Impact of the Mode of Extraction on the Lipidomic Profile of Oils Obtained from Selected Amazonian Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Collection and Suitability
2.2. Extraction of Oil from Selected Palms Species
2.3. Physicochemical Characterization
2.4. Experimental Design and Data Analysis
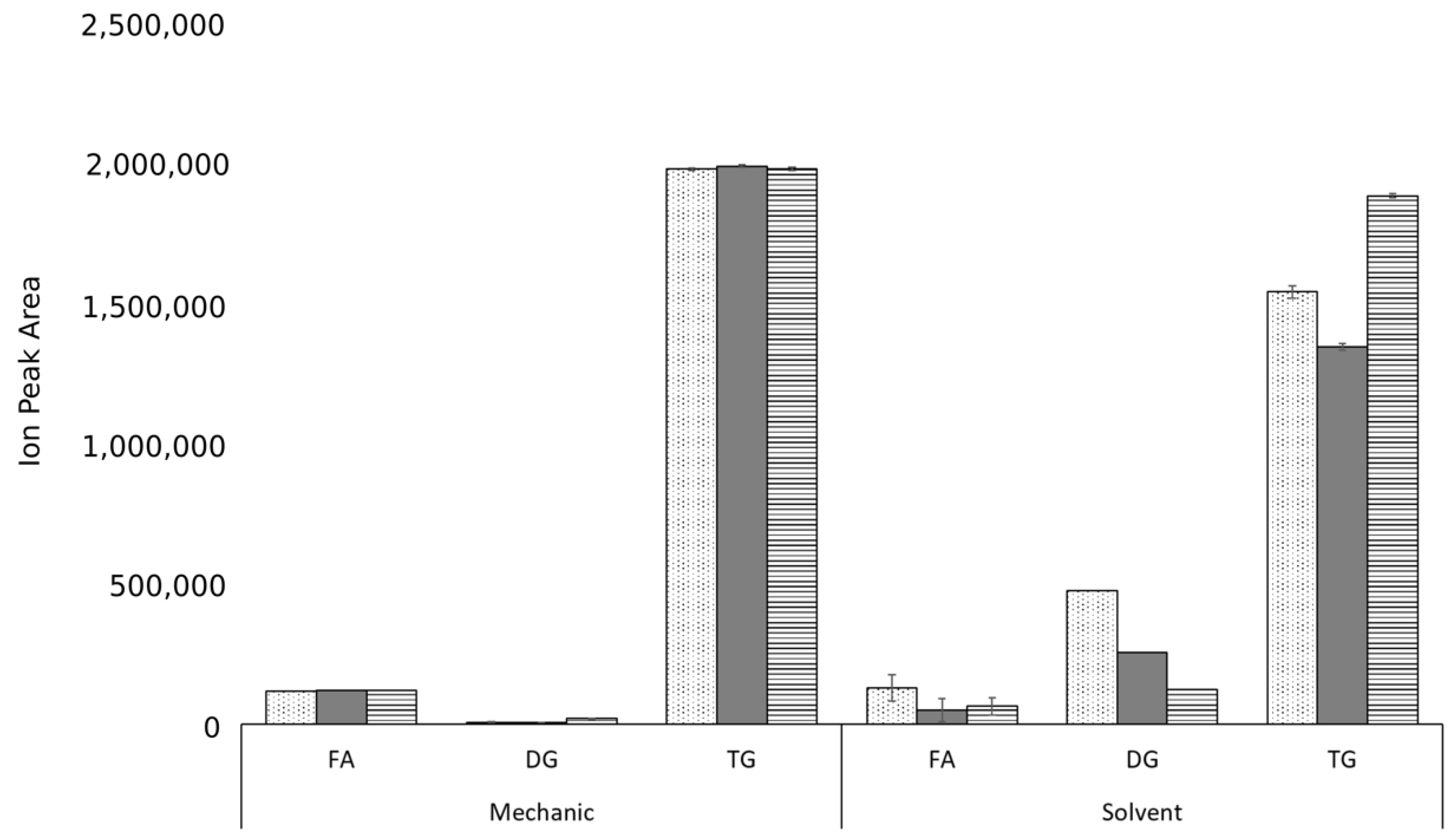
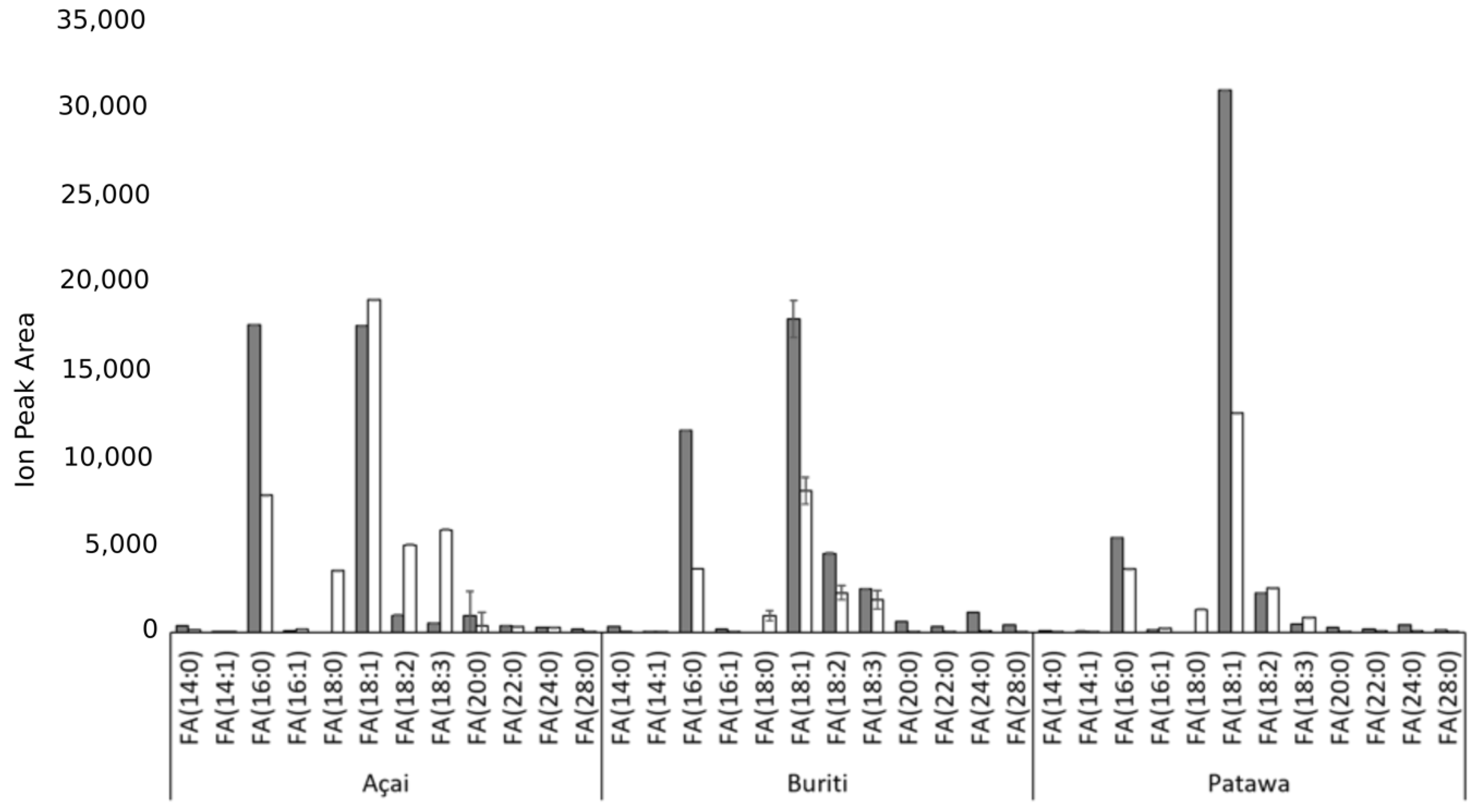
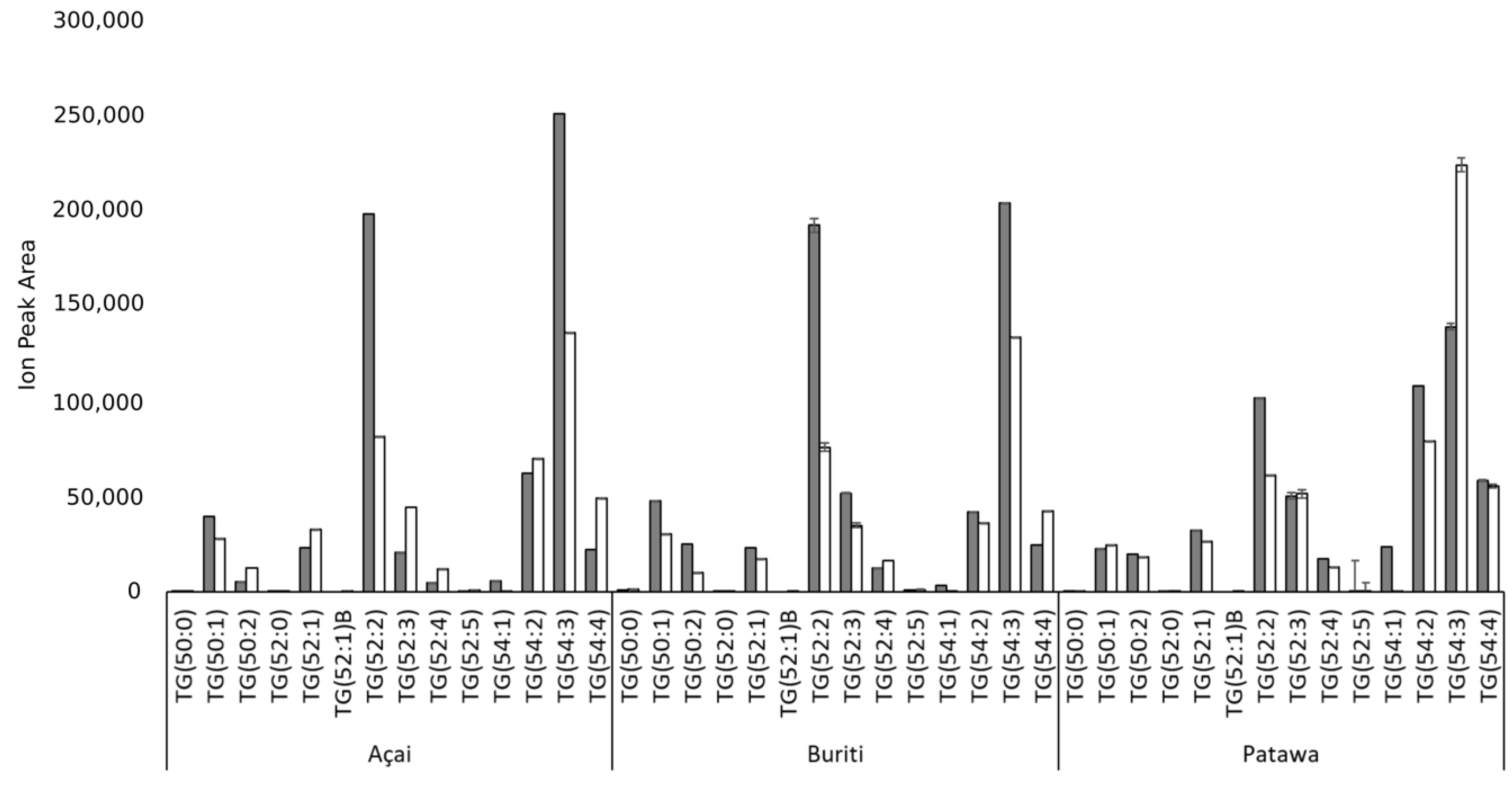
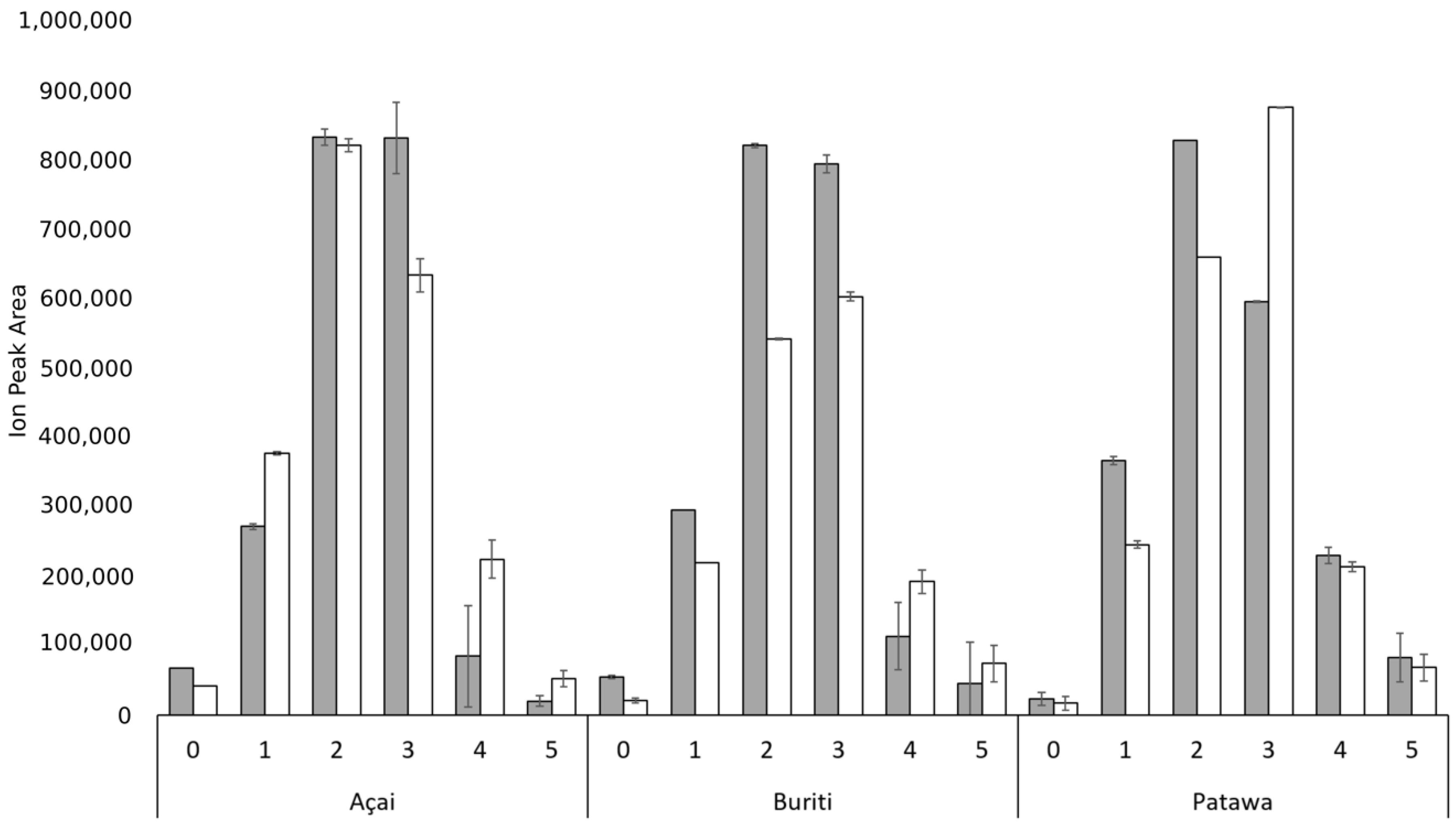
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Secchi, M.; Castellani, V.; Collina, E.; Mirabella, N.; Sala, S. Assessing eco-innovations in green chemistry: Life Cycle Assessment (LCA) of a cosmetic product with a bio-based ingredient. J. Clean. Prod. 2016, 129, 269–281. [Google Scholar] [CrossRef]
- Sakamoto, K.; Lochhead, R.Y.; Maibach, H.I.; Yamashita, Y.; Ifuku, O. Chapter 20—Botanical Ingredients. In Cosmetic Science and Technology; Elsevier: Amsterdam, The Netherland, 2017; pp. 305–320. ISBN 9780128020050. [Google Scholar]
- Cizauskaite, U.; Marksiene, R.; Viliene, V.; Gruzauskas, R.; Bernatoniene, J. New strategy of multiple emulsion formation based on the interactions between polymeric emulsifier and natural ingredients. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 515, 22–33. [Google Scholar] [CrossRef]
- Angerhofer, C.K.; Maes, D.; Giacomoni, P.U. Chapter 10—The Use of Natural Compounds and Botanicals in the Development of Anti-Aging Skin Care Products. In Skin Aging Handbook; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 205–263. ISBN 9780815515845. [Google Scholar]
- Bernal, R.; Torres, C.; García, N.; Isaza, C.; Navarro, J.; Vallejo, M.I.; Galeano, G.; Balslev, H. Palm Management in South America. Bot. Rev. 2011, 77, 607–646. [Google Scholar] [CrossRef]
- Lee, R.; Balick, M.J. Palms, People, and Health. Explore 2008, 4, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Macía, M.; Armesilla, P.; Cámara-Leret, R.; Paniagua-Zambrana, N.; Villalba, S.; Balslev, H.; Pardo-de-Santayana, M. Palm Uses in Northwestern South America: A Quantitative Review. Bot. Rev. 2011, 77, 462–570. [Google Scholar] [CrossRef]
- Balslev, H.; Copete, J.C.; Pedersen, D.; Bernal, R.; Galeano, G.; Duque, Á.; Berrio, J.C.; Sanchéz, M. Palm Diversity and Abundance in the Colombian Amazon. In Forest structure, function and dynamics in Western Amazonia; Myster, R.W., Ed.; Wiley-Blackwel: West Sussex, UK, 2017; pp. 101–123. ISBN 9781119090670. [Google Scholar]
- Isaza, C.; Bernal, R.; Galeano, G.; Martorell, C. Demography of Euterpe precatoria and Mauritia flexuosa in the Amazon: application of integral projection models for their harvest. Biotropica 2017, 49, 653–664. [Google Scholar] [CrossRef]
- Carrillo, M.P.; Cardona, J.E.C.; Peña, L.F.; Díaz, R.O.; Mosquera, L.E.; Hernández, M.S. El rol de la ingeniería en el aprovechamiento sostenible de la biodiversidad de la amazonia. Rev. Ing. 2015, 42, 67–70. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Cardenas, D.; Ramirez, J.G. Plantas útiles y su incorporación a los sistemas productivos del departamento del Guaviare (Amazonia Colombiana). Caldasia 2004, 26, 95–110. [Google Scholar]
- Castro, S.Y.; Barrera, J.A.; Carrillo, M.P.; Hernández, M.S. Asaí (Euterpe precatoria); Sinchi, Instituto Amazónico de Investigaciones Científicas; Ministerio de Ambiente y Desarrollo Sostenible: Bogotá, Colombia, 2015.
- Vieira De Abreu, Y.; Gualberto De Ávila, R. The buriti agro-extractivism: an alternative for the development of the Brazilian Amazon region. Int. J. Soc. Sci. Entrep. 2014, 1, 189–197. [Google Scholar]
- Virapongse, A.; Endress, B.A.; Gilmore, M.P.; Horn, C.; Romulo, C. Ecology, livelihoods, and management of the Mauritia flexuosa palm in South America. Glob. Ecol. Conserv. 2017, 10, 70–92. [Google Scholar] [CrossRef]
- Escriche, I.; Restrepo, J.; Serra, J.A.; Herrera, L.F. Composition and Nutritive Value of Amazonian Palm Fruits. Food Nutr. Bull. 1999, 20, 361–364. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Maria do Socorro, M.R.; Pérez-Jiménez, J.; Arranz, S.; Alves, R.E.; de Brito, E.S.; Oliveira, M.S.; Saura-Calixto, F. Açaí (Euterpe oleraceae) [‘]BRS Pará’: a tropical fruit source of antioxidant dietary fiber and high antioxidant capacity oil. Food Res. Int. 2011, 44, 2100–2106. [Google Scholar]
- da Costa, P.A.; Ballus, C.A.; Teixeira-Filho, J.; Godoy, H.T. Phytosterols and tocopherols content of pulps and nuts of Brazilian fruits. Food Res. Int. 2010, 43, 1603–1606. [Google Scholar] [CrossRef]
- Ferreira de França, L.; Reber, G.; Meireles, M.A.A.; Machado, N.T.; Brunner, G. Supercritical extraction of carotenoids and lipids from buriti (Mauritia flexuosa), a fruit from the Amazon region. J. Supercrit. Fluids 1999, 14, 247–256. [Google Scholar] [CrossRef]
- Aquino, J.D.S.; Pessoa, D.C.; Araújo, K.D.L.G.; Epaminondas, P.S.; Schuler, A.R.P.; Souza, A.G.D.; Stamford, T.L.M. Refining of buriti oil (Mauritia flexuosa) originated from the Brazilian Cerrado: physicochemical, thermal-oxidative and nutritional implications. J. Braz. Chem. Soc. 2012, 23, 212–219. [Google Scholar] [CrossRef]
- Bataglion, G.A.; da Silva, F.M.A.; Eberlin, M.N.; Koolen, H.H.F. Simultaneous quantification of phenolic compounds in buriti fruit (Mauritia flexuosa L.f.) by ultra-high performance liquid chromatography coupled to tandem mass spectrometry. Food Res. Int. 2014, 66, 396–400. [Google Scholar] [CrossRef]
- Silva, S.M.; Sampaio, K.A.; Taham, T.; Rocco, S.A.; Ceriani, R.; Meirelles, A.J.A. Characterization of oil extracted from buriti fruit (Mauritia flexuosa) grown in the Brazilian Amazon Region. J. Am. Oil Chem. Soc. 2009, 86, 611–616. [Google Scholar] [CrossRef]
- Rezaire, A.; Robinson, J.-C.; Bereau, D.; Verbaere, A.; Sommerer, N.; Khan, M.K.; Durand, P.; Prost, E.; Fils-Lycaon, B. Amazonian palm Oenocarpus bataua (“patawa”): Chemical and biological antioxidant activity--phytochemical composition. Food Chem. 2014, 149, 62–70. [Google Scholar] [CrossRef]
- Montúfar, R.; Laffargue, A.; Pintaud, J.-C.C.; Hamon, S.; Avallone, S.; Dussert, S.; Montufar, R.; Laffargue, A.; Pintaud, J.-C.C.; Hamon, S.; et al. Oenocarpus bataua Mart. (Arecaceae): Rediscovering a Source of High Oleic Vegetable Oil from Amazonia. J. Am. Oil Chem. Soc. 2010, 87, 167–172. [Google Scholar] [CrossRef]
- De Cássia Rodrigues Batista, C.; De Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; Da Silva Souza Filho, A.P.; Machado, N.T.; Carvalho, R.N. Supercritical CO2extraction of açaí (Euterpe oleracea) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids 2015, 107, 364–369. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J.P. Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae Mart. (Acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.C.; Meda, V.; Rout, P.K.; Naik, S.; Dalai, A.K. Supercritical CO2 extraction of fatty oil from flaxseed and comparison with screw press expression and solvent extraction processes. J. Food Eng. 2010, 98, 393–397. [Google Scholar] [CrossRef]
- Panadare, D.C.; Rathod, V.K. Three phase partitioning for extraction of oil: A review. Trends Food Sci. Technol. 2017, 68, 145–151. [Google Scholar] [CrossRef]
- Chemat, F.; Fabiano-Tixier, A.S.; Vian, M.A.; Allaf, T.; Vorobiev, E. Solvent-free extraction of food and natural products. TrAC Trends Anal. Chem. 2015, 71, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Delfan-Hosseini, S.; Nayebzadeh, K.; Mirmoghtadaie, L.; Kavosi, M.; Hosseini, S.M. Effect of extraction process on composition, oxidative stability and rheological properties of purslane seed oil. Food Chem. 2017, 222, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Fabiano-Tixier, A.S.; Abert Vian, M.; Allaf, T.; Vorobiev, E. Chapter Eight—Solvent-Free Extraction. In Green Extraction Techniques; Ibáñez, E., Cifuentes, A.B.T.-C.A.C., Eds.; Elsevier: Amsterdam, The Netherland, 2017; Volume 76, pp. 225–254. ISBN 0166-526X. [Google Scholar]
- Fetzer, D.L.; Cruz, P.N.; Hamerski, F.; Corazza, M.L. Extraction of baru (Dipteryx alata vogel) seed oil using compressed solvents technology. J. Supercrit. Fluids 2018, 137, 23–33. [Google Scholar] [CrossRef]
- Perrier, A.; Delsart, C.; Boussetta, N.; Grimi, N.; Citeau, M.; Vorobiev, E. Effect of ultrasound and green solvents addition on the oil extraction efficiency from rapeseed flakes. Ultrason. Sonochem. 2017, 39, 58–65. [Google Scholar] [CrossRef]
- Teixeira, C.B.; Macedo, G.A.; Macedo, J.A.; da Silva, L.H.M.; da, C.; Rodrigues, A.M. Simultaneous extraction of oil and antioxidant compounds from oil palm fruit (Elaeis guineensis) by an aqueous enzymatic process. Bioresour. Technol. 2013, 129, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. J. Supercrit. Fluids 2018, 135, 45–51. [Google Scholar] [CrossRef]
- Trentini, C.P.; Santos, K.A.; Da Silva, A.; Dos, A.; Garcia, S.; Cardozo-Filho, L.; Da Silva, C. Oil extraction from macauba pulp using compressed propane. J. Supercrit. Fluids 2017, 126, 72–78. [Google Scholar] [CrossRef]
- Kumar, R.C.; Benal, M.M.; Prasad, B.D.; Krupashankara, M.S.; Kulkarni, R.S.; Siddaligaswamy, N.H. Microwave assisted extraction of oil from pongamia pinnata seeds. Mater. Today Proc. 2018, 5, 2960–2964. [Google Scholar] [CrossRef]
- Fornasari, C.H.; Secco, D.; Santos, R.F.; da Silva, T.R.B.; Galant Lenz, N.B.; Tokura, L.K.; Lenz, M.L.; de Souza, S.N.M.; Zanão Junior, L.A.; Gurgacz, F. Efficiency of the use of solvents in vegetable oil extraction at oleaginous crops. Renew. Sustain. Energy Rev. 2017, 80, 121–124. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Comparison of Different Methods for the Determination of the Oil Content in Oilseeds. J. Am. Oil Chem. Soc. 2001, 78, 95–102. [Google Scholar] [CrossRef]
- Brühl, L.; Matthäus, B. Extraction of oilseeds by SFE-A comparison with other methods for the determination of the oil content. Fresenius’ J. Anal. Chem. 1999, 364, 631–634. [Google Scholar]
- Nasa, J.L.; Degano, I.; Brandolini, L.; Modugno, F.; Bonaduce, I. A novel HPLC-ESI-Q-ToF approach for the determination of fatty acids and acylglycerols in food samples. Anal. Chim. Acta 2018, 1013, 98–109. [Google Scholar] [CrossRef]
- Sirbu, D.; Corno, M.; Ullrich, M.S.; Kuhnert, N. Characterization of triacylglycerols in unfermented cocoa beans by HPLC- ESI mass spectrometry. Food Chem. 2018, 254, 232–240. [Google Scholar] [CrossRef]
- Firestone, D. (Ed.) Official Methods and Recommended Practices of the AOCS. Analytical Methods, 5th ed.; Firestone, D. (Ed.) American Oil Chemists’ Society: Urbana, IL, USA, 1998; ISBN 9780935315974. [Google Scholar]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef]
- Maxime Vaz, F.; Pras-Raves, M.L.; Van Kampen, A. Principles and practice of lipidomics. J. Inherit. Metab. Dis. 2014. [CrossRef]
- Hernández, G.M.S.; Barrera, G.J.A.; Páez, B.D.; Oviedo, A.E.; Romero, R.H. Aspectos biológicos y conservación poscosecha de la cocona (Solanum sessiliflorum) en la Amazonia Occidental Colombiana. In Aspectos biológicos y conservación de frutas promisorias de la amazonia colombiana; González, D.V., Ed.; Instituto Amazónico de Investigaciones Científicas, SINCHI; Universidad de la Amazonia: Bogotá, Colombia, 2004; pp. 127–150. [Google Scholar]
- Alves, E.; Rey, F.; Costa, E.D.; Moreira, A.S.P.; Pato, L.; Pato, L.; Domingues, M.D.R.M.; Domingues, P. Olive (Olea europaea L. cv. Galega vulgar) Seed Oil: A First Insight into the Major Lipid Composition of a Promising Agro-Industrial By-Product at Two Ripeness Stages. Eur. J. Lipid Sci. Technol. 2018, 120, 1700381. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.-J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry—A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Miyahara, R. Emollients. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherland, 2017; pp. 245–253. ISBN 9780128020548. [Google Scholar]
- Wei, F.; Hu, N.; Lv, X.; Dong, X.-Y.; Chen, H. Quantitation of triacylglycerols in edible oils by off-line comprehensive two-dimensional liquid chromatography–atmospheric pressure chemical ionization mass spectrometry using a single column. J. Chromatogr. A 2015, 1404, 60–71. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Lusardi, R.; Chiavaro, E.; Cerretani, L.; Bendini, A.; Ramis-Ramos, G.; Simó-Alfonso, E.F. Use of triacylglycerol profiles established by high performance liquid chromatography with ultraviolet-visible detection to predict the botanical origin of vegetable oils. J. Chromatogr. A 2011, 1218, 7521–7527. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Profiling triacylglycerols, fatty acids and tocopherols in hazelnut varieties grown in Turkey. J. Food Compos. Anal. 2015, 44, 115–121. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Triacylglycerols: Structures and Properties. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldra, F., Eds.; Elsevier: Amsterdam, The Netherland, 2015; pp. 351–356. ISBN 9780123849533. [Google Scholar]
- Youzbachi, N.; Trabelsi, H.; Elfalleh, W.; Khaldi, A.; Nasri, N.; Tlili, N. Fatty acids and triacylglycerols composition from Tunisian Acacia species seed oil. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Somerville, C.; Browse, J. Plant lipids: Metabolism, mutants, and membranes. Science 1991, 252, 80–87. [Google Scholar] [CrossRef]
- Loi, C.C.; Eyres, G.T.; Birch, E.J. Effect of mono- and diglycerides on physical properties and stability of a protein-stabilised oil-in-water emulsion. J. Food Eng. 2019, 240, 56–64. [Google Scholar] [CrossRef]
- Albuquerque, M.L.S.; Guedes, I.; Alcantara, P.; Moreira, S.G.C. Infrared absorption spectra of Buriti (Mauritia flexuosa L.) oil. Vib. Spectrosc. 2003, 33, 127–131. [Google Scholar] [CrossRef]
- Wycoff, W.; Luo, R.; Schauss, A.G.; Neal-Kababick, J.; Sabaa-Srur, A.U.O.; Maia, J.G.S.; Tran, K.; Richards, K.M.; Smith, R.E. Chemical and nutritional analysis of seeds from purple and white açaí (Euterpe oleracea Mart.). J. Food Compos. Anal. 2015, 41, 181–187. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Vearasilp, S.; Trakhtenberg, S.; Gorinstein, S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food Res. Int. 2011, 44, 1671–1701. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.; Nolasco, S.M.; Tomás, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]

| Fruit | Mechanical Extract | Solvent Extract |
|---|---|---|
| Açai | 10% | 18% |
| Buriti | 22% | 33% |
| Patawa | 28% | 62% |
| EXTRACTION | OIL | IODINE INDEX | SAPONIFICATION INDEX | ACIDITY INDEX % | DENSITY |
|---|---|---|---|---|---|
| MECHANICAL | Açai | 68.3 ± 2.11 | 186.0 ± 3.1 | 4.83 ± 0.05 | 0.925 ± 0.02 |
| Buriti | 76.4 ± 4.02 | 189.2 ± 0.4 | 6.13 ± 0.02 | 0.911 ± 0.04 | |
| Patawa | 76.4 ± 1.52 | 164.9 ± 5.1 | 3.92 ± 0.05 | 0.870 ± 0.04 | |
| SOLVENT | Açai | 69.2 ± 1.89 | 184.0 ± 2.0 | 1.87 ± 0.05 | 0.912 ± 0.04 |
| Buriti | 75.3 ± 2.32 | 187.5 ± 0.2 | 2.71 ± 0.02 | 0.910 ± 0.04 | |
| Patawa | 74.2 ± 3.40 | 160.7 ± 3.2 | 1.96 ± 0.05 | 0.871 ± 0.03 |
| Açai | Buriti | Patawa | ||||
|---|---|---|---|---|---|---|
| FAME | Mechanical | Solvent | Mechanical | Solvent | Mechanical | Solvent |
| C12:0 | 0.00% | 0.80% | 0.00% | 0.00% | 0.00% | 0.16% |
| C14:0 | 0.00% | 0.00% | 0.00% | 0.00% | 0.10% | 0.92% |
| C14:1 | 0.10% | 1.40% | 12.20% | 13.61% | 0.00% | 0.00% |
| C16:0 | 20.50% | 17.30% | 25.60% | 22.87% | 13.00% | 13.31% |
| C16:1 | 0.00% | 0.00% | 0.00% | 0.00% | 0.40% | 0.00% |
| C18:0 | 4.40% | 6.60% | 0.00% | 0.00% | 3.80% | 0.00% |
| C18:1n9c | 64.70% | 68.20% | 62.20% | 63.52% | 80.00% | 85.62% |
| C20:0 | 0.00% | 0.00% | 0.00% | 0.00% | 0.10% | 0.00% |
| C18:2n6c | 9.40% | 4.40% | 0.00% | 0.00% | 1.80% | 0.00% |
| C18:3n6 | 0.00% | 0.00% | 0.00% | 0.00% | 0.60% | 0.00% |
| C20:1 | 0.00% | 0.00% | 0.00% | 0.00% | 0.10% | 0.00% |
| C20:2 | 0.30% | 0.50% | 0.00% | 0.00% | 0.00% | 0.00% |
| C22:0 | 0.10% | 0.10% | 0.00% | 0.00% | 0.00% | 0.00% |
| C20:3n6 | 0.00% | 0.10% | 0.00% | 0.00% | 0.00% | 0.00% |
| SAT | 25.00% | 24.80% | 25.60% | 22.87% | 17.00% | 13.39% |
| UNS | 75.00% | 75.20% | 74.40% | 77.13% | 83.00% | 86.61% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardona Jaramillo, J.E.C.; Carrillo Bautista, M.P.; Alvarez Solano, O.A.; Achenie, L.E.K.; González Barrios, A.F. Impact of the Mode of Extraction on the Lipidomic Profile of Oils Obtained from Selected Amazonian Fruits. Biomolecules 2019, 9, 329. https://doi.org/10.3390/biom9080329
Cardona Jaramillo JEC, Carrillo Bautista MP, Alvarez Solano OA, Achenie LEK, González Barrios AF. Impact of the Mode of Extraction on the Lipidomic Profile of Oils Obtained from Selected Amazonian Fruits. Biomolecules. 2019; 9(8):329. https://doi.org/10.3390/biom9080329
Chicago/Turabian StyleCardona Jaramillo, Juliana Erika Cristina, Marcela Piedad Carrillo Bautista, Oscar Alberto Alvarez Solano, Luke E. K. Achenie, and Andrés Fernando González Barrios. 2019. "Impact of the Mode of Extraction on the Lipidomic Profile of Oils Obtained from Selected Amazonian Fruits" Biomolecules 9, no. 8: 329. https://doi.org/10.3390/biom9080329
APA StyleCardona Jaramillo, J. E. C., Carrillo Bautista, M. P., Alvarez Solano, O. A., Achenie, L. E. K., & González Barrios, A. F. (2019). Impact of the Mode of Extraction on the Lipidomic Profile of Oils Obtained from Selected Amazonian Fruits. Biomolecules, 9(8), 329. https://doi.org/10.3390/biom9080329






