Ziziphora taurica subsp. taurica: Analytical Characterization and Biological Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Solvent Extraction
2.3. Chemicals
2.4. Quantification of Phenolic Compounds in the Extracts
2.5. Biological Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic (TPC) and Flavonoid (TFC) Content
3.2. LC-ESI-MS/MS Analysis
3.3. Biological Activities
3.3.1. Antioxidant Activities
3.3.2. Enzyme Inhibitory Activities
3.4. Correlations among Phenolic Compounds and Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shahbazi, Y. Chemical compositions, antioxidant and antimicrobial properties of Ziziphora clinopodioides Lam. essential oils collected from different parts of Iran. J. Food Sci. Technol. 2017, 54, 3491–3503. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Diuzheva, A.; Badarau, S.; Moldovan, C.; Andruch, V.; Carradori, S.; Campestre, C.; Tartaglia, A.; De Simone, M.; Vodnar, D.; et al. Liquid phase and microwave-assisted extractions for multicomponent phenolic pattern determination of five Romanian Galium species coupled with bioassays. Molecules 2019, 24, 1226. [Google Scholar] [CrossRef] [PubMed]
- Bekut, M.; Brkic, S.; Kladar, N.; Dragovic, G.; Gavaric, N.; Bozin, B. Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Šmejkal, K.; Malaník, M.; Zhaparkulova, K.; Sakipova, Z.; Ibragimova, L.; Ibadullaeva, G.; Žemlicka, M. Kazakh Ziziphora species as sources of bioactive substances. Molecules 2016, 21, 826. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M. The ethnobotanical, phytochemical and pharmacological properties and medicinal applications of essential oils and extracts of different Ziziphora species. Ind. Crop. Prod. 2017, 105, 164–192. [Google Scholar] [CrossRef]
- Ozturk, S.; Ercisli, S. Antibacterial activity and chemical constitutions of Ziziphora clinopodioides. Food Control 2007, 18, 535–540. [Google Scholar] [CrossRef]
- Senejoux, F.; Demougeot, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bevalot, F.; Girard-Thernier, C. Bioassay-guided isolation of vasorelaxant compounds from Ziziphora clinopodioides Lam. (Lamiaceae). Fitoterapia 2012, 83, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Wang, H.M.; Chen, C.Y.; Chen, C.Y.; Ho, M.L.; Chou, Y.T.; Chang, H.C.; Lee, C.H.; Wang, C.Z.; Chu, I.M. (-)-N-Formylanonaine from Michelia alba as a human tyrosinase inhibitor and antioxidant. Bioorg. Med. Chem. 2010, 18, 5241–5247. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Kirkan, B.; Ozer, M.S.; Ceylan, O.; Atilgan, N.; Cengiz, M.; Tepe, B. Chemical characterization and biological activity of Onosma gigantea extracts. Ind. Crop. Prod. 2018, 115, 323–329. [Google Scholar] [CrossRef]
- Genovese, S.; Fiorito, S.; Locatelli, M.; Carlucci, G.; Epifano, F. Analysis of biologically active oxyprenylated ferulic acid derivatives in Citrus fruits. Plant Food Hum. Nutr. 2014, 69, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Llorent-Martínez, E.J.; Córdova, M.L.F.-D.; Bahadori, M.B.; Mocan, A.; Locatelli, M.; Aktumsek, A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crop. Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B.; Kocak, M.S.; Uren, M.C. Metal concentration and antioxidant activity of edible mushrooms from Turkey. Food Chem. 2015, 175, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Tlili, N.; Kirkan, B.; Sarikurkcu, C. LC–ESI–MS/MS characterization, antioxidant power and inhibitory effects on α-amylase and tyrosinase of bioactive compounds from hulls of Amygdalus communis: The influence of the extracting solvents. Ind. Crop. Prod. 2019, 128, 147–152. [Google Scholar] [CrossRef]
- Cittan, M.; Çelik, A. Development and validation of an analytical methodology based on liquid chromatography–electrospray tandem mass spectrometry for the simultaneous determination of phenolic compounds in olive leaf extract. J. Chromatogr. Sci. 2018, 56, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçaǧ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Kocak, M.S.; Sarikurkcu, C.; Cengiz, M.; Kocak, S.; Uren, M.C.; Tepe, B. Salvia cadmica: Phenolic composition and biological activity. Ind. Crop. Prod. 2016, 85, 204–212. [Google Scholar] [CrossRef]
- Odabas Kose, E.; Aktaş, O.; Deniz, I.G.; Sarikürkçü, C. Chemical composition, antimicrobial and antioxidant activity of essential oil of endemic Ferula lycia Boiss. J. Med. Plants Res. 2010, 4, 1698–1703. [Google Scholar]
- Zengin, G.; Sarikurkcu, C.; Uyar, P.; Aktumsek, A.; Uysal, S.; Kocak, M.S.; Ceylan, R. Crepis foetida L. subsp rhoeadifolia (Bleb.) Celak. as a source of multifunctional agents: Cytotoxic and phytochemical evaluation. J. Funct. Foods 2015, 17, 698–708. [Google Scholar] [CrossRef]
- Kuo, C.T.; Liu, T.H.; Hsu, T.H.; Lin, F.Y.; Chen, H.Y. Antioxidant and antiglycation properties of different solvent extracts from Chinese olive (Canarium album L.) fruit. Asian. Pac. J. Trop. Med. 2015, 8, 1013–1021. [Google Scholar] [CrossRef]
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, G.; Yue, J.; Qian, B.; Liu, Z.; Wang, D.; Zhong, Y.; Zhao, Y. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control 2014, 38, 184–191. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungord, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC–DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef] [PubMed]

| Extracts | Abbreviation | Yield (%) | Total Phenolics (mg GAEs/g extract) | Total Flavonoids (mg QEs/g extract) |
|---|---|---|---|---|
| Ethyl Acetate | ZTT-EtOAc | 3.23 | 34.82 ± 0.79 a | 20.87 ± 1.38 b |
| Methanol | ZTT-MeOH | 8.93 | 27.49 ± 0.74 b | 37.39 ± 2.54 a |
| Water | ZTT-W | 13.69 | 15.10 ± 0.45 c | 7.08 ± 0.58 c |
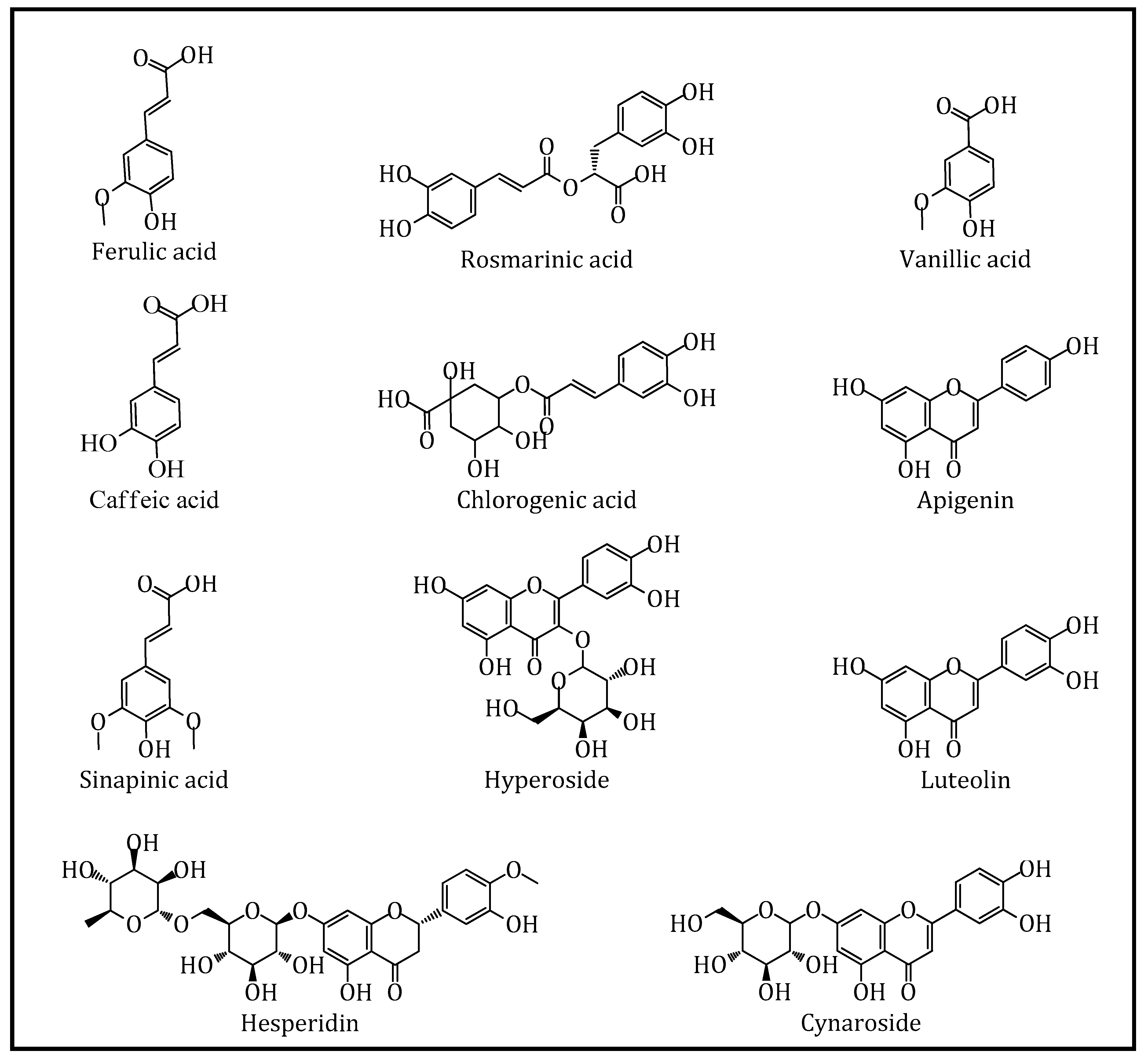
| Compound | ZTT-EtOAc | ZTT-MeOH | ZTT-W | Linear Equation | R2 | LOD (μg/L) | LOQ (μg/L) |
|---|---|---|---|---|---|---|---|
| (-)-Epicatechin | nd | nd | nd | y = 9.11x−9.99 | 0.9971 | 1.85 | 6.18 |
| (+)-Catechin | nd | 11.79 ± 0.21 | nd | y = 1.45x+1.95 | 0.9974 | 3.96 | 13.20 |
| 2,5-Dihydroxybenzoic Acid | 15.98 ± 0.84 c | 60.48 ± 1.77 b | 268.65 ± 5.24 a | y = 3.79x−14.12 | 0.9980 | 2.12 | 7.08 |
| 2-Hydroxycinnamic Acid | nd | nd | nd | y = 16.72x−26.94 | 0.9996 | 0.61 | 2.03 |
| 3,4-Dihydroxyphenylacetic Acid | nd | 3.20 ± 0.21 b | 17.87 ± 0.11 a | y = 5.13x−12.39 | 0.9990 | 1.35 | 4.51 |
| 3-Hydroxybenzoic Acid | 10.75 ± 0.38 a | 9.16 ± 0.58 ab | 8.12 ± 0.72 c | y = 3.69x−12.29 | 0.9991 | 1.86 | 6.20 |
| 4-Hydroxybenzoic Acid | 74.92 ± 0.60 b | 76.71 ± 0.88 b | 94.84 ± 1.44 a | y = 7.62x+22.79 | 0.9996 | 1.72 | 5.72 |
| Apigenin | 1457.99 ± 24.48 a | 1270.90 ± 0.30 b | 201.25 ± 3.53 c | y = 11.29x+38.05 | 0.9987 | 0.96 | 3.20 |
| Apigenin 7-glucoside | 293.07 ± 1.22 b | 847.22 ± 5.96 a | nd | y = 21.33x−31.69 | 0.9983 | 0.41 | 1.35 |
| Caffeic acid | 17.42 ± 0.07 c | 44.12 ± 0.92 b | 415.94 ± 2.44 a | y = 11.09x+16.73 | 0.9997 | 3.15 | 10.50 |
| Chlorogenic Acid | 48.33 ± 13.46 c | 136.14 ± 1.56 b | 2135.92 ± 2.02 a | y = 12.14x+32.34 | 0.9995 | 0.55 | 1.82 |
| Eriodictyol | 82.66 ± 0.31 a | 31.32 ± 0.54 b | 7.39 ± 0.25 c | y = 14.24x−0.50 | 0.9998 | 0.80 | 2.68 |
| Ferulic Acid | 1478.13 ± 24.89 b | 1540.91 ± 1.31 a | 1118.92 ± 7.20 c | y = 3.32x−4.30 | 0.9992 | 1.43 | 4.76 |
| Gallic Acid | nd | 4.13 ± 0.10 a | 3.66 ± 0.07 b | y = 4.82x−26.48 | 0.9988 | 1.46 | 4.88 |
| Hesperidin | 63.47 ± 3.16 c | 2389.83 ± 16.55 a | 158.20 ± 0.13 b | y = 5.98x+0.42 | 0.9993 | 1.73 | 5.77 |
| Hyperoside | 117.67 ± 0.07 c | 956.75 ± 3.28 a | 139.13 ± 4.04 b | y = 16.32x−1.26 | 0.9998 | 0.99 | 3.31 |
| Kaempferol | nd | nd | 3.17 ± 0.36 | y = 0.82x−3.06 | 0.9959 | 3.30 | 10.99 |
| Luteolin | 5347.32 ± 54.96 a | 5339.91 ± 72.76 a | 780.53 ± 32.25 b | y = 8.96x+26.80 | 0.9992 | 1.34 | 4.46 |
| Luteolin 7-glucoside | 270.77 ± 3.42 b | 1281.14 ± 39.03 a | 9.80 ± 0.66 c | y = 45.25x+156.48 | 0.9996 | 0.45 | 1.51 |
| p-Coumaric Acid | 97.06 ± 1.72 c | 109.55 ± 0.73 b | 174.73 ± 2.53 a | y = 17.51x+53.73 | 0.9997 | 1.93 | 6.44 |
| Pinoresinol | 262.78 ± 0.40 a | 79.11 ± 0.85 c | 107.54 ± 0.52 b | y = 0.80x−2.69 | 0.9966 | 3.94 | 13.12 |
| Protocatechuic Acid | 74.35 ± 1.88 c | 175.75 ± 1.86 b | 378.73 ± 1.26 a | y = 5.65x−9.99 | 0.9990 | 1.17 | 3.88 |
| Pyrocatechol | nd | nd | nd | y = 0.11x−0.52 | 0.9916 | 9.62 | 32.08 |
| Quercetin | nd | 12.82 ± 0.20 b | 57.12 ± 0.70 a | y = 14.68x−18.25 | 0.9997 | 1.23 | 4.10 |
| Rosmarinic Acid | 29.39 ± 1.49 c | 524.65 ± 14.24 b | 2074.40 ± 3.55 a | y = 9.82x−17.98 | 0.9989 | 0.57 | 1.89 |
| Sinapic Acid | 449.82 ± 15.76 a | 35.79 ± 0.30 b | 23.75 ± 0.77 b | y = 2.09x−6.79 | 0.9974 | 2.64 | 8.78 |
| Syringic Acid | 76.05 ± 3.45 c | 103.67 ± 2.51 b | 157.88 ± 2.09 a | y = 0.74x−1.54 | 0.9975 | 3.75 | 12.50 |
| Taxifolin | nd | nd | 0.95 ± 0.32 | y = 12.32x+9.98 | 0.9993 | 1.82 | 6.05 |
| Vanillic Acid | 694.01 ± 64.38 a | 735.80 ± 24.67 a | 476.23 ± 9.98 b | y = 0.49x−1.61 | 0.9968 | 2.56 | 8.54 |
| Vanillin | 31.36 ± 1.39 a | 15.89 ± 0.29 b | nd | y = 2.02x+135.49 | 0.9926 | 15.23 | 50.77 |
| Verbascoside | 41.47 ± 12.61 a | 4.57 ± 0.31 b | 41.99 ± 0.66 a | y = 8.59x−28.05 | 0.9988 | 0.82 | 2.75 |
| Assays | ZTT-EtOAc | ZTT-MeOH | ZTT-W | Trolox | EDTA |
|---|---|---|---|---|---|
| Inhibition Concentration (IC50: mg/mL) | |||||
| DPPH Radical Scavenging | 15.75 ± 0.66 c | 5.74 ± 0.08 b | 7.02 ± 0.23 b | 0.25 ± 0.01 a | - |
| ABTS Radical Scavenging | 6.30 ± 0.03 d | 2.74 ± 0.10 c | 2.39 ± 0.10 b | 0.26 ± 0.01 a | - |
| Phosphomolybdenum | 2.60 ± 0.03 c | 1.84 ± 0.08 b | 3.80 ± 0.17 d | 1.15 ± 0.01 a | - |
| CUPRAC Reducing Power | 2.39 ± 0.10 b | 2.24 ± 0.11 b | 2.80 ± 0.02 c | 0.31 ± 0.02 a | - |
| FRAP Reducing Power | 3.41 ± 0.28 c | 1.42 ± 0.04 b | 1.71 ± 0.02 b | 0.1 ± 0.01 a | - |
| Ferrous Ion Chelating | 51.40 ± 1.32 c | 3.77 ± 0.09 b | 1.04 ± 0.01 b | - | 0.034 ± 0.003 a |
| Antioxidant Activity | |||||
| DPPH Radical Scavenging (mg TE/g extract) | 15.01 ± 0.68 c | 42.15 ± 0.6 1a | 33.99 ± 1.21 b | - | - |
| ABTS Radical Scavenging (mg TE/g extract) | 42.06 ± 0.22 c | 97.01 ± 3.45 b | 111.94 ± 4.74 a | - | - |
| Phosphomolybdenum (mg TE/g extract) | 446.22 ± 4.94 b | 630.08 ± 28.80 a | 304.30 ± 13.99 c | - | - |
| CUPRAC Reducing Power (mg TE/g extract) | 131.89 ± 5.95 a | 134.66 ± 7.00 a | 105.47 ± 0.70 b | - | - |
| FRAP Reducing Power (mg TE/g extract) | 30.79 ± 2.55 c | 73.62 ± 1.85 a | 61.27 ± 0.67 b | - | - |
| Ferrous Ion Chelating (mg EDTAE/g extract) | 1.03 ± 0.04 c | 18.53 ± 0.47 b | 69.31 ± 0.18 a | - | - |
| Assays | ZTT-EtOAc | ZTT-MeOH | ZTT-W | Kojic acid | Acarbose |
|---|---|---|---|---|---|
| Inhibition Concentration (IC50: mg/mL) | |||||
| Tyrosinase Inhibition | 1.37 ± 0.07 b | 1.46 ± 0.06 b | 2.29 ± 0.13 c | 0.37 ± 0.02 a | - |
| α-Amylase Inhibition | 1.82 ± 0.08 ab | 2.69 ± 0.14 b | 62.56 ± 0.56 c | - | 1.21 ± 0.07 a |
| Enzyme inhibition activity | |||||
| Tyrosinase Inhibition (mg KAE/g extract) | 262.76 ± 13.82 a | 246.27 ± 9.75 a | 156.88 ± 8.88 b | - | - |
| α-Amylase Inhibition (mg ACE/g extract) | 672.87 ± 28.68 a | 452.44 ± 23.81 b | 16.03 ± 0.18 c | - | - |
| Assays and Compounds | Tyrosinase | α-Amylase | CUPRAC | FRAP | ABTS | DPPH | Phosphomolybdenum | Ferrous Ion Chelating |
|---|---|---|---|---|---|---|---|---|
| α-Amylase | 0.982 | 1 | ||||||
| CUPRAC | 0.973 | 0.912 | 1 | |||||
| FRAP | –0.376 | –0.544 | –0.153 | 1 | ||||
| ABTS | –0.766 | –0.874 | –0.599 | 0.883 | 1 | |||
| DPPH | –0.363 | –0.533 | –0.14 | 0.999 z | 0.877 | 1 | ||
| Phosphomolybdenum | 0.736 | 0.595 | 0.872 | 0.350 | –0.13 | 0.363 | 1 | |
| Ferrous Ion Chelating | –0.995 | –0.996 | –0.944 | 0.470 | 0.829 | 0.458 | –0.662 | 1 |
| Total Flavonoid | 0.751 | 0.612 | 0.883 | 0.330 | –0.152 | 0.342 | 0.999 y | –0.679 |
| Total Phenolic | 0.973 | 0.999 y | 0.895 | –0.578 | –0.893 | –0.567 | 0.562 | –0.992 |
| Caffeic Acid | –0.996 | –0.962 | –0.989 | 0.295 | 0.709 | 0.282 | –0.791 | 0.982 |
| Vanillic Acid | 0.957 | 0.884 | 0.988 | –0.09 | –0.543 | –0.091 | 0.901 | –0.919 |
| Chlorogenic Acid | –0.994 | –0.956 | –0.992 | 0.273 | 0.693 | 0.260 | –0.805 | 0.978 |
| Sinapic Acid | 0.639 | 0.774 | 0.446 | –0.953 | –0.984 | –0.949 | –0.049 | –0.716 |
| Ferulic Acid | 0.960 | 0.890 | 0.999 y | –0.101 | –0.556 | –0.088 | 0.896 | –0.926 |
| Luteolin 7-glucoside | 0.543 | 0.374 | 0.721 | 0.574 | 0.124 | 0.585 | 0.968 | –0.453 |
| Hesperidin | 0.336 | 0.151 | 0.543 | 0.747 | 0.348 | 0.756 | 0.884 | –0.236 |
| Hyperoside | 0.348 | 0.164 | 0.554 | 0.738 | 0.335 | 0.747 | 0.891 | –0.249 |
| Rosmarinic Acid | –0.996 | –0.995 | –0.949 | 0.456 | 0.820 | 0.444 | –0.673 | 0.999 z |
| Luteolin | 0.990 | 0.945 | 0.996 | –0.239 | –0.666 | –0.226 | 0.826 | –0.969 |
| Apigenin | 0.999 z | 0.981 | 0.975 | –0.369 | –0.762 | –0.357 | 0.741 | –0.994 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, M.; Ceylan, O.; Locatelli, M.; Tartaglia, A.; Ferrone, V.; Sarikurkcu, C. Ziziphora taurica subsp. taurica: Analytical Characterization and Biological Activities. Biomolecules 2019, 9, 367. https://doi.org/10.3390/biom9080367
Tomczyk M, Ceylan O, Locatelli M, Tartaglia A, Ferrone V, Sarikurkcu C. Ziziphora taurica subsp. taurica: Analytical Characterization and Biological Activities. Biomolecules. 2019; 9(8):367. https://doi.org/10.3390/biom9080367
Chicago/Turabian StyleTomczyk, Michał, Olcay Ceylan, Marcello Locatelli, Angela Tartaglia, Vincenzo Ferrone, and Cengiz Sarikurkcu. 2019. "Ziziphora taurica subsp. taurica: Analytical Characterization and Biological Activities" Biomolecules 9, no. 8: 367. https://doi.org/10.3390/biom9080367
APA StyleTomczyk, M., Ceylan, O., Locatelli, M., Tartaglia, A., Ferrone, V., & Sarikurkcu, C. (2019). Ziziphora taurica subsp. taurica: Analytical Characterization and Biological Activities. Biomolecules, 9(8), 367. https://doi.org/10.3390/biom9080367







