Insights on the Use of α-Lipoic Acid for Therapeutic Purposes
Abstract
:1. Introduction
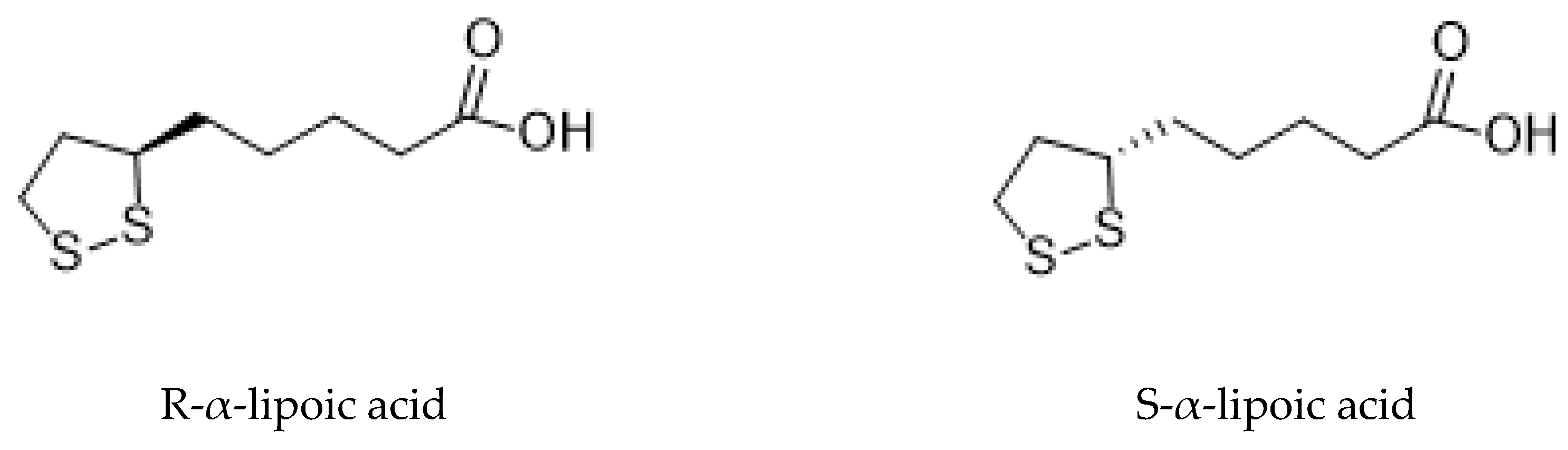
1.1. Forms of Lipoic Acid
1.1.1. R-α-Lipoic Acid
1.1.2. S-α-Lipoic Acid
2. Research Methodology
3. α-Lipoic Acid Pharmacological Activities: An Overview
3.1. α-Lipoic Acid Antioxidant Potential
3.2. α-Lipoic Acid Antidiabetic Potential
3.3. α-Lipoic Acid and Alzheimer’s Disease
3.4. α-Lipoic Acid and Cancer
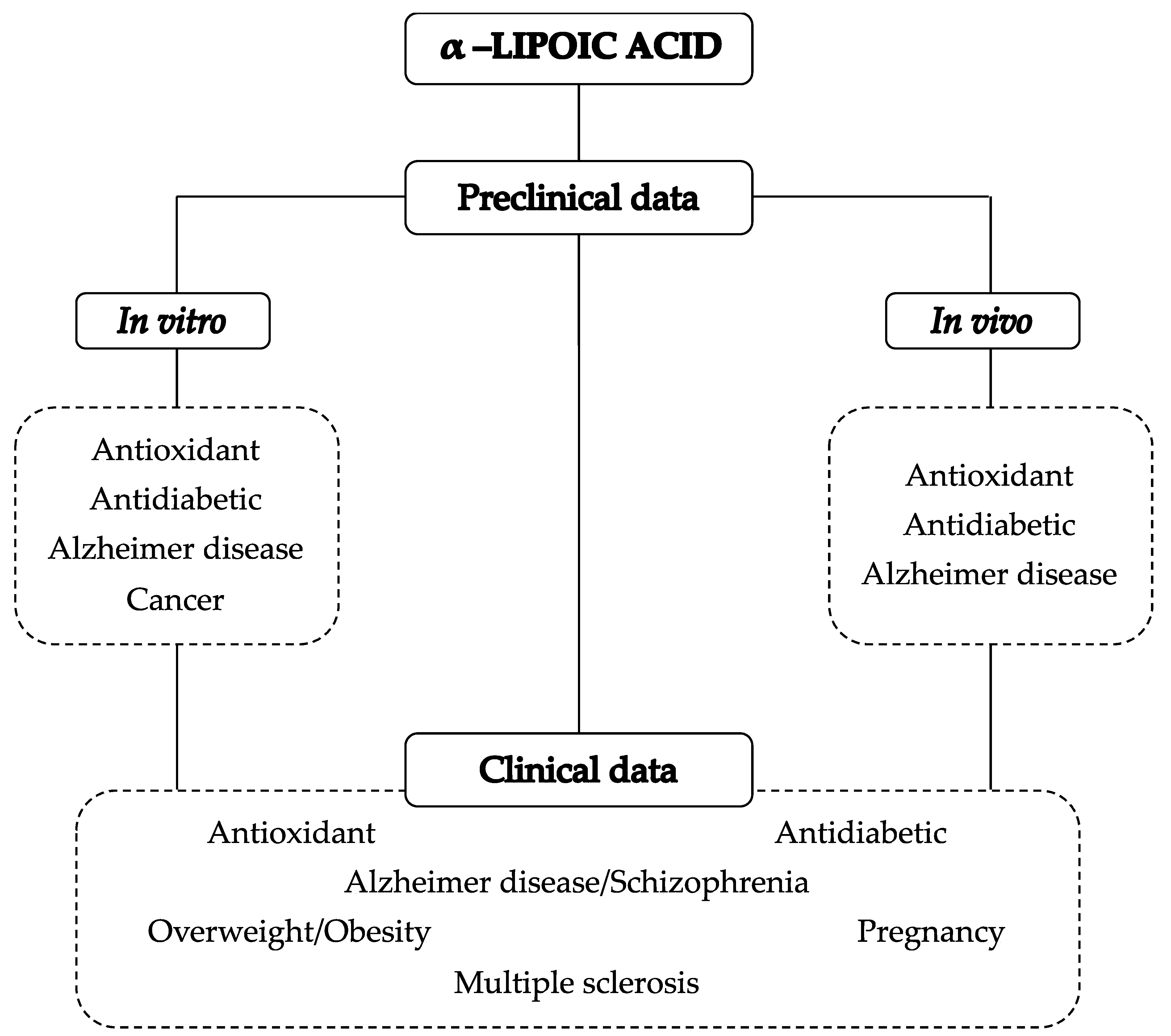
4. Preclinical Actability of α-Lipoic Acid
4.1. Anti-diabetic Properties of α-Lipoic Acid
4.2. α-Lipoic Acid and Alzheimer’s Disease
4.3. α-Lipoic Acid and Pregnancy
5. Pharmacokinetics of α-Lipoic Acid
5.1. Bioavailability of Lipoic Acid Through Food Sources
5.2. Lipoic Acid Absorption and Plasma Concentrations
5.3. Effect of Different Formulations on Lipoic acid Bioavailability
5.4. Age- and Gender-Dependent α-Lipoic Acid Bioavailability
6. α-Lipoic Acid in Clinical Trials
6.1. The Effects of α-Lipoic Acid on Diabetic Patients with Neuropathy
6.2. Effects of α-Lipoic Acid in Overweight/Obese Patients
6.3. Effects of α-Lipoic Acid on Patients with Schizophrenia
6.4. Effects of α-Lipoic Acid in Patients with Multiple Sclerosis
6.5. Effects of α-Lipoic Acid on Abnormalities in Pregnancy
6.6. Other Trials
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reed, L.J.; DeBusk, B.G.; Gunsalus, I.C.; Hornberger, C.S. Crystalline α-lipoic acid: A catalytic agent associated with pyruvate dehydrogenase. Science 1951, 114, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Bock, E.; Schneeweiss, J. Ein Beitrag zur Therapie der Neuropathia diabetica. Munch. Med. Wochenschr. 1959, 43, 1911–1912. [Google Scholar]
- Brookes, M.H.; Golding, B.T.; Howes, D.A.; Hudson, A.T. Proof that the absolute configuration of natural α-lipoic acid is R by the synthesis of its enantiomer [(S)-(–)-α-lipoic acid] from (S)-malic acid. J. Chem. Soc. Chem. Commun. 1983, 19, 1051–1053. [Google Scholar] [CrossRef]
- Ghibu, S.; Richard, C.; Vergely, C.; Zeller, M.; Cottin, Y.; Rochette, L. Antioxidant properties of an endogenous thiol: Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J. Cardiovasc. Pharmacol. 2009, 54, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Brufani, M. Acido α-lipoico farmaco o integratore. Una panoramica sulla farmacocinetica, le formulazioni disponibili e le evidenze cliniche nelle complicanze del diabete. Prog. Nutr. 2014, 16, 62–74. [Google Scholar]
- Singh, U.; Jialal, I. Retracted: Alpha-lipoic acid supplementation and diabetes. Nutr. Rev. 2008, 66, 646–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maglione, E.; Marrese, C.; Migliaro, E.; Marcuccio, F.; Panico, C.; Salvati, C.; Citro, G.; Quercio, M.; Roncagliolo, F.; Torello, C.; et al. Increasing bioavailability of (R)-alpha-lipoic acid to boost antioxidant activity in the treatment of neuropathic pain. Acta Bio-Medica Atenei Parm. 2015, 86, 226–233. [Google Scholar]
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2010, 48, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Konrad, D.; Somwar, R.; Sweeney, G.; Yaworsky, K.; Hayashi, M.; Ramlal, T.; Klip, A. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: Potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001, 50, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Kang, C.-H.; Wang, S.-G.; Lee, H.-M. α-Lipoic acid regulates lipid metabolism through induction of sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia 2012, 55, 1824–1835. [Google Scholar] [CrossRef]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid–biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Han, D.; Handelman, G.; Marcocci, L.; Sen, C.K.; Roy, S.; Kobuchi, H.; Tritschler, H.J.; Flohé, L.; Packer, L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors 1997, 6, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, P.; Tritschler, H.J.; Wolff, S.P. Thioctic (lipoic) acid: A therapeutic metal-chelating antioxidant? Biochem. Pharmacol. 1995, 50, 123–126. [Google Scholar] [CrossRef]
- Bilska, A.; Wlodek, L. Lipoic acid-the drug of the future. Pharmacol. Rep. 2005, 57, 570–577. [Google Scholar]
- Castro, M.C.; Villagarcía, H.G.; Massa, M.L.; Francini, F. Alpha-lipoic acid and its protective role in fructose induced endocrine-metabolic disturbances. Food Funct. 2019, 10, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.J.; Butler, J.A.; Bemer, B.; Dixon, B.; Johnson, S.; Garrard, M.; Sudakin, D.L.; Christensen, J.M.; Pereira, C.; Hagen, T.M. Age and gender dependent bioavailability of R- and R,S-alpha-lipoic acid: A pilot study. Pharmacol. Res. 2012, 66, 199–206. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. alpha-Lipoic acid as a biological antioxidant. Free Radic. Boil. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Carreau, J.-P. [32] Biosynthesis of lipoic acid via unsaturated fatty acids. Meth. Enzymol. 1979, 62, 152–158. [Google Scholar]
- Ziegler, D. Thioctic acid for patients with symptomatic diabetic polyneuropathy: A critical review. Treat Endocrino 2004, 3, 173–179. [Google Scholar] [CrossRef]
- Henriksen, E.J. Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic. Boil. Med. 2006, 40, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.; Bakal, U. The effect of lipoic acid on macro and trace metal levels in living tissues exposed to oxidative stress. Anti-Cancer Agents Med. Chem. 2009, 9, 560–568. [Google Scholar] [CrossRef]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Szeląg, M.; Mikulski, D.; Molski, M. Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites. J. Mol. Modeling 2012, 18, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Akiba, S.; Matsugo, S.; Packer, L.; Konishi, T. Assay of protein-bound lipoic acid in tissues by a new enzymatic method. Anal. Biochem. 1998, 258, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.A.; de Andrade, K.Q.; dos Santos, J.C.; Goulart, M.O. Lipoic acid: Its antioxidant and anti-inflammatory role and clinical applications. Curr. Topics Med. Chem. 2015, 15, 458–483. [Google Scholar]
- Gomes, M.B.; Negrato, C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6, 80. [Google Scholar] [CrossRef]
- Smith, A.R.; Shenvi, S.V.; Widlansky, M.; Suh, J.H.; Hagen, T.M. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004, 11, 1135–1146. [Google Scholar] [CrossRef]
- Liu, J.; Head, E.; Gharib, A.M.; Yuan, W.; Ingersoll, R.T.; Hagen, T.M.; Cotman, C.W.; Ames, B.N. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-L-carnitine and/or R-α-lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 2356–2361. [Google Scholar] [CrossRef]
- Han, D.; Sen, C.K.; Roy, S.; Kobayashi, M.S.; Tritschler, H.J.; Packer, L. Protection against glutamate-induced cytotoxicity in C6 glial cells by thiol antioxidants. Am. J. Physiol. Integr. Comp. Physiol. 1997, 273, 1771–1778. [Google Scholar] [CrossRef]
- Wray, D.W.; Nishiyama, S.K.; Harris, R.A.; Zhao, J.; McDaniel, J.; Fjeldstad, A.S.; Richardson, R.S. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 2012, 59, 818–824. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.M.; Davison, G.W.; Murphy, M.H.; Nadeem, N.; Trinick, T.; Duly, E.; McEneny, J. Effect of α-lipoic acid and exercise training on cardiovascular disease risk in obesity with impaired glucose tolerance. Lipids Heal. Dis. 2011, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Kherada, N.; Farrar, B.; Kampfrath, T.; Chung, Y.; Simonetti, O.; Deiuliis, J.; Desikan, R.; Khan, B.; Villamena, F.; et al. Lipoic acid effects on established atherosclerosis. Life Sci. 2010, 86, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Karunakaran, U.; Jeoung, N.H.; Jeon, J.-H.; Lee, I.-K. Physiological effect and therapeutic application of alpha lipoic acid. Curr. Med. Chem. 2014, 21, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- El Barky, A.R.; Hussein, S.A.; Mohamed, T.M. The potent antioxidant alpha lipoic acid. J. Plant Chem. Ecophysiol. 2017, 2, 1016. [Google Scholar]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. Vasc. Syst. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Goralska, M.; Dackor, R.; Holley, B.; McGahan, M.C. Alpha lipoic acid changes iron uptake and storage in lens epithelial cells. Exp. Eye Res. 2003, 76, 241–248. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Tsuchiya, M.; Packer, L. Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Radic. Res. Commun. 1991, 15, 255–263. [Google Scholar] [CrossRef]
- Scott, B.C.; Aruoma, O.I.; Evans, P.J.; O’Neill, C.; Van der Vliet, A.; Cross, C.E.; Tritschler, H.; Halliwell, B. Lipoic and dihydrolipoic acids as antioxidants. A critical evaluation. Free Radic. Res. 1994, 20, 119–133. [Google Scholar] [CrossRef]
- Islam, M.T. Antioxidant activities of dithiol alpha-lipoic acid. Bangladesh J. Med. Sci. 2009, 8, 34–49. [Google Scholar] [CrossRef]
- WHO. Diabetes; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Moodley, K.; Joseph, K.; Naidoo, Y.; Islam, S.; Mackraj, I. Antioxidant, antidiabetic and hypolipidemic effects of Tulbaghia violacea Harv. (wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement. Altern. Med. 2015, 15, 408. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. J. Am. Med. Assoc. 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative Stress in Diabetes: Implications for Vascular and Other Complications. Int. J. Mol. Sci. 2013, 14, 21525–21550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Eason, R.C.; Archer, H.E.; Akhtar, S.; Bailey, C.J. Lipoic acid increases glucose uptake by skeletal muscles of obesediabetic ob/ob mice. Diabetes Obes. Metab. 2002, 4, 29–35. [Google Scholar] [CrossRef] [PubMed]
- García-Osta, A.; Cuadrado-Tejedor, M.; García-Barroso, C.; Oyarzábal, J.; Franco, R. Phosphodiesterases as therapeutic targets for Alzheimer’s disease. ACS Chem. Neurosci. 2012, 3, 832–844. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Huang, Y.Y.; Wu, D.; Luo, H.B. Novel phosphodiesterase inhibitors for cognitive improvement in Alzheimer’s disease. J. Med. Chem. 2018, 61, 5467–5483. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.; Cash, A.D.; Smith, M.A. Alzheimer disease and oxidative stress. J. Biomed. Biotechnol. 2002, 2, 120–123. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Cacciatore, I.; Marinelli, L.; Fornasari, E.; Cerasa, L.S.; Eusepi, P.; Türkez, H.; Pomilio, C.; Reale, M.; D’Angelo, C.; Costantini, E.; et al. Novel NSAID-derived drugs for the potential treatment of Alzheimer’s disease. Int. J. Mol. Sci. 2016, 17, 1035. [Google Scholar] [CrossRef] [PubMed]
- Hagen, T.M.; Ingersoll, R.T.; Lykkesfeldt, J.; Liu, J.; Wehr, C.M.; Vinarsky, V.; Bartholomew, J.C.; Ames, A.B. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999, 13, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Poon, H.F.; Dogrukol-Ak, D.; Drake, J.; Banks, W.A.; Eyerman, E.; Allan Butterfield, D.; Morley, J.E. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J. Neurochem. 2003, 84, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hirohata, M.; Yamada, M. α-Lipoic acid exhibits anti-amyloidogenicity for β-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2006, 341, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Frei, B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001, 15, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.; Xiong, S.; Markesbery, W. Protection against amyloid beta peptide and iron/hydrogen peroxide toxicity by alpha lipoic acid. J. Alzheimer’s Dis. 2003, 5, 229–239. [Google Scholar] [CrossRef]
- Holmquist, L.; Stauchbury, G.; Berbaum, K.; Muscat, S.; Young, S.; Hager, K.; Engel, J.; Münch, G. Lipoic acid as a novel treatment for Alzheimer’s disease and related demenias. Pharmacol. Ther. 2007, 113, 154–164. [Google Scholar] [CrossRef]
- Haugaard, N.; Levin, R.M. Regulation of the activity of choline acetyl transferase by lipoic acid. Mol. Cell. Biochem. 2000, 213, 61–63. [Google Scholar] [CrossRef]
- Meraz-Ríos, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernández, J.; Campos-Peña, V. Inflammatory process in Alzheimer’s disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef]
- Ooi, L.; Patel, M.; Münch, G. The thiol antioxidant lipoic acid and Alzheimer’s disease. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 2275–2288. [Google Scholar]
- Suh, J.H.; Wang, H.; Liu, R.-M.; Liu, J.K.; Hagena, T.M. (R)-α-Lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: Evidence for increased cysteine requirement for zGSH synthesis. Arch. Biochem. Biophys. 2015, 423, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hardas, S.S.; Sultana, R.; Clark, A.M.; Beckett, T.L.; Szweda, L.I.; Murphy, P.; Butterfielda, D.A. Oxidative modification of lipoic acid by HNE in Alzheimer disease brain. Redox Biol. 2013, 1, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Dukic-Stefanovic, S.; Gasic-Milenkovic, J.; Schinzel, R.; Wiesinger, H.; Riederer, P.; Münch, G. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur. J. Neurosci. 2001, 14, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Proietti, S.; Cucina, A.; Bizzarri, M.; Fuso, A.J.A. Alpha-Lipoic Acid Downregulates IL-1β and IL-6 by DNA Hypermethylation in SK-N-BE Neuroblastoma Cells. Antioxidant 2017, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Abolhassani, M.; Guais, A.; Sanders, E.; Steyaert, J.M.; Campion, F.; Israël, M. A combination of alpha lipoic acid and calcium hydroxycitrate is efficient against mouse cancer models: Preliminary results. Oncol. Rep. 2010, 23, 1407–1420. [Google Scholar] [CrossRef]
- Na, M.H.; Seo, E.Y.; Kim, W.K. Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr. Res. Pract. 2009, 3, 265–271. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Liang, Y.; Wu, R.; Zhao, Y.; Hong, X.; Lin, M.; Yu, H.; Liu, L.; Levine, A.J.; et al. Tumour-associated mutant p53 drives the Warburg effect. Nat. Commun. 2013, 4, 2935. [Google Scholar] [CrossRef]
- Feuerecker, B.; Pirsig, S.; Seidl, C.; Aichler, M.; Feuchtinger, A.; Bruchelt, G.; Senekowitsch-Schmidtke, R. Lipoic acid inhibits cell proliferation of tumor cells in vitro and in vivo. Cancer Biol. Ther. 2012, 13, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.J.; Kim, W.G.; Lim, S.; Choi, H.J.; Sim, S.; Kim, T.Y.; Shong, Y.K.; Kim, W.B. Alpha lipoic acid inhibits proliferation and epithelial mesenchymal transition of thyroid cancer cells. Mol. Cell. Endocrinol. 2016, 419, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, Y.; Lv, G.; Lin, Y.; Tang, J.; Lu, J.; Zhang, M.; Liu, W.; Sun, X. a-Lipoic acid inhibits human lung cancer cell proliferation through Grb2-mediated EGFR down regulation. Biochem. Biophys. Res. Commun. 2017, 494, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Moungjaroen, J.; Nimmannit, U.; Callery, P.S.; Wang, L.; Azad, N.; Lipipun, V.; Chanvorachote, P.; Rojanasakul, Y. Reactive oxygen species mediate caspase activation and apoptosis induced by lipoic acid in human lung epithelial cancer cells through Bcl-2 down-regulation. J. Pharmacol. Exp. Ther. 2006, 319, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Ruscica, M.; Passafaro, L.; Dogliotti, G.; Steffani, L.; Marthyn, P.; Pagani, A.; Demartini, G.; Esposti, D.; Fraschini, F.; et al. The natural antioxidant alpha-lipoic acid induces p27(Kip1)-dependent cell cycle arrest and apoptosis in MCF-7 human breast cancer cells. Eur. J. Pharmacol. 2010, 641, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Nickel, A.; Daniel, H. α-Lipoic acid induces apoptosis in human colon cancer cells by increasing mitochondrial respiration with a concomitant O2-*-generation. Apoptosis 2005, 10, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.P.; Jena, G.B. Role of α-lipoic acid in dextran sulfate sodium-induced ulcerative colitis in mice: Studies on inflammation, oxidative stress, DNA damage and fibrosis. Food Chem. Toxicol. 2013, 59, 339–355. [Google Scholar] [CrossRef]
- Tripathy, J.; Tripathy, A.; Thangaraju, M.; Suar, M.; Elangovan, S. alpha-Lipoic acid inhibits the migration and invasion of breast cancer cells through inhibition of TGFbeta signaling. Life Sci. 2018, 207, 15–22. [Google Scholar] [CrossRef]
- Lee, W.J.; Song, K.H.; Koh, E.H.; Won, J.C.; Kim, H.S.; Park, H.S.; Kim, M.S.; Kim, S.W.; Lee, K.U.; Park, J.Y. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 885–891. [Google Scholar] [CrossRef]
- Bitar, M.S.; Ayed, A.K.; Abdel-Halim, S.M.; Isenovic, E.R.; Al-Mulla, F. Inflammation and apoptosis in aortic tissues of aged type II diabetes: Amelioration with lipoic acid through phosphatidylinositol 3-kinase/Akt-dependent mechanism. Life Sci. 2010, 86, 844–853. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes1. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef]
- Smith, A.R.; Hagen, T.M. Vascularendothelialdys- function inaging: Loss of Akt- dependent endothelial nitricoxide synthase phosphorylation and partial restoration by (R)-alpha-lipoic acid. Biochem. Soc. Trans. 2003, 31, 1447–1449. [Google Scholar] [CrossRef] [PubMed]
- Artwohl, M.; Muth, K.; Kosulin, K.; de Martin, R.; Holzenbein, T.; Rainer, G.; Freudenthaler, A.; Huttary, N.; Schmetterer, L.; Waldhausl, W.K.; et al. R-(+)-alpha-lipoic acid inhibits endothelial cell apoptosis and proliferation: Involvement of Akt and retinoblastoma protein/E2F-1. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Wei, H.; Hagen, T.; Frei, B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Packer, L. Alpha-lipoate can protect against glycation of serum albumin, but not low density lipoprotein. Biochem. Biophys. Res. Commun. 1994, 203, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, V.; Nandhini, A.A.T.; Anuradha, C.V. Lipoic acid improves glucose utilisation and prevents protein glycation and AGE formation. Die Pharm. 2005, 60, 772–775. [Google Scholar]
- Quinn, J.F.; Bussiere, J.R.; Hammond, R.S.; Montine, T.J.; Henson, E.; Jones, R.E.; Stackman, R.W. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol. Aging 2007, 28, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Zhu, B.Z.; de Szoeke, E.; Frei, B.; Hagen, T.M. Dihydrolipoic acid lowers the redox activity of transition metal ions but does not remove them from the active site of enzymes. Redox Rep. 2004, 9, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Micili, S.C.; Goker, A.; Kuscu, K.; Ergur, B.U.; Fuso, A.J.R.S. α-Lipoic Acid Vaginal Administration Contrasts Inflammation and Preterm Delivery in Rats. Reprod. Sci. 2019, 26, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Brufani, M.; Figliola, R. (R)-α-lipoic acid oral liquid formulation: Pharmacokinetic parameters and therapeutic efficacy. Acta Bio-Medica: Atenei Parm. 2014, 85, 108–115. [Google Scholar]
- Gleiter, C.H.; Schug, B.S.; Hermann, R.; Elze, M.; Blume, H.H.; Gundert-Remy, U. Influence of food intake on the bioavailability of thioctic acid enantiomers. Eur. J. Clin. Pharmacol. 1996, 50, 513–514. [Google Scholar] [CrossRef]
- Hermann, R.; Niebch, G.; Borbe, H.O.; Fieger-Büschges, H.; Ruus, P.; Nowak, H.; Riethmüller-Winzen, H.; Peukert, M.; Blume, H. Enantioselective pharmacokinetics and bioavailability of different racemic α-lipoic acid formulations in healthy volunteers. Eur. J. Pharmacol. Sci. 1996, 4, 167–174. [Google Scholar] [CrossRef]
- Teichert, J.; Tuemmers, T.; Achenbach, H.; Preiss, C.; Hermann, R.; Ruus, P.; Preiss, R. Pharmacokinetics of alpha-lipoic acid in subjects with severe kidney damage and end-stage renal disease. J. Clin. Pharmacol. 2005, 45, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt-Grogler, K.; Niebch, G.; Schneider, E.; Erb, K.; Hermann, R.; Blume, H.H.; Schug, B.S.; Belz, G.G. Dose-proportionality of oral thioctic acid--coincidence of assessments via pooled plasma and individual data. Eur. J. Pharm. Sci. 1999, 8, 57–65. [Google Scholar] [CrossRef]
- Uchida, R.; Iwamoto, K.; Nagayama, S.; Miyajima, A.; Okamoto, H.; Ikuta, N.; Fukumi, H.; Terao, K.; Hirota, T. Effect of gamma-Cyclodextrin Inclusion Complex on the Absorption of R-alpha-Lipoic Acid in Rats. Int. J. Mol. Sci. 2015, 16, 10105–10120. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Okamoto, H.; Ikuta, N.; Terao, K.; Hirota, T. Enantioselective Pharmacokinetics of alpha-Lipoic Acid in Rats. Int. J. Mol. Sci. 2015, 16, 22781–22794. [Google Scholar] [CrossRef] [PubMed]
- Mignini, F.; Nasuti, C.; Gioventu, G.; Napolioni, V.; Martino, P.D. Human bioavailability and pharmacokinetic profile of different formulations delivering alpha lipoic acid. Open Access Sci. Rep. 2012, 1, 418. [Google Scholar] [CrossRef]
- Hermann, R.; Mungo, J.; Cnota, P.J.; Ziegler, D. Enantiomer-selective pharmacokinetics, oral bioavailability, and sex effects of various alpha-lipoic acid dosage forms. Clin. Pharmacol. 2014, 6, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Reed, L.J. The chemistry and function of lipoic acid. Adv. Enzymol. Related Areas Mol. Biol. 1957, 18, 319–347. [Google Scholar]
- Kim, N.W.; Song, Y.M.; Kim, E.; Cho, H.S.; Cheon, K.A.; Kim, S.J.; Park, J.Y. Adjunctive α-lipoic acid reduces weight gain compared with placebo at 12 weeks in schizophrenic patients treated with atypical antipsychotics: A double-blind randomized placebo-controlled study. Int. Clin. Psychopharmacol. 2016, 31, 265–274. [Google Scholar] [CrossRef]
- Sun, H.; Yao, W.; Tang, Y.; Zhuang, W.; Wu, D.; Huang, S.; Sheng, H. Urinary exosomes as a novel biomarker for evaluation of α-lipoic acid’s protective effect in early diabetic nephropathy. J. Clin. Lab. Anal. 2017, 31, e22129. [Google Scholar] [CrossRef]
- De Sousa, C.N.S.; da Silva Leite, C.M.G.; da Silva Medeiros, I.; Vasconcelos, L.C.; Cabral, L.M.; Patrocínio, C.F.V.; Patrocínio, M.L.V.; Mouaffak, F.; Kebir, O.; Macedo, D.; et al. Alpha-lipoic acid in the treatment of psychiatric and neurological disorders: A systematic review. Metab. Brain Dis. 2019, 34, 39–52. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Vinik, A.; Casellini, C.; Nevoret, M.L. Diabetic Neuropathies. In South Dartmouth; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ziegler, D.; Low, P.A.; Freeman, R.; Tritschler, H.J.; Vinik, A.I. Predictors of improvement and progression of diabetic polyneuropathy following treatment with α-lipoic acid for 4 years in the NATHAN 1 trial. J. Diabetes Its Complicat. 2016, 30, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alcala, H.; Santos Vichido, C.I.; Islas Macedo, S.; Genestier-Tamborero, C.N.; Minutti-Palacios, M.; Hirales Tamez, O.; Garcia, C.; Ziegler, D. Treatment with alpha-Lipoic Acid over 16 Weeks in Type 2 Diabetic Patients with Symptomatic Polyneuropathy Who Responded to Initial 4-Week High-Dose Loading. J. Diabetes Res. 2015, 2015, 189857. [Google Scholar] [CrossRef]
- Agathos, E.; Tentolouris, A.; Eleftheriadou, I.; Katsaouni, P.; Nemtzas, I.; Petrou, A.; Papanikolaou, C.; Tentolouris, N. Effect of α-lipoic acid on symptoms and quality of life in patients with painful diabetic neuropathy. J. Int. Med. Res. 2018, 46, 1779–1790. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007, 298, 2028–2037. [Google Scholar] [CrossRef]
- Van Kruijsdijk, R.C.M.; Van Der Wall, E.; Visseren, F.L.J. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef]
- Escoté, X.; Félix-Soriano, E.; Gayoso, L.; Huerta, A.E.; Alvarado, M.A.; Ansorena, D.; Astiasarán, I.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of EPA and lipoic acid supplementation on circulating FGF21 and the fatty acid profile in overweight/obese women following a hypocaloric diet. Food Funct. 2018, 9, 3028–3036. [Google Scholar] [CrossRef]
- Huerta, A.E.; Navas-Carretero, S.; Prieto-Hontoria, P.L.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity 2015, 23, 313–321. [Google Scholar] [CrossRef]
- Li, N.; Yan, W.; Hu, X.; Huang, Y.; Wang, F.; Zhang, W.; Wang, Q.; Wang, X.; Sun, K. Effects of oral α-lipoic acid administration on body weight in overweight or obese subjects: A crossover randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2017, 86, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Arjmand, S.; Amirkhizi, F.; Ebrahimi-Mameghani, M. The effect of alpha-lipoic acid on inflammatory markers and body composition in obese patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Clin. Pharm. Ther. 2019, 44, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Romo-Hualde, A.; Huerta, A.E.; González-Navarro, C.J.; Ramos-López, O.; Moreno-Aliaga, M.J.; Martínez, J.A. Untargeted metabolomic on urine samples after α-lipoic acid and/or eicosapentaenoic acid supplementation in healthy overweight/obese women. Lipids Health Dis. 2018, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- The American Psychiatric Association. Diagnostic and statistical manual of mental disorders; The American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Gold, J.M. Cognitive deficits as treatment targets in schizophrenia. Schizophr. Res. 2004, 72, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.I.; Wallenstein, S.; Moshier, E.; Parrella, M.; White, L.; Bowler, S.; Gottlieb, S.; Harvey, P.D.; McGinn, T.G.; Flanagan, L. The effects of hypertension and body mass index on cognition in schizophrenia. Am. J. Psychiatry 2010, 167, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Goughari, A.S.; Mazhari, S.; Pourrahimi, A.M.; Sadeghi, M.M.; Nakhaee, N. Associations between components of metabolic syndrome and cognition in patients with schizophrenia. J. Psychiatr. Pract. 2015, 21, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.L.O.; de Souza Menezes, C.E.; Chaves Filho, A.J.M.; de Almeida Viana, G.; Fechine, F.V.; de Queiroz, M.G.R.; da Cruz Fonseca, S.G.; Vasconcelos, S.M.M.; de Moraes, M.E.A.; Gama, C.S. α-Lipoic acid as adjunctive treatment for Schizophrenia: An open-label trial. J. Clin. Psychopharmacol. 2017, 37, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Vidović, B.; Milovanović, S.; Stefanović, A.; Kotur-Stevuljević, J.; Takić, M.; Debeljak-Martačić, J.; Pantović, M.; Đorđević, B. Effects of alpha-lipoic acid supplementation on plasma adiponectin levels and some metabolic risk factors in patients with schizophrenia. J. Med. Food 2017, 20, 79–85. [Google Scholar] [CrossRef]
- Confavreux, C.; Vukusic, S.; Moreau, T.; Adeleine, P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 2000, 343, 1430–1438. [Google Scholar] [CrossRef]
- Marracci, G.H.; Jones, R.E.; McKeon, G.P.; Bourdette, D.N. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2002, 131, 104–114. [Google Scholar] [CrossRef]
- Yadav, V.; Marracci, G.; Lovera, J.; Woodward, W.; Bogardus, K.; Marquardt, W.; Shinto, L.; Morris, C.; Bourdette, D. Lipoic acid in multiple sclerosis: A pilot study. Mult. Scler. J. 2005, 11, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Trapp, B.D. Relapsing and progressive forms of multiple sclerosis–insights from pathology. Curr. Opin. Neurol. 2014, 27, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Azimi, A.; Izadi, V.; Eghtesadi, S.; Mirshafiey, A.; Sahraian, M.A.; Motevalian, A.; Norouzi, A.; Sanoobar, M.; Eskandari, G.; et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: A double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation 2014, 21, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Loy, B.D.; Fling, B.W.; Horak, F.B.; Bourdette, D.N.; Spain, R.I. Effects of lipoic acid on walking performance, gait, and balance in secondary progressive multiple sclerosis. Complement. Ther. Med. 2018, 41, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.E.; Yadav, V.; Kerns, A.R.; Tsang, C.; Markwardt, S.; Kim, E.; Spain, R.; Bourdette, D.; Salinthone, S. Lipoic acid stimulates cAMP production in healthy control and secondary progressive MS subjects. Mol. Neurobiol. 2018, 55, 6037–6049. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Atsuki, Y.; Wakasaya, A.; Kobayashi, M.; Hirano, Y.; Ohwada, M. Characteristics of patients with subchorionic hematomas in the second trimester. J. Obstet. Gynaecol. Res. 2012, 38, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Şükür, Y.E.; Göç, G.; Köse, O.; Açmaz, G.; Özmen, B.; Atabekoğlu, C.S.; Koç, A.; Söylemez, F. The effects of subchorionic hematoma on pregnancy outcome in patients with threatened abortion. J. Turkish German Gynecol. Assoc. 2014, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Carp, H.J.A. Progestogens and pregnancy loss. Climacteric 2018, 21, 380–384. [Google Scholar] [CrossRef]
- Porcaro, G.; Brillo, E.; Giardina, I.; Di Iorio, R. Alpha Lipoic Acid (ALA) effects on subchorionic hematoma: Preliminary clinical results. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3426–3432. [Google Scholar]
- Grandi, G.; Pignatti, L.; Ferrari, F.; Dante, G.; Neri, I.; Facchinetti, F. Vaginal alpha-lipoic acid shows an anti-inflammatory effect on the cervix, preventing its shortening after primary tocolysis. A pilot, randomized, placebo-controlled study. J. Matern. Fetal Neonatal Med. 2017, 30, 2243–2249. [Google Scholar] [CrossRef]
- Costantino, M.; Guaraldi, C.; Costantino, D. Resolution of subchorionic hematoma and symptoms of threatened miscarriage using vaginal alpha lipoic acid or progesterone: Clinical evidences. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1656–1663. [Google Scholar] [PubMed]
- Ambrosi, N.; Arrosagaray, V.; Guerrieri, D.; Uva, P.D.; Petroni, J.; Herrera, M.B.; Iovanna, J.L.; Leon, L.; Incardona, C.; Chuluyan, H.E.; et al. alpha-Lipoic acid protects against ischemia-reperfusion injury in simultaneous kidney-pancreas transplantation. Transplantation 2016, 100, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Casciato, P.; Ambrosi, N.; Caro, F.; Vazquez, M.; Müllen, E.; Gadano, A.; de Santibañes, E.; de Santibañes, M.; Zandomeni, M.; Chahdi, M. α-Lipoic acid reduces postreperfusion syndrome in human liver transplantation-a pilot study. Transpl. Int. 2018, 31, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jones, D.; Palmer, J.L.; Forman, A.; Dakhil, S.R.; Velasco, M.R.; Weiss, M.; Gilman, P.; Mills, G.M.; Noga, S.J.; et al. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: A randomized, double-blind, placebo-controlled trial. Support. Care Cancer 2014, 22, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
| Patients (n) | Design | Treatment | Key Effects | References |
|---|---|---|---|---|
| Diabetic patients with mild-to-moderate polyneuropathy Age range: n.s. n = 429 | Clinical trial Randomized Double-blind Placebo-controlled Multicenter Two-arm | 600 mg/day ALA or placebo, orally Duration: 4 years | - Prevention of neuropathic improvements progression with regular and long-term administration | [107] |
| Type 2 diabetic patients with symptomatic polyneuropathy Age range: n.s. n = 45 | Clinical trial Randomized Withdrawal Open-label study | 600 mg ALA 3 times per day in phase 1, orally 600 mg ALA daily or ALA withdrawal in phase 2, orally Duration: 4 weeks (phase 1) 16 weeks (phase 2) | - Phase 1: Total Symptom Score (TSS) decreased - Phase 2: TSS decreased in ALA-treated group and improved neuropathic symptoms | [108] |
| Diabetic patients with early nephropathy Age range: n.s. n = 62 | Clinical trial Randomized Controlled | 600 mg/day ALA, intravenously with routine treatment or routine treatment (control group) Duration: 8 weeks | - Decline in urinary albumin excretion rates, serum creatinine and malonaldehyde - Increased plasma SOD activity and improved endothelium-dependent flow mediated vasodilation flexibility | [102] |
| Diabetic patients with neuropathy Age range: 18–75 n = 72 | Clinical trial Clinical report Interventional study | 600 mg/day ALA, orally Duration: 40 days | - Reduction in neuropathic symptoms and triglycerides levels | [109] |
| Patients (n) | Design | Treatment | Key Effects | References |
|---|---|---|---|---|
| Overweight/ obese women Age range: 20–50 n = 77 | Clinical trial Randomized Double-blind Placebo-Controlled Parallel design | 1300 mg/day EPA or 300 mg/day ALA or both 1300 mg/day EPA + 300 mg/day ALA or placebo, orally 30% energy-restricted diet Duration: 10 weeks | - Significantly higher body weight loss in ALA treated groups - Significantly attenuated decrease in leptin levels in ALA treated groups during weight loss | [114] |
| Overweight/ obese women Age range: 20–50 n = 73 | Clinical trial Randomized Double-blind Placebo-controlled Parallel design | 1300 mg/day EPA or 300 mg/day ALA or both 1300 mg/day EPA + 300 mg/day ALA or placebo 30% energy-restricted diet Duration: 10 weeks | - A high reduction in body weight, BMI and fat mass was stated in ALA treated groups - Significant reduction in glucose levels for only control group and EPA + ALA group - No significant differences in irisin changes between groups | [114] |
| Overweight or obese patients Age range: 38–47 n = 170 | Clinical trial Single-center Randomized Double-blind Crossover controlled | 1200 mg/day ALA or placebo, orally Duration: 8 weeks | - Significant reduction in body weight and waist circumference | [115] |
| Obese patients with non-alcoholic fatty liver disease (NAFLD) Age range: 20–50 n = 45 | Clinical trial Randomized Double-blind Placebo-controlled | 1200 mg/day ALA + 400 mg/day vitamin E or vitamin E (placebo), orally Duration: 12 weeks | - Significant improvement in serum adiponectin and IL-6 levels | [116] |
| Overweight/obese women Age range: not specified n = 57 | Clinical trial Randomized Double-blind Placebo-controlled | 300 mg/day ALA or 1300 mg/day EPA or 1300 mg/day EPA + 300 mg/day ALA or placebo, orally Hypocaloric diet Duration: 10 weeks | - A significant reduction in the circulating levels of saturated fatty acid and total n-6-PUFAs | [113] |
| Overweight/ obese sedentary females Age range: n.s. n = 65 | Clinical trial Randomized Double-blind Placebo-controlled | 300 mg/day ALA or 1300 mg/day EPA or 1300 mg/day EPA + 300 mg/day ALA or placebo, orally Energy restricted diet Duration: 10 weeks | - Significant reduction in BMI and fat mass in ALA treated groups | [117] |
| Patients (n) | Design | Treatment | Key Effects | References |
|---|---|---|---|---|
| Schizophrenia with antipsychotic induced weight gain Age range: n.s n = 15 | Clinical trial Randomized Double-blind Placebo-controlled | 600–1800 mg/day ALA or placebo, orally Duration: 12 weeks | - Reduction in body weight and BMI - Significantly reduced visceral fat areas - No severe side effects except gastrointestinal symptoms and mild dermatologic symptoms | [101] |
| Schizophrenia Age range: 18–60 n = 10 | Clinical trial Open-Label Trial | 100 mg/day ALA, orally Duration: 4 months | - Significant improvement in neurocognitive parameters - No significant differences in BMI, abdominal circumference, blood count and liver enzymes | [122] |
| Schizophrenia Age range: 25–60 n = 18 | Clinical trial Controlled | 500 mg/day ALA, orally Duration: 3 months | - Significant increase in plasma adiponectin levels - Decrease in fasting glucose and aspartate aminotransferase activity | [123] |
| Patients (n) | Design | Treatment | Key Effects | References |
|---|---|---|---|---|
| Relapsing-remitting MS Age range: 18–50 n = 52 | Clinical trial Randomized Double-blind Placebo-controlled | 1200 mg/day ALA or placebo, orally Duration: 12 weeks | - Significant reduction in serum levels of INF-γ, ICAM-1 TGF-β and IL-4 - No significant changes in TNF-α, IL-6, EDSS and MMP-9 levels | [128] |
| Secondary progressive multiple sclerosis (SPMS) Age range: 40–70 n = 21 | Clinical trial Randomized Double-blind Placebo-controlled Pilot study | 1200 mg/day ALA or placebo, orally Duration: 2 years | - Significant improvements in walking performance in patients | [129] |
| Relapsing and remitting MS (RRMS), secondary progressive MS (SPMS) Age range: age ≥ 18 n = 57 | Clinical trial Controlled | 1200 mg racemic ALA once Duration: 48 h | - Increased cAMP at 2 and 4 h of ALA treatment in healthy and SPMS patients - Decrease cAMP in RRMS patients | [130] |
| Patients (n) | Design | Treatment | Key Effects | References |
|---|---|---|---|---|
| Threatened miscarriage and subchorionic hematoma Age range: 20–40 n = 16 | Preliminary Clinical trial Randomized | 600 mg/day ALA + 400 mg/day Progesterone or 400 mg/day Progesterone (control group), orally Duration: until complete resolution of the clinical picture | - Effective determination in major signs of threatened miscarriage in ALA-treated group - Significant improvements for hematoma resorption in ALA-treated group - No adverse effects on mother or fetus | [134] |
| Singleton pregnancy, at a gestational age ranging 24–30 weeks, hospitalized for a first preterm labor episode Age range: n.s. n = 32 | Clinical trial Randomized Placebo-controlled Pilot study | 400 mg/day ALA (active ingredient 10 mg) or placebo, vaginal tablets Duration: 30 days | - Significant increase in anti-inflammatory interleukins in the cervical vaginal liquids of undelivered women after a preterm labor episode | [135] |
| Threatened miscarriage Age range: 24–40 n = 62 | Clinical trial Randomized Controlled | 10 mg/day ALA (vaginal capsule) or 400 mg/day progesterone (vaginal soft gel) or placebo Duration: 60 days | - quick reabsorption of sub-chorionic hematoma in ALA-treated group - Smaller number of miscarriages in ALA-treated group | [136] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. https://doi.org/10.3390/biom9080356
Salehi B, Berkay Yılmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, Akram M, Riaz M, Capanoglu E, Sharopov F, et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules. 2019; 9(8):356. https://doi.org/10.3390/biom9080356
Chicago/Turabian StyleSalehi, Bahare, Yakup Berkay Yılmaz, Gizem Antika, Tugba Boyunegmez Tumer, Mohamad Fawzi Mahomoodally, Devina Lobine, Muhammad Akram, Muhammad Riaz, Esra Capanoglu, Farukh Sharopov, and et al. 2019. "Insights on the Use of α-Lipoic Acid for Therapeutic Purposes" Biomolecules 9, no. 8: 356. https://doi.org/10.3390/biom9080356






