Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Conjugate Palmitate-BSA Preparation
2.4. Cytotoxicity Assays
2.5. Cell Growth Curves
2.6. EdU Proliferation Assay
2.7. Wound Healing Migration Assay
2.8. Flow Cytometry Assay for Cell Cycle Distribution
2.9. Extracellular Flux Analyzer Experiments
2.10. FACS Analysis of Glucose and Palmitate Uptake
2.11. Glutamine Consumption and Oxidation
2.12. Lactate production
2.13. RNA Isolation and Purification and cDNA Synthesis
2.14. qPCR
2.15. Western Blot
2.16. Statistical Analysis
3. Results
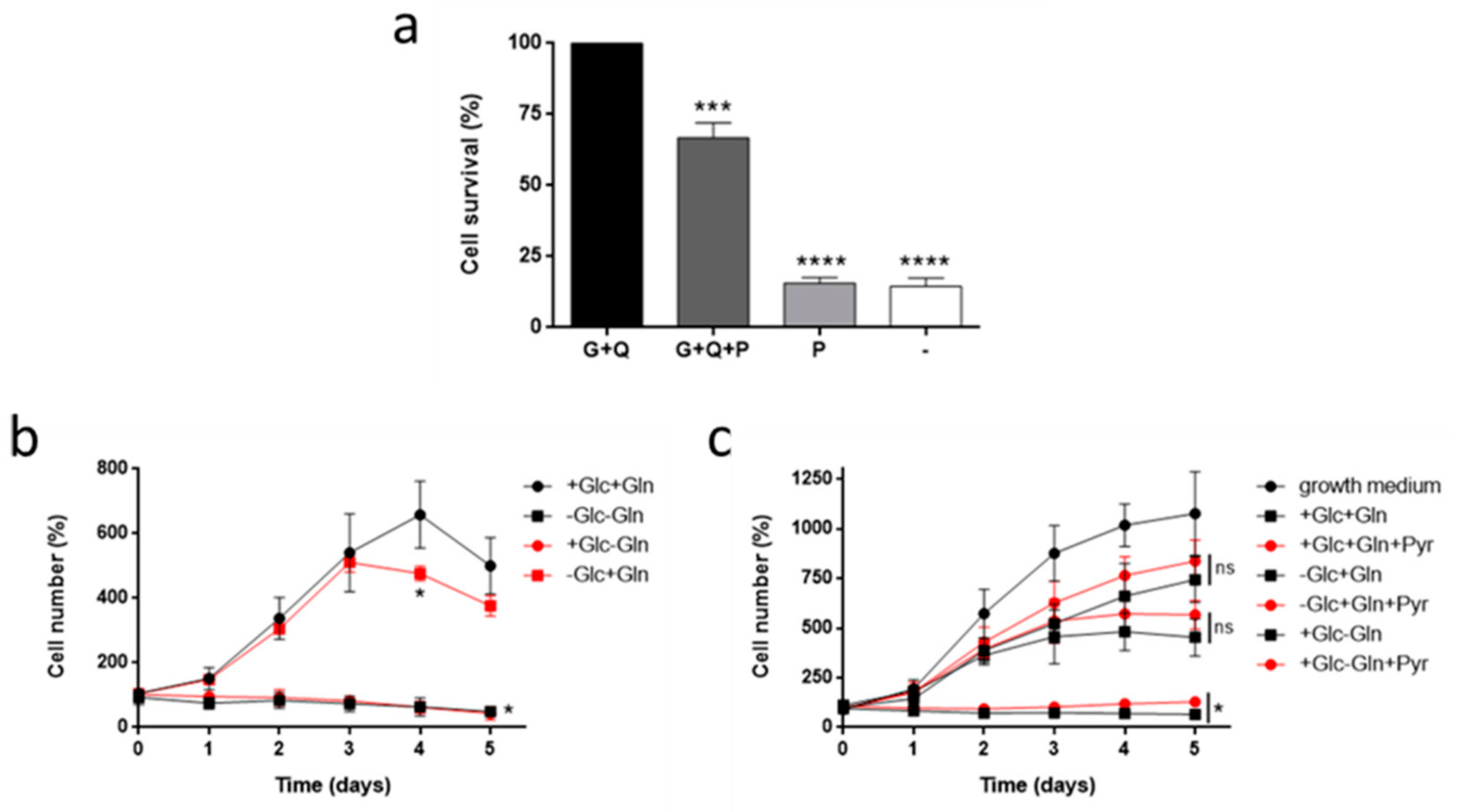
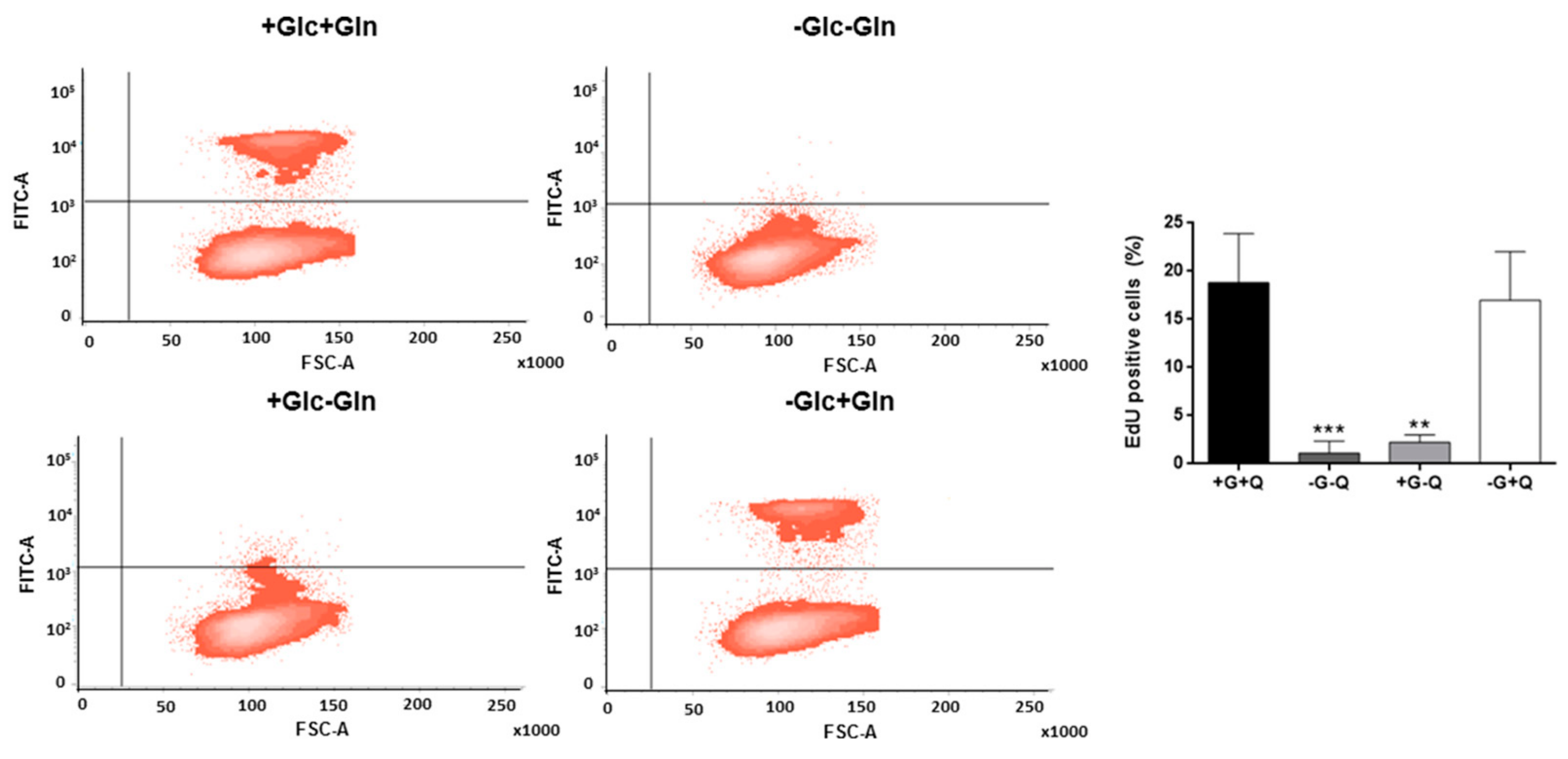
3.1. Glutamine, but not Glucose, is Essential for HMEC Growth
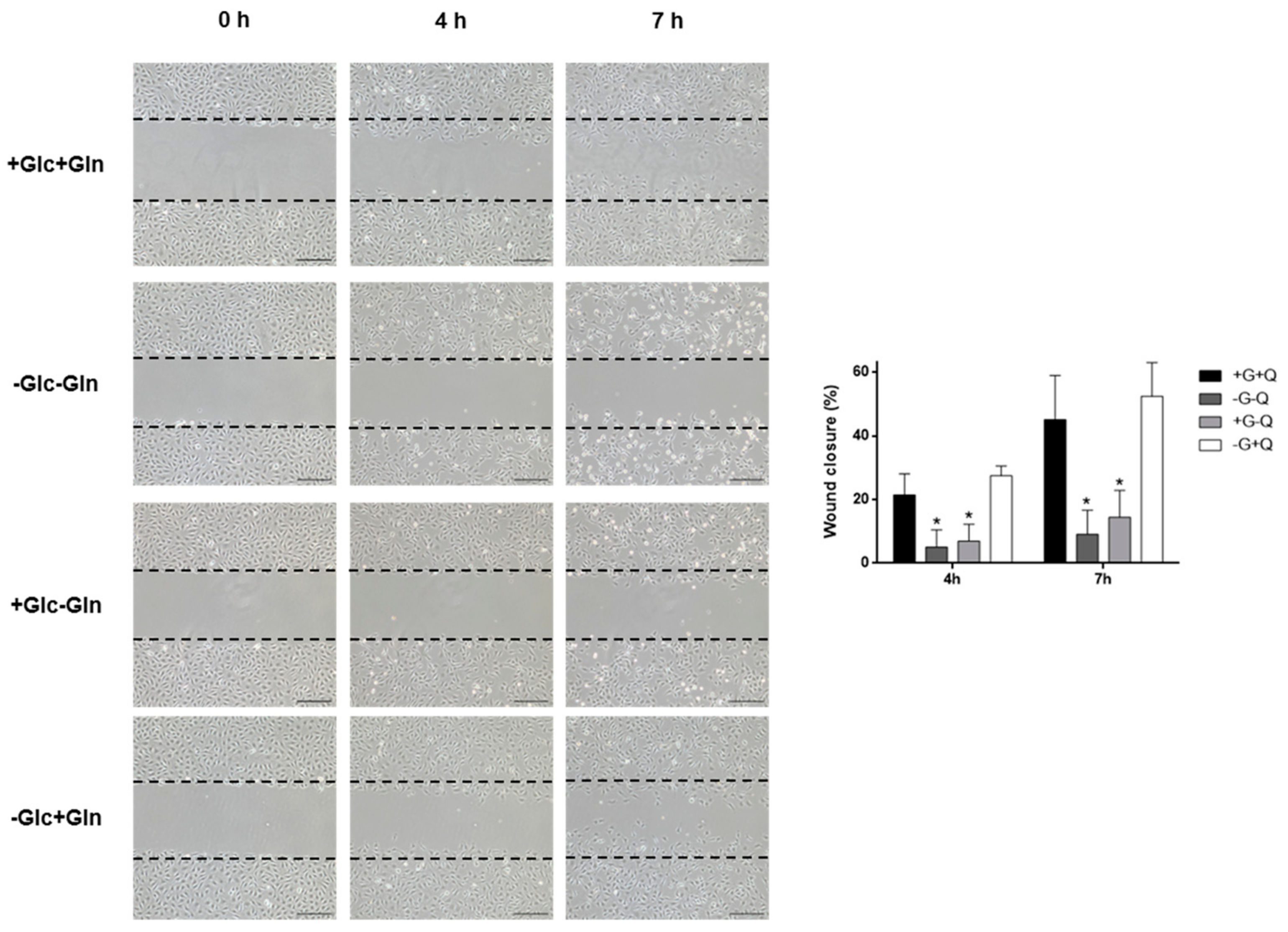
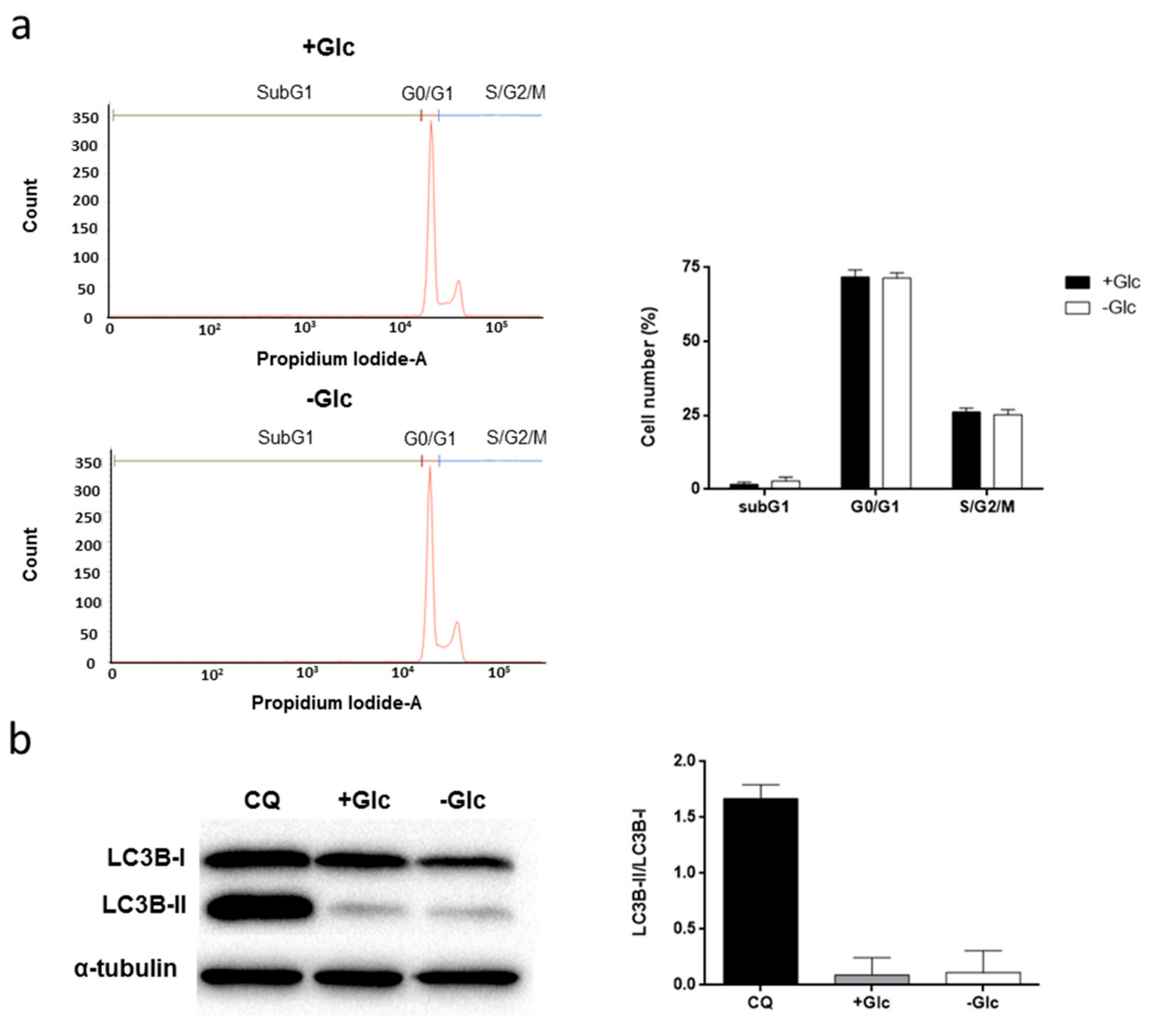
3.2. Glucose Starvation Does Not Affect Cell Migration, Cell Cycle Distribution and Does Not Induce Autophagy
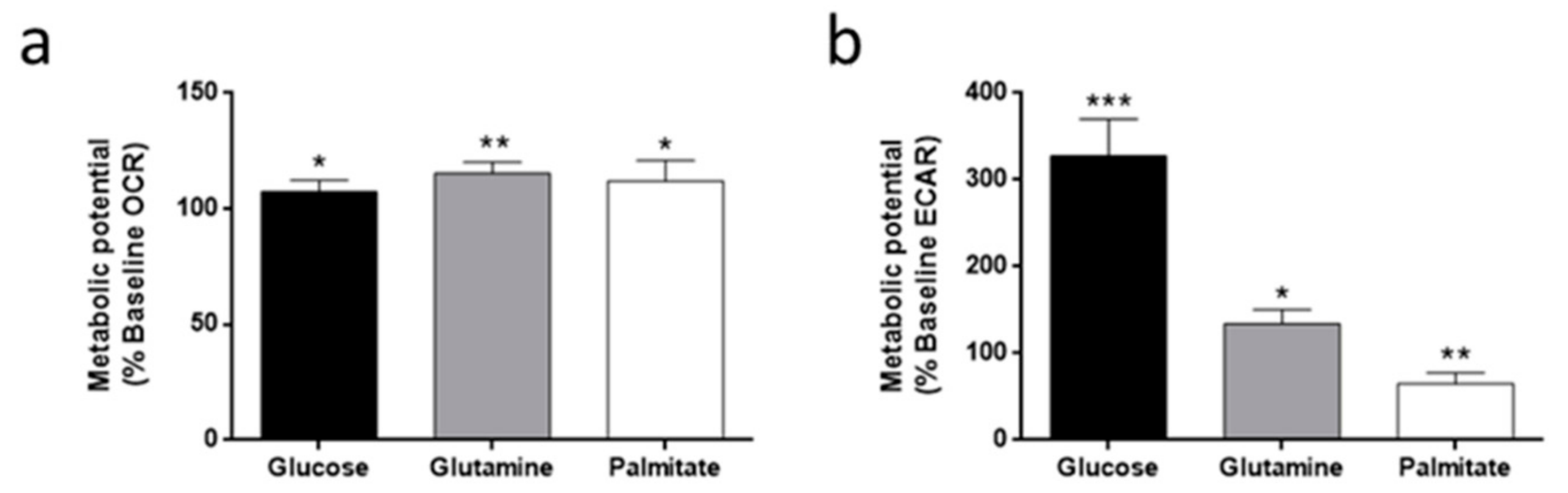
3.3. Oxygen Consumption and Extracellular Acidification Rates in HMEC
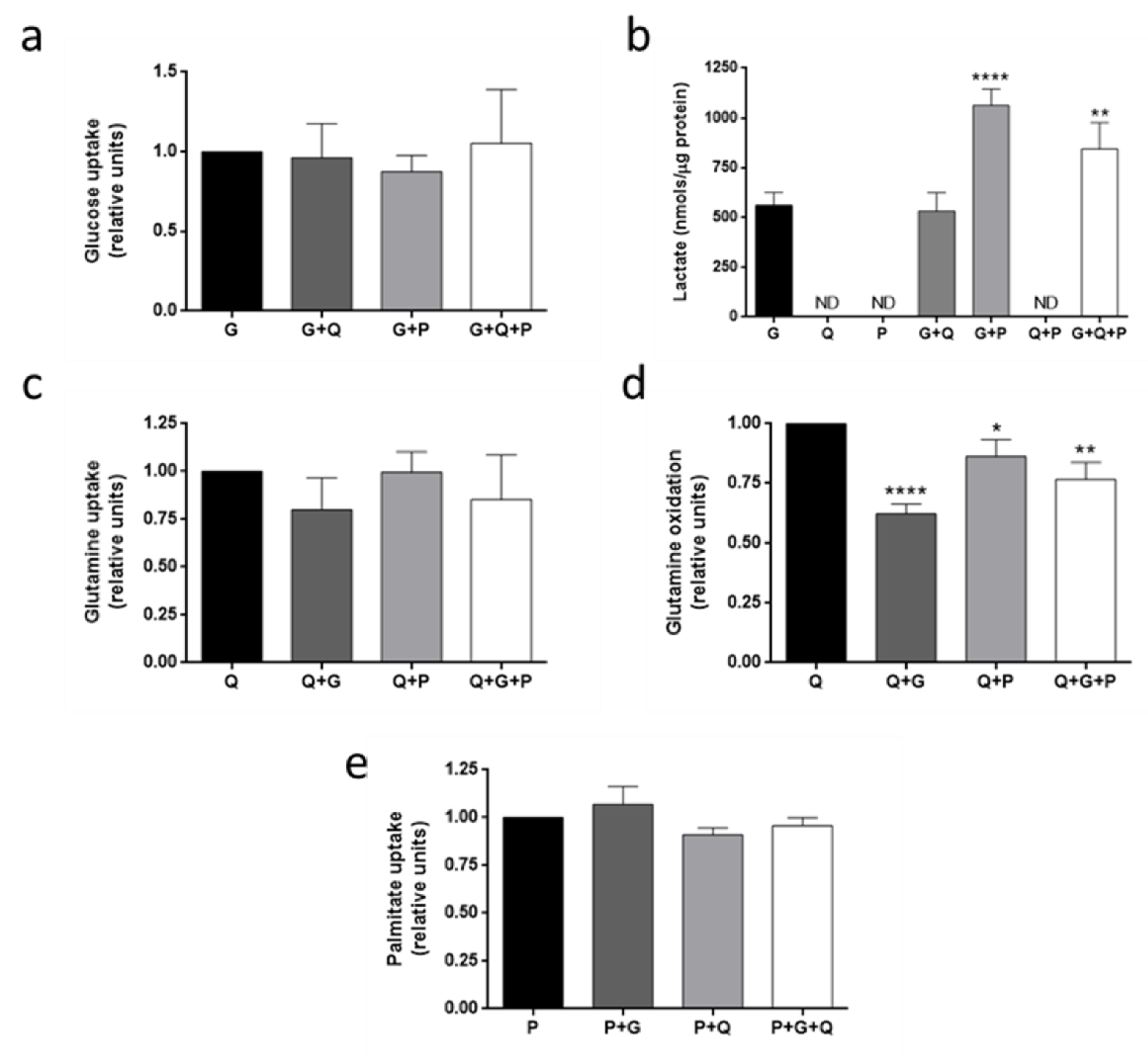
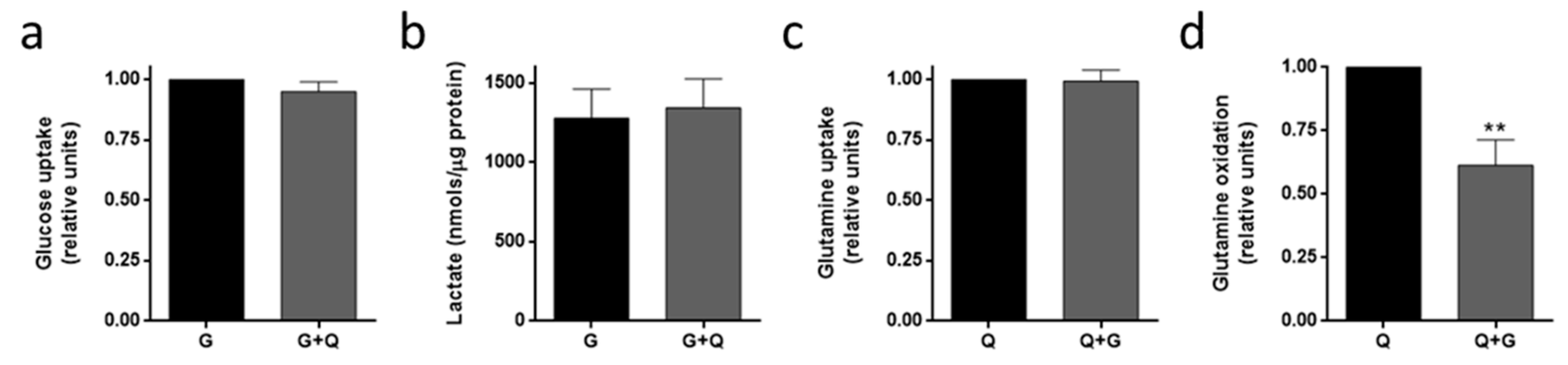
3.4. Metabolic Fuel Uptake and Metabolism in HMEC
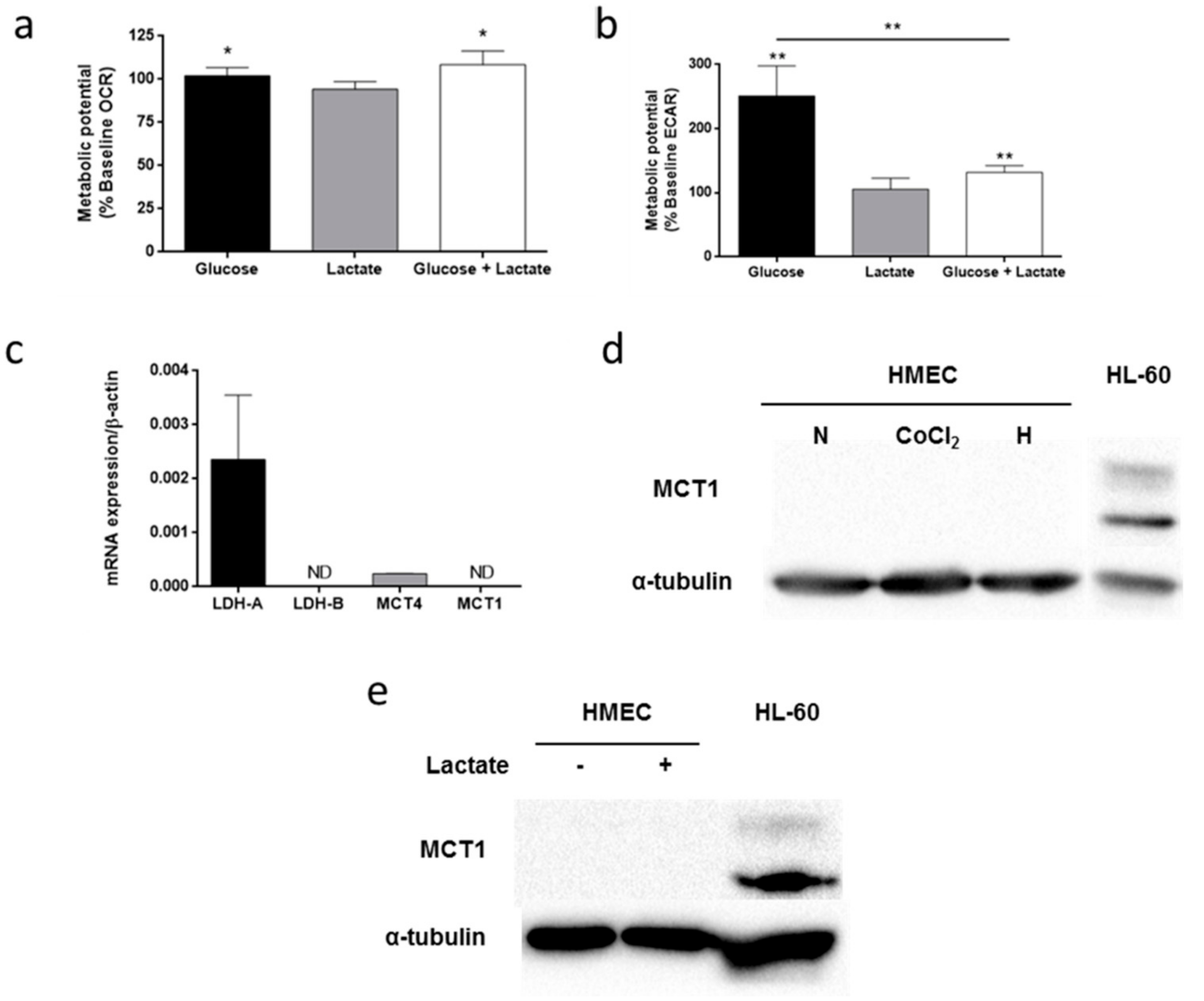
3.5. Lactate Metabolism in HMEC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.R.; Medina, M.Á.; Alba, E. Playing only one instrument may be not enough: Limitations and future of the antiangiogenic treatment of cancer. BioEssays 2007, 29, 1159–1168. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Brüning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.S. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 2015, 520, 192–197. [Google Scholar] [CrossRef]
- Goveia, J.; Stapor, P.; Carmeliet, P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol. Med. 2014, 6, 1105–1120. [Google Scholar] [CrossRef]
- Potente, M.; Carmeliet, P. The link between angiogenesis and endothelial metabolism. Annu. Rev. Physiol. 2017, 79, 43–66. [Google Scholar] [CrossRef]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef]
- Spolarics, Z.; Lang, C.H.; Bagby, G.J.; Spitzer, J.J. Glutamine and fatty acid oxidation are the main sources of energy for Kupffer and endothelial cells. Am. J. Physiol. 1991, 261, G185–G190. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, J.D.; Fernandes-Siqueira, L.O.; Carvalho, A.S.; Cararo-Lopes, E.; Dias, M.H.; Ketzer, L.A.; Galina, A.; Da Poian, A.T. Short-term starvation is a strategy to unravel the cellular capacity of oxidizing specific exogenous/endogenous substrates in mitochondria. J. Biol. Chem. 2017, 292, 14176–14187. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.R.; Vander Heiden, M.G. Famine versus feast: Understanding the metabolism of tumors in vivo. Trends Biochem. Sci. 2015, 40, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, S.; Moreno-Altamirano, M.M.B.; Prado-García, H.; Sánchez-García, F.J. Lactate contribution to the tumor microenvironment: Mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef]
- Sonveaux, P.; Copetti, T.; de Saedeleer, C.J.; Végran, F.; Verrax, J.; Kennedy, K.M.; Moon, E.J.; Dhup, S.; Danhier, P.; Frérart, F.; et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 2012, 7, e33418. [Google Scholar] [CrossRef]
- Ruan, G.X.; Kazlauskas, A. Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor receptor 2 to activate phosphoinositide 3-kinase/AKT and promote angiogenesis. J. Biol. Chem. 2013, 288, 21161–21172. [Google Scholar] [CrossRef]
- Miranda-Gonçalves, V.; Bezerra, F.; Costa-Almeida, R.; Freitas-Cunha, M.; Soares, R.; Martinho, O.; Reis, R.M.; Pinheiro, C.; Baltazar, F. Monocarboxylate transporter 1 is a key player in glioma-endothelial cell crosstalk. Mol. Carcinog. 2017, 56, 2630–2642. [Google Scholar] [CrossRef]
- Kishimoto, A.; Takahashi-Iwanaga, H.; Watanabe, M.M.; Iwanaga, T. Differential expression of endothelial nutrient transporters (MCT1 and GLUT1) in the developing eyes of mice. Exp. Eye Res. 2016, 153, 170–177. [Google Scholar] [CrossRef][Green Version]
- Cárdenas, C.; Quesada, A.; Medina, M. Evaluation of the anti-angiogenic effect of aloe-emodin. Cell. Mol. Life Sci. 2006, 63, 3083–3089. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Kleinman, H.K.; Martin, G.R.; Lawley, T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988, 107, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Huynh, F.K.; Green, M.F.; Koves, T.R.; Hirschey, M.D. Measurement of fatty acid oxidation rates in animal tissues and cell lines. Methods Enzymol. 2014, 542, 391–405. [Google Scholar] [PubMed]
- Zou, C.; Wang, Y.; Shen, Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J. Biochem. Biophys. Methods 2005, 64, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Dubikovskaya, E.; Chudnovskiy, R.; Karateev, G.; Park, H.M.; Stahl, A. Measurement of long-chain fatty acid uptake into adipocytes. Methods Enzymol. 2014, 538, 107–134. [Google Scholar] [PubMed]
- Ruiz-Pérez, M.V.; Medina, M.Á.; Urdiales, J.L.; Keinänen, T.A.; Sánchez-Jiménez, F. Polyamine metabolism is sensitive to glycolysis inhibition in human neuroblastoma cells. J. Biol. Chem. 2015, 290, 6106–6119. [Google Scholar] [CrossRef]
- Lopes-Coehlo, F.; Nunes, C.; Gouveia-Fernandes, S.; Rosas, R.; Silva, F.; Gameiro, P.; Carvalho, T.; Gomes da Silva, M.; Cabeçadas, J.; Dias, S.; et al. Monocarboxylate transporter 1 (MCT1), a tool to stratify acute myeloid leukemia (AML) patients and a vehicle to kill cancer cells. Oncotarget 2017, 8, 82803–82823. [Google Scholar] [CrossRef]
- Missiaen, R.; Rodriguez, F.M.; Eelen, G.; Carmeliet, P. Targeting endothelial metabolism for anti-angiogenesis therapy: A pharmacological perspective. Vascul. Pharmacol. 2017, 90, 8–18. [Google Scholar] [CrossRef]
- Ghesquière, B.; Wong, B.W.; Kuchnio, A.; Carmeliet, P. Metabolism of stromal and immune cells in health and disease. Nature 2014, 511, 167–176. [Google Scholar] [CrossRef]
- Ocaña, M.C.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Metabolism within the tumor microenvironment and its implication on cancer progression: An ongoing therapeutic target. Med. Res. Rev. 2019, 39, 70–113. [Google Scholar] [CrossRef]
- Wilhelm, K.; Happel, K.; Eelen, G.; Schoors, S.; Oellerich, M.F.; Lim, R.; Zimmermann, B.; Aspalter, I.M.; Franco, C.A.; Braun, T.; et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 2017, 529, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Schoors, S.; De Bock, K.; Cantelmo, A.R.; Georgiadou, M.; Ghesquière, B.; Cauwenberghs, S.; Kuchnio, A.; Wong, B.W.; Quaegebeur, A.; Goveia, J.; et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014, 19, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Koziel, A.; Woyda-Ploszczyca, A.; Kicinska, A.; Jarmuszkiewicz, W. The influence of high glucose on the aerobic metabolism of endothelial EA. hy926 cells. Pflügers Arch. Eur. J. Physiol. 2012, 464, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [PubMed]
- Du, J.Y.; Yuan, F.; Zhao, L.Y.; Zhu, J.; Huang, Y.Y.; Zhang, G.S.; Wei, Y.; Liu, Y.; Yi, Q.; Tu, Y.S.; et al. Suppression of Kv1.5 protects against endothelial apoptosis induced by palmitate and in type 2 diabetes mice. Life Sci. 2017, 168, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.I.; Okamoto, T.; Amano, S.; Inagaki, Y.; Koga, K.; Koga, M.; Choei, H.; Sasaki, N.; Kikuchi, S.; Takeuchi, M.; et al. Palmitate-induced apoptosis of microvascular endothelial cells and pericytes. Mol. Med. 2002, 8, 179–184. [Google Scholar] [CrossRef]
- Hardy, S.; El-Assaad, W.; Przybytkowski, E.; Joly, E.; Prentki, M.; Langelier, Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2003, 278, 31861–31870. [Google Scholar] [CrossRef]
- Haywood, J.; Yammani, R.R. Free fatty acid palmitate activates unfolded protein response pathway and promotes apoptosis in meniscus cells. Osteoarthr. Cartil. 2016, 24, 942–945. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, Y.M.; Lee, K.H.; Jeong, S.W.; Kwon, O.J. Oleate protects macrophages from palmitate-induced apoptosis through the downregulation of CD36 expression. Biochem. Biophys. Res. Commun. 2017, 488, 477–482. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, Y.; Li, Y.; Luo, X.; Shi, H. Palmitate impairs angiogenesis via suppression of cathepsin activity. Mol. Med. Rep. 2017, 15, 3644–3650. [Google Scholar] [CrossRef]
- Makita, J.; Hosoya, K.; Zhang, P.; Kador, P.F. Response of rat retinal capillary pericytes and endothelial cells to glucose. J. Ocul. Pharmacol. Ther. 2011, 27, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017, 36, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kamphorst, J.J.; Mathew, R.; Chung, M.K.; White, E.; Shlomi, T.; Rabinowitz, J.D. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 2013, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Arreola, A.; Cowey, C.L.; Coloff, J.L.; Rathmell, J.C.; Rathmell, W.K. HIF1α and HIF2α exert distinct nutrient preferences in renal cells. PLoS ONE 2014, 9, e98705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karbach, S.; Jansen, T.; Horke, S.; Heeren, T.; Scholz, A.; Coldewey, M.; Karpi, A.; Hausding, M.; Kröller-Schön, S.; Oelze, M.; et al. Hyperglycemia and oxidative stress in cultured endothelial cells—A comparison of primary endothelial cells with an immortalized endothelial cell line. J. Diabetes Complicat. 2012, 26, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.M.; Ghosh, S.; Majeed, Y.; Arunachalam, G.; Emara, M.M.; Ding, H.; Triggle, C.R. Metformin represses glucose starvation induced autophagic response in microvascular endothelial cells and promotes cell death. Biochem. Pharmacol. 2017, 132, 118–132. [Google Scholar] [CrossRef]
- Culic, O.; Decking, U.K.; Schrader, J. Metabolic adaptation of endothelial cells to substrate deprivation. Am. J. Physiol. 1999, 276, C1061–C1068. [Google Scholar] [CrossRef]
- Vizán, P.; Sánchez-Tena, S.; Alcarraz-Vizán, G.; Soler, M.; Messeguer, R.; Pujol, M.D.; Lee, W.N.P.; Cascante, M. Characterization of the metabolic changes underlying growth factor angiogenic activation: Identification of new potential therapeutic targets. Carcinogenesis 2009, 30, 946–952. [Google Scholar] [CrossRef]
- Artwohl, M.; Brunmair, B.; Fürnsinn, C.; Hölzenbein, T.; Rainer, G.; Freudenthaler, A.; Porod, E.M.; Huttary, N.; Baumgartner-Parzer, S.M. Insulin does not regulate glucose transport and metabolism in human endothelium. Eur. J. Clin. Investig. 2007, 37, 643–650. [Google Scholar] [CrossRef]
- Medina, M.Á.; Sánchez-Jiménez, F.; Quesada, A.R.; Márquez, F.J.; Núñez de Castro, I. Effect of palmitate, acetate and glucose on glutamine metabolism in Ehrlich ascites tumor cells. Biochimie 1988, 70, 833–834. [Google Scholar] [CrossRef]
- Medina, M.Á.; Sánchez-Jiménez, F.; Márquez, J.; Pérez-Rodríguez, J.; Quesada, A.R.; Núñez de Castro, I. Glutamine and glucose as energy substrates for Ehrlich ascites tumour cells. Biochem. Int. 1988, 16, 339–347. [Google Scholar] [PubMed]
- Spector, A.A.; Steinberg, D. Relationship between fatty acid and glucose utilization in Ehrlich ascites tumor cells. J. Lipid Res. 1966, 7, 657–663. [Google Scholar] [PubMed]
- Eaton, P.; Steinberg, D. Effects of medium fatty acid concentration, epinephrine, and glucose on palmitate-l-C14 oxidation and incorporation into neutral lipids by skeletal muscle in vitro. J. Lipid Res. 1961, 2, 376–382. [Google Scholar]
- Marchut, E.; Guminska, M.; Kedryna, T. The inhibitory effect of various fatty acids on aerobic glycolysis in Ehrlich ascites tumour cells. Acta Biochim. Pol. 1986, 33, 7–16. [Google Scholar] [PubMed]
- Krützfeldt, A.; Spahr, R.; Mertens, S.; Siegmund, B.; Piper, H.M. Metabolism of exogenous substrates by coronary endothelial cells in culture. J. Mol. Cell. Cardiol. 1990, 22, 1393–1404. [Google Scholar] [CrossRef]
- Spahr, R.; Krtitzfeldt, A.; Mertens, S.; Siegmund, B.; Piper, H.M. Fatty acids are not an important fuel for coronary microvascular endothelial cells. Mol. Cell. Biochem. 1989, 88, 59–64. [Google Scholar] [CrossRef]
- Dagher, Z.; Ruderman, N.; Tornheim, K.; Ido, Y. Acute regulation of fatty acid oxidation and AMP-activated protein kinase in human umbilical vein endothelial cells. Circ. Res. 2001, 88, 1276–1282. [Google Scholar] [CrossRef]
- Broniarek, I.; Koziel, A.; Jarmuszkiewicz, W. The effect of chronic exposure to high palmitic acid concentrations on the aerobic metabolism of human endothelial EA. hy926 cells. Pflügers Arch. Eur. J. Physiol. 2016, 468, 1541–1554. [Google Scholar] [CrossRef]
- Dobrina, A.; Rossi, F. Metabolic properties of freshly isolated bovine endothelial cells. Biochim. Biophys. Acta 1983, 762, 295–301. [Google Scholar] [CrossRef]
- Peters, K.; Kamp, G.; Berz, A.; Unger, R.E.; Barth, S.; Salamon, A.; Rychly, J.; Kirkpatric, C.J. Changes in human endothelial cell energy metabolic capacities during in vitro cultivation. The role of aerobic glycolysis and proliferation. Cell. Physiol. Biochem. 2009, 24, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Goncalves, R.L.S.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 2015, 1847, 171–181. [Google Scholar] [CrossRef]
- Rattigan, Y.I.; Patel, B.B.; Ackerstaff, E.; Sukenick, G.; Koutcher, J.A.; Glod, J.W.; Banerjee, D. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp. Cell Res. 2012, 318, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.S.; Davies, A.J.; Halestrap, A.P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem. 2006, 281, 9030–9037. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The SCL16 gene family – Structure, role and regulation in health and disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.; Nguyen, M. Human microvascular endothelial cells differ from macrovascular endothelial cells in their expression of matrix metalloproteinases. Int. J. Biochem. Cell Biol. 1997, 29, 1167–1177. [Google Scholar] [CrossRef]
- Lang, I.; Pabst, M.A.; Hiden, U.; Blaschitz, A.; Dohr, G.; Hahn, T.; Desoye, G. Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. Eur. J. Cell Biol. 2003, 82, 163–173. [Google Scholar] [CrossRef]
| Gene | Primers | Annealing Temperature (°C) | Amplicon Size (bp) |
|---|---|---|---|
| β-actin | Fw: GACGACATGGAGAAAATCTG Rv: ATGATCTGGGTCATCTTCTC | 60 | 131 |
| LDH-A | Fw: CACCATGATTAAGGGTCTTTAC Rv: AGGTCTGAGATTCCATTCTG | 60 | 87 |
| LDH-B | Fw: CAACAATGGTAAAGGGGATG Rv: TCACTAGTCACAGGTCTTTTAG | 60 | 189 |
| MCT1 | Fw: GAGGTCCTATCAGCAGTATC Rv: CAATGACTCCAATACAGACG | 60 | 144 |
| MCT4 | Fw: CAGTTCGAGGTGCTCATGG Rv: ATGTAGACGTGGGTCGCAT | 60 | 140 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocaña, M.C.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions. Biomolecules 2019, 9, 332. https://doi.org/10.3390/biom9080332
Ocaña MC, Martínez-Poveda B, Quesada AR, Medina MÁ. Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions. Biomolecules. 2019; 9(8):332. https://doi.org/10.3390/biom9080332
Chicago/Turabian StyleOcaña, Mª Carmen, Beatriz Martínez-Poveda, Ana R. Quesada, and Miguel Ángel Medina. 2019. "Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions" Biomolecules 9, no. 8: 332. https://doi.org/10.3390/biom9080332
APA StyleOcaña, M. C., Martínez-Poveda, B., Quesada, A. R., & Medina, M. Á. (2019). Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions. Biomolecules, 9(8), 332. https://doi.org/10.3390/biom9080332






