of minimalist squaramide-based compounds. Exp. Parasitol. 2016, 170, 36–49. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Espadinha, M.; Machado, M.; Gut, J.; Gonçalves, L.M.; Rosenthal, P.J.; Prudêncio, M.; Moreira, R.; Santos, M.M.M. Novel squaramides with in vitro liver stage antiplasmodial activity. Bioorg. Med. Chem. 2016, 24, 1786–1792. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Marín, C.; Vega, M.; Martin-Montes, Á.; Medina-Carmona, E.; López, C.; Rotger, C.; Costa, A.; Sánchez-Moreno, M. Synthesis and biological evaluation of new long-chain squaramides as anti-chagasic agents in the BALB/c mouse model. Bioorg. Med. Chem. 2019, 27, 865–879. [Google Scholar] [CrossRef]
- Fu, H.; Li, W.; Luo, J.; Lee, N.T.K.; Li, M.; Tsim, K.W.K.; Pang, Y.; Youdim, M.B.H.; Han, Y. Promising anti-Alzheimer’s dimer bis(7)-tacrine reduces β-amyloid generation by directly inhibiting BACE-1 activity. Biochem. Biophys. Res. Commun. 2008, 366, 631–636. [Google Scholar] [CrossRef]
- Li, C.; Carlier, P.R.; Ren, H.; Kan, K.K.W.; Hui, K.; Wang, H.; Li, W.; Li, Z.; Xiong, K.; Clement, E.C.; et al. Alkylene tether-length dependent γ-aminobutyric acid type A receptor competitive antagonism by tacrine dimers. Neuropharmacology 2007, 52, 436–443. [Google Scholar] [CrossRef]
- Ros, E.; Aleu, J.; Gomez De Aranda, I.; Cantí, C.; Pang, Y.-P.; Marsal, J.; Solsona, C. Effects of Bis(7)-Tacrine on Spontaneous Synaptic Activity and on the Nicotinic ACh Receptor of Torpedo Electric Organ. J. Neurophysiol. 2001, 86, 183–189. [Google Scholar] [CrossRef]
- Minarini, A.; Milelli, A.; Tumiatti, V.; Rosini, M.; Simoni, E.; Bolognesi, M.L.; Andrisano, V.; Bartolini, M.; Motori, E.; Angeloni, C.; et al. Cystamine-tacrine dimer: A new multi-target-directed ligand as potential therapeutic agent for Alzheimer’s disease treatment. Neuropharmacology 2012, 62, 997–1003. [Google Scholar] [CrossRef]
- Han, Y.-F.; Wu, D.-C.; Xiao, X.-Q.; Chen, P.M.Y.; Chung, W.; Lee, N.T.K.; Pang, Y.-P.; Carlier, P.R. Protection against ischemic injury in primary cultured astrocytes of mouse cerebral cortex by bis(7)-tacrine, a novel anti-Alzheimer’s agent. Neurosci. Lett. 2000, 288, 95–98. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Chow, P.C.Y.; Lau, D.T.K.; Lee, N.T.K.; Pang, Y.; Zhang, X.; Wang, X.; Han, Y. Bis(7)-tacrine, a promising anti-Alzheimer’s dimer, affords dose- and time-dependent neuroprotection against transient focal cerebral ischemia. Neurosci. Lett. 2008, 439, 160–164. [Google Scholar] [CrossRef]
- Li, J.; Lu, Z.; Xu, L.; Wang, Q.; Zhang, Z.; Fang, J. Neuroprotective effects of bis(7)-tacrine in a rat model of pressure-induced retinal ischemia. Cell Biochem. Biophys. 2014, 68, 275–282. [Google Scholar] [CrossRef]
- Xiao, X.Q.; Lee, N.T.; Carlier, P.R.; Pang, Y.; Han, Y.F. Bis(7)-tacrine, a promising anti-Alzheimer’s agent, reduces hydrogen peroxide-induced injury in rat pheochromocytoma cells: comparison with tacrine. Neurosci. Lett. 2000, 290, 197–200. [Google Scholar] [CrossRef]
- Li, W.; Xue, J.; Niu, C.; Fu, H.; Lam, C.S.C.; Luo, J.; Chan, H.H.N.; Xue, H.; Kan, K.K.W.; Lee, N.T.K.; et al. Synergistic Neuroprotection by Bis(7)-tacrine via Concurrent Blockade of N-Methyl-d-aspartate Receptors and Neuronal Nitric-Oxide Synthase. Mol. Pharmacol. 2007, 71, 1258–1267. [Google Scholar] [CrossRef]
- Li, W.; Lee, N.T.K.; Fu, H.; Kan, K.K.W.; Pang, Y.-P.; Li, M.; Tsim, K.W.K.; Li, X.; Han, Y. Neuroprotection via inhibition of nitric oxide synthase by bis(7)-tacrine. NeuroReport 2006, 17, 471–474. [Google Scholar] [CrossRef]
- Li, W.; Pi, R.; Chan, H.H.N.; Fu, H.; Lee, N.T.K.; Tsang, H.W.; Pu, Y.; Chang, D.C.; Li, C.; Luo, J.; et al. Novel dimeric acetylcholinesterase inhibitor bis7-tacrine, but not donepezil, prevents glutamate-induced neuronal apoptosis by blocking N-methyl-D-aspartate receptors. J. Biol. Chem. 2005, 280, 18179–18188. [Google Scholar] [CrossRef]
- Nepovimova, E.; Korabecny, J.; Dolezal, R.; Babkova, K.; Ondrejicek, A.; Jun, D.; Sepsova, V.; Horova, A.; Hrabinova, M.; Soukup, O.; et al. Tacrine–Trolox Hybrids: A Novel Class of Centrally Active, Nonhepatotoxic Multi-Target-Directed Ligands Exerting Anticholinesterase and Antioxidant Activities with Low In Vivo Toxicity. J. Med. Chem. 2015, 58, 8985–9003. [Google Scholar] [CrossRef]
- Spilovska, K.; Korabecny, J.; Kral, J.; Horova, A.; Musilek, K.; Soukup, O.; Drtinova, L.; Gazova, Z.; Siposova, K.; Kuca, K. 7-Methoxytacrine-adamantylamine heterodimers as cholinesterase inhibitors in Alzheimer’s disease treatment--synthesis, biological evaluation and molecular modeling studies. Molecules 2013, 18, 2397–2418. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pohanka, M.; Jun, D.; Kuca, K. Improvement of acetylcholinesterase-based assay for organophosphates in way of identification by reactivators. Talanta 2008, 77, 451–454. [Google Scholar] [CrossRef]
- Sepsova, V.; Karasova, J.Z.; Korabecny, J.; Dolezal, R.; Zemek, F.; Bennion, B.J.; Kuca, K. Oximes: inhibitors of human recombinant acetylcholinesterase. A structure-activity relationship (SAR) study. Int. J. Mol. Sci. 2013, 14, 16882–16900. [Google Scholar] [CrossRef]
- Pohanka, M.; Karasova, J.Z.; Kuca, K.; Pikula, J.; Holas, O.; Korabecny, J.; Cabal, J. Colorimetric dipstick for assay of organophosphate pesticides and nerve agents represented by paraoxon, sarin and VX. Talanta 2010, 81, 621–624. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Cavalli, A.; Valgimigli, L.; Bartolini, M.; Rosini, M.; Andrisano, V.; Recanatini, M.; Melchiorre, C. Multi-Target-Directed Drug Design Strategy: From a Dual Binding Site Acetylcholinesterase Inhibitor to a Trifunctional Compound against Alzheimer’s Disease. J. Med. Chem. 2007, 50, 6446–6449. [Google Scholar] [CrossRef]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Nepovimova, E.; Uliassi, E.; Korabecny, J.; Peña-Altamira, L.E.; Samez, S.; Pesaresi, A.; Garcia, G.E.; Bartolini, M.; Andrisano, V.; Bergamini, C.; et al. Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects. J. Med. Chem. 2014, 57, 8576–8589. [Google Scholar] [CrossRef]
- Spilovska, K.; Korabecny, J.; Horova, A.; Musilek, K.; Nepovimova, E.; Drtinova, L.; Gazova, Z.; Siposova, K.; Dolezal, R.; Jun, D.; et al. Design, synthesis and in vitro testing of 7-methoxytacrine-amantadine analogues: a novel cholinesterase inhibitors for the treatment of Alzheimer’s disease. Med. Chem. Res. 2015, 24, 2645–2655. [Google Scholar] [CrossRef]
- Muckova, L.; Pejchal, J.; Jost, P.; Vanova, N.; Herman, D.; Jun, D. Cytotoxicity of acetylcholinesterase reactivators evaluated in vitro and its relation to their structure. Drug Chem. Toxicol. 2019, 42, 252–256. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Li, C.; Wainhaus, S.; Uss, A.S.; Cheng, K.-C. High-Throughput Screening Using Caco-2 Cell and PAMPA Systems. In Drug Absorption Studies: In Situ, In Vitro and In Silico Models; Ehrhardt, C., Kim, K.-J., Eds.; Biotechnology: Pharmaceutical Aspects; Springer US: Boston, MA, USA, 2008; pp. 418–429. ISBN 978-0-387-74901-3. [Google Scholar]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Hepnarova, V.; Korabecny, J.; Matouskova, L.; Jost, P.; Muckova, L.; Hrabinova, M.; Vykoukalova, N.; Kerhartova, M.; Kucera, T.; Dolezal, R.; et al. The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 150, 292–306. [Google Scholar] [CrossRef]
- Rydberg, E.H.; Brumshtein, B.; Greenblatt, H.M.; Wong, D.M.; Shaya, D.; Williams, L.D.; Carlier, P.R.; Pang, Y.-P.; Silman, I.; Sussman, J.L. Complexes of alkylene-linked tacrine dimers with Torpedo californica acetylcholinesterase: Binding of Bis5-tacrine produces a dramatic rearrangement in the active-site gorge. J. Med. Chem. 2006, 49, 5491–5500. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- León, R.; Garcia, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019, 62, 420–444. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Sittampalam, G.S., Coussens, N.P., Nelson, H., Arkin, M., Auld, D., Austin, C., Bejcek, B., Glicksman, M., Inglese, J., Iversen, P.W., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Liu, B.; Wang, L.; Jin, Y.-H. An effective PSO-based memetic algorithm for flow shop scheduling. IEEE Trans. Syst. Man Cybern. Part B Cybern. Publ. IEEE Syst. Man Cybern. Soc. 2007, 37, 18–27. [Google Scholar] [CrossRef]
| Compound | R1 | R2 | n | IC50 (nM) a | SI b | |
|---|---|---|---|---|---|---|
| hAChE | hBChE | |||||
| 3a | H | H | 2 | 3.8 | 62 | 16 |
| 3b | H | H | 3 | 8.4 | 55 | 6.5 |
| 3c | H | H | 4 | 5.5 | 75 | 14 |
| 3d | H | H | 5 | 8.1 | 69 | 8.5 |
| 3e | H | H | 6 | 13 | 21 | 1.6 |
| 3f | H | H | 7 | 32 | 32 | 1 |
| 3g | H | H | 8 | 72 | 50 | 0.7 |
| 4a | Cl | H | 2 | 4.2 | 150 | 36 |
| 4b | Cl | H | 3 | 2.0 | 110 | 55 |
| 4c | Cl | H | 4 | 4.6 | 170 | 37 |
| 4d | Cl | H | 5 | 8.2 | 310 | 38 |
| 4e | Cl | H | 6 | 3.2 | 540 | 170 |
| 4f | Cl | H | 7 | 16 | 450 | 28 |
| 4g | Cl | H | 8 | 10 | ˃100,000 | ˃10,000 |
| 5a | H | OMe | 2 | 170 | 130 | 0.7 |
| 5b | H | OMe | 3 | 1100 | 8900 | 8.1 |
| 5c | H | OMe | 4 | 4500 | 4700 | 0.9 |
| 5d | H | OMe | 5 | 120 | 680 | 5.7 |
| 5e | H | OMe | 6 | 150 | 1100 | 7.3 |
| 5f | H | OMe | 7 | 170 | 1300 | 7.6 |
| 5g | H | OMe | 8 | 490 | 2900 | 5.9 |
| THA c | H | H | - | 320 | 80 | 0.3 |
| 6-Cl-THA c | Cl | H | - | 20 | 1800 | 90 |
| 7-MEOTA c | H | OMe | - | 10,000 | 17,000 | 1.7 |
| Compound | R1 | R2 | n | IC50 (µM) a |
|---|---|---|---|---|
| 3a | H | H | 2 | >256 |
| 3b | H | H | 3 | >32 |
| 3c | H | H | 4 | 150 |
| 3d | H | H | 5 | 69 |
| 3e | H | H | 6 | 76 |
| 3f | H | H | 7 | 23 |
| 3g | H | H | 8 | 6.6 |
| 4a | Cl | H | 2 | >128 |
| 4b | Cl | H | 3 | >64 |
| 4c | Cl | H | 4 | >64 |
| 4d | Cl | H | 5 | >64 |
| 4e | Cl | H | 6 | >64 |
| 4f | Cl | H | 7 | >64 |
| 4g | Cl | H | 8 | >64 |
| 5a | H | OMe | 2 | >256 |
| 5b | H | OMe | 3 | >64 |
| 5c | H | OMe | 4 | 190 |
| 5d | H | OMe | 5 | 98 |
| 5e | H | OMe | 6 | 26 |
| 5f | H | OMe | 7 | 9 |
| 5g | H | OMe | 8 | 3.5 |
| THA | H | H | - | 169 |
| 6-Cl-THA | Cl | H | - | 43 |
| 7-MEOTA | H | OMe | - | 44 |
| Donepezil | - | - | - | 150 |
| Rivastigmine | - | - | - | 3400 |
| Galantamine | - | - | - | 4200 |
| Compound | R1 | R2 | n | Pe (× 10−6 cm.s−1) a | CNS (+/−) b |
|---|---|---|---|---|---|
| 3a | H | H | 3 | 1.1 | CNS − |
| 3e | H | H | 3 | 2.6 | CNS +/− |
| 4b | Cl | H | 3 | 2.5 | CNS +/− |
| 4e | Cl | H | 6 | 17.0 | CNS + |
| 5a | H | OMe | 2 | 2.3 | CNS +/− |
| 5d | H | OMe | 5 | 1.3 | CNS − |
| THA | H | H | - | 6.0 | CNS + |
| donepezil | 21.0 | CNS + | |||
| ibuprofen | 12.0 | CNS + | |||
| furosemide | 0.0 | CNS − | |||
| chlorothiazide | 0.3 | CNS − | |||
| ranitidine | 0.0 | CNS − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svobodova, B.; Mezeiova, E.; Hepnarova, V.; Hrabinova, M.; Muckova, L.; Kobrlova, T.; Jun, D.; Soukup, O.; Jimeno, M.L.; Marco-Contelles, J.; et al. Exploring Structure-Activity Relationship in Tacrine-Squaramide Derivatives as Potent Cholinesterase Inhibitors. Biomolecules 2019, 9, 379. https://doi.org/10.3390/biom9080379
Svobodova B, Mezeiova E, Hepnarova V, Hrabinova M, Muckova L, Kobrlova T, Jun D, Soukup O, Jimeno ML, Marco-Contelles J, et al. Exploring Structure-Activity Relationship in Tacrine-Squaramide Derivatives as Potent Cholinesterase Inhibitors. Biomolecules. 2019; 9(8):379. https://doi.org/10.3390/biom9080379
Chicago/Turabian StyleSvobodova, Barbora, Eva Mezeiova, Vendula Hepnarova, Martina Hrabinova, Lubica Muckova, Tereza Kobrlova, Daniel Jun, Ondrej Soukup, María Luisa Jimeno, José Marco-Contelles, and et al. 2019. "Exploring Structure-Activity Relationship in Tacrine-Squaramide Derivatives as Potent Cholinesterase Inhibitors" Biomolecules 9, no. 8: 379. https://doi.org/10.3390/biom9080379
APA StyleSvobodova, B., Mezeiova, E., Hepnarova, V., Hrabinova, M., Muckova, L., Kobrlova, T., Jun, D., Soukup, O., Jimeno, M. L., Marco-Contelles, J., & Korabecny, J. (2019). Exploring Structure-Activity Relationship in Tacrine-Squaramide Derivatives as Potent Cholinesterase Inhibitors. Biomolecules, 9(8), 379. https://doi.org/10.3390/biom9080379