Genome-Wide Identification and Expression Profiling of CBL-CIPK Gene Family in Pineapple (Ananas comosus) and the Role of AcCBL1 in Abiotic and Biotic Stress Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of CBL-CIPK Genes in Pineapple Genome
2.2. Phylogenetic Analysis
2.3. Gene Structure Analysis and Conserved Motif Identification
2.4. Chromosome Location of CBL and CIPK Genes in Pineapple
2.5. Plant Materials and Growth Condition and Treatments
2.5.1. Pineapple Growth and Treatments
2.5.2. Arabidopsis Treatments and Root Growth Assay
2.6. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.7. RNA-Seq and Data Analysis
2.8. Vector Constructs
2.9. Live Cell Imaging
2.10. Statistical Analysis
3. Results
3.1. Identification of the CBL-CIPK Genes in Pineapple
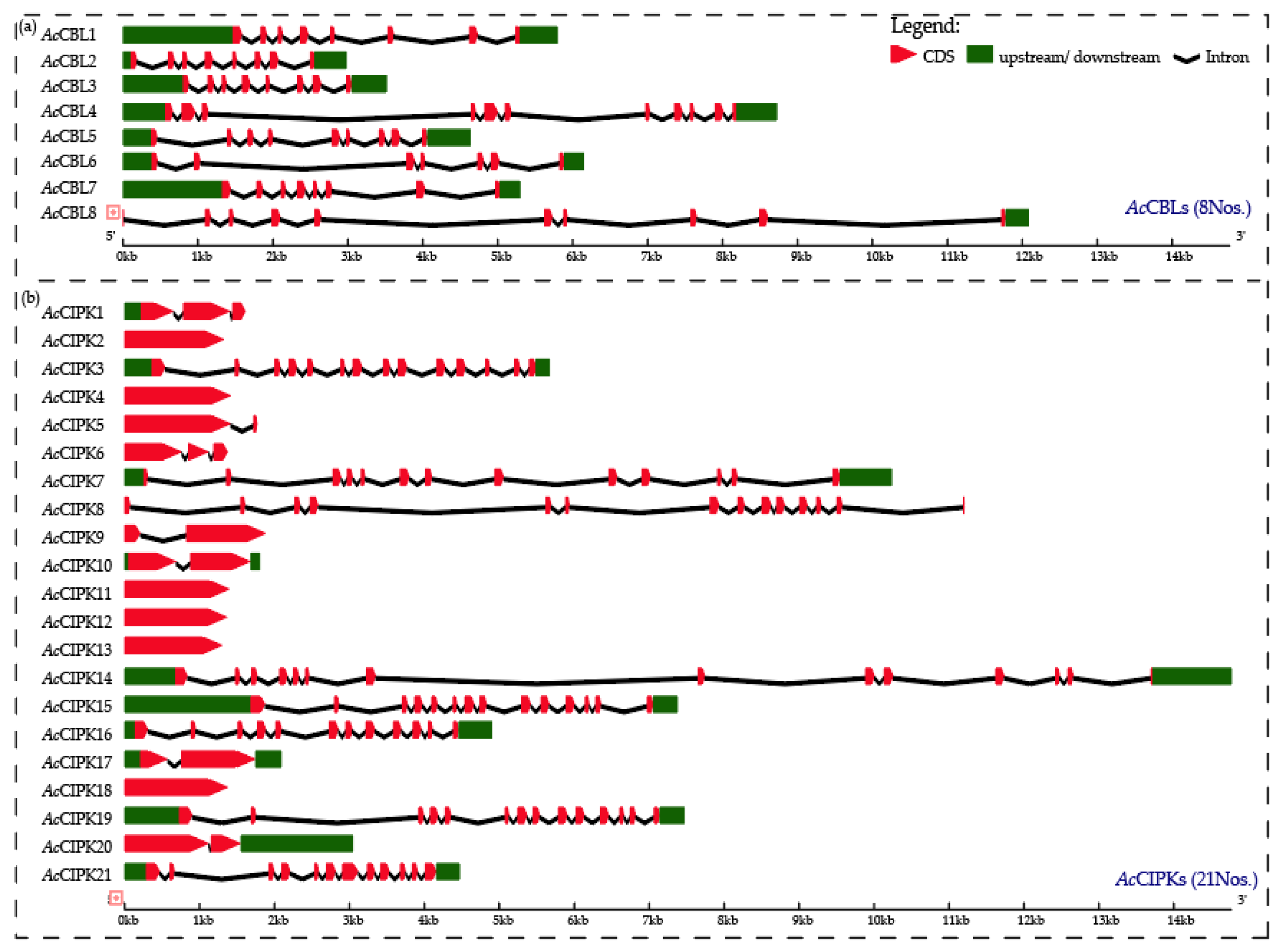
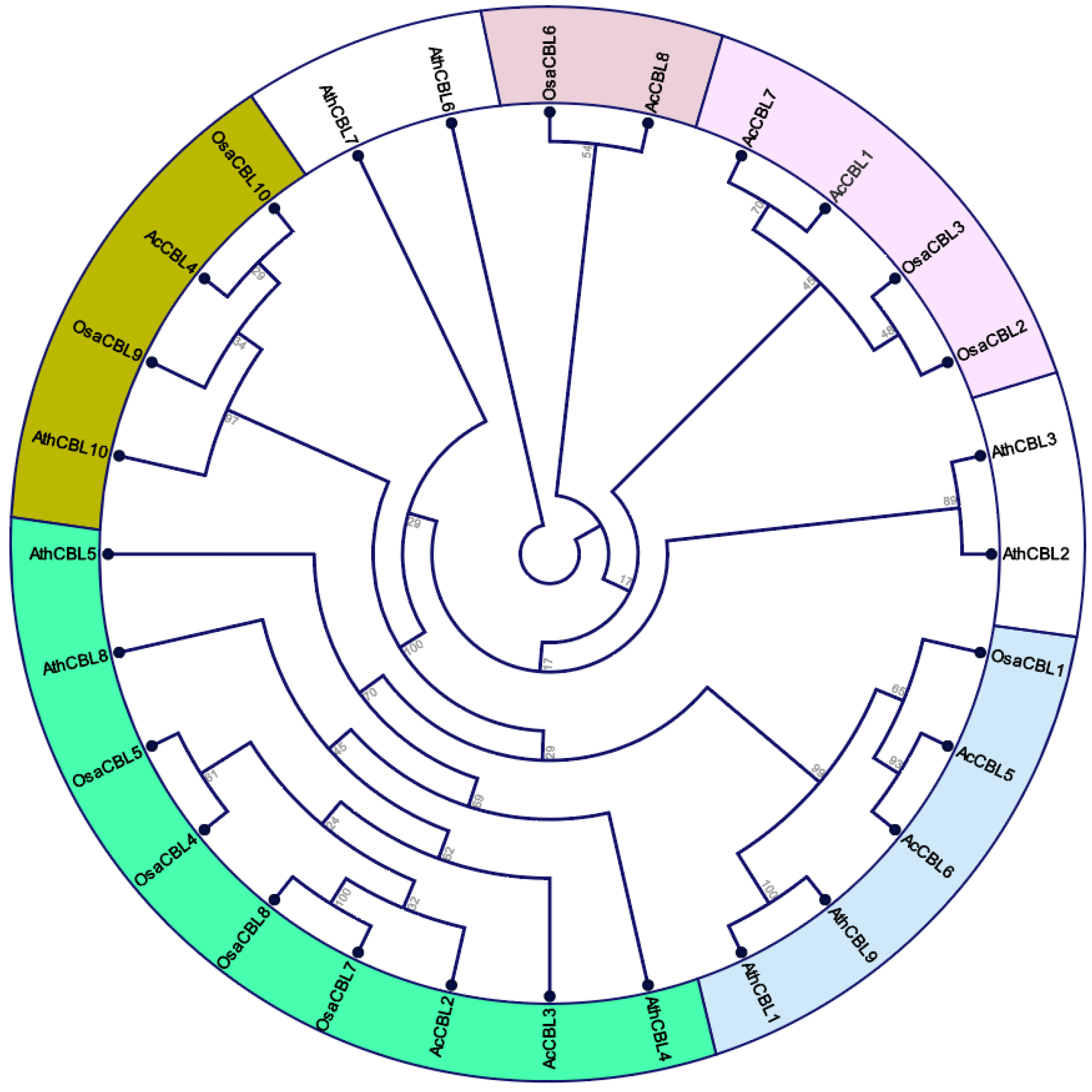
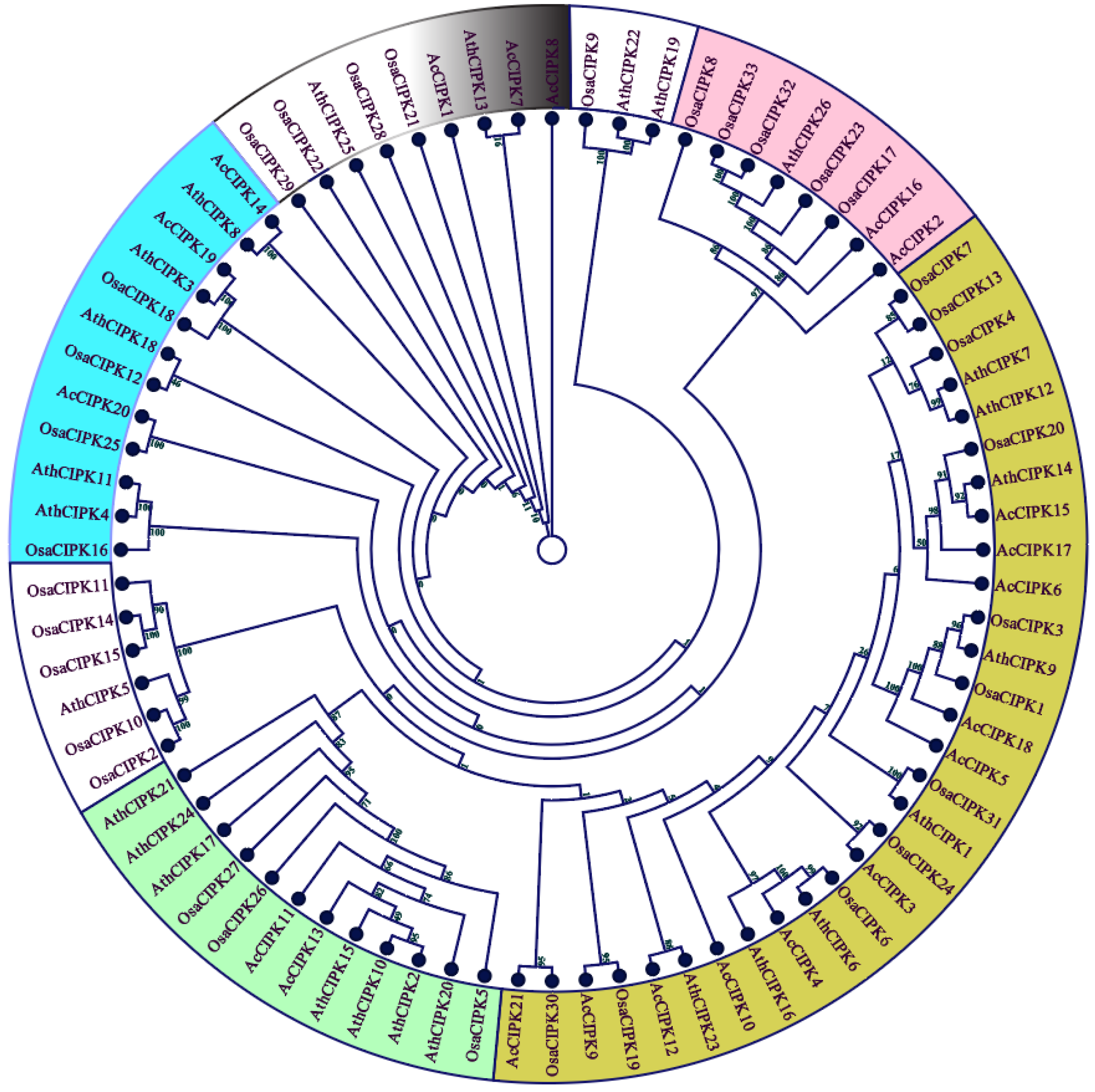
3.2. Gene Structure and Phylogenetic Analysis of Pineapple CBL-CIPK Genes
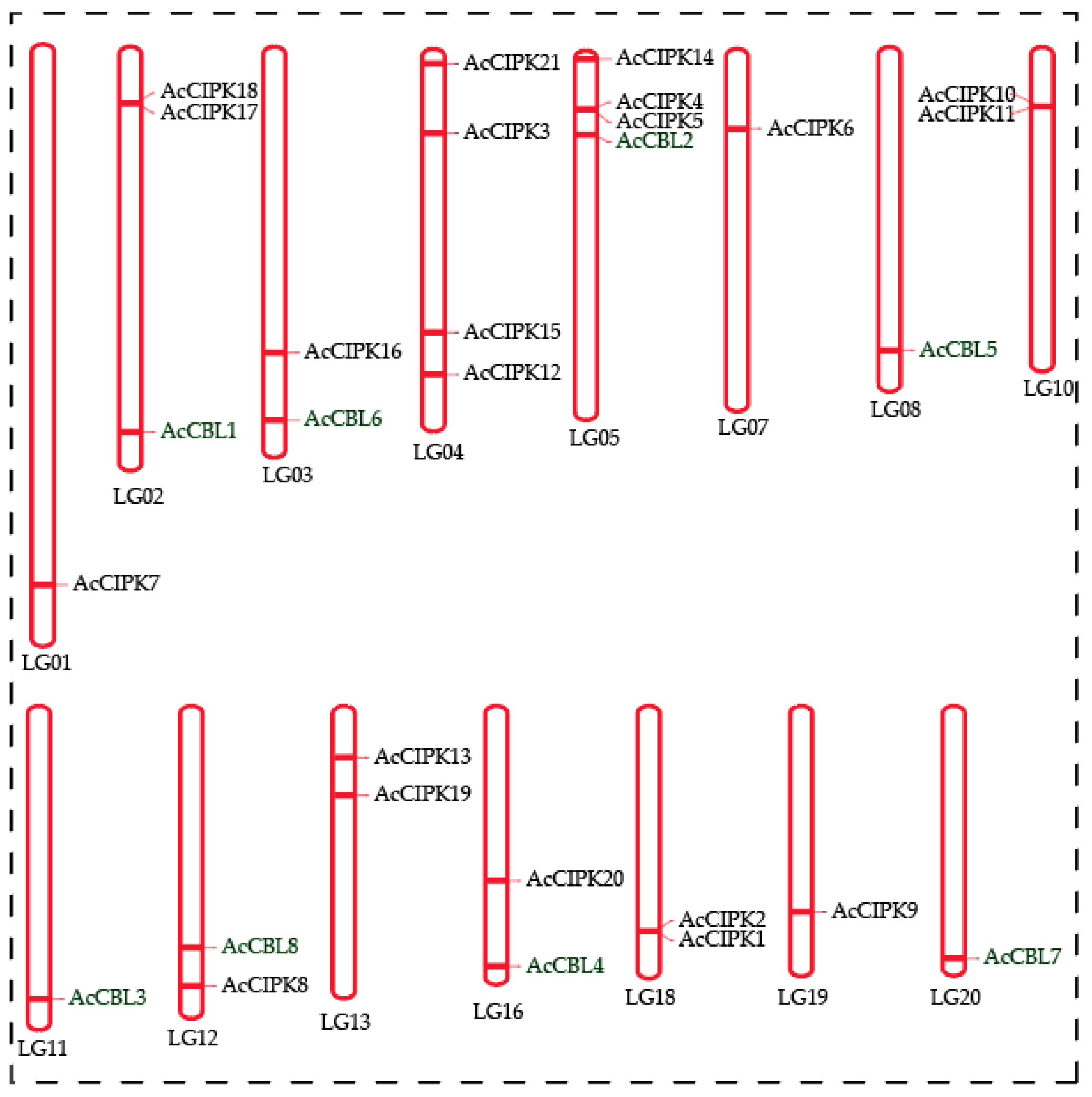
3.3. Chromosomal Distribution of Pineapple CBL-CIPK Genes
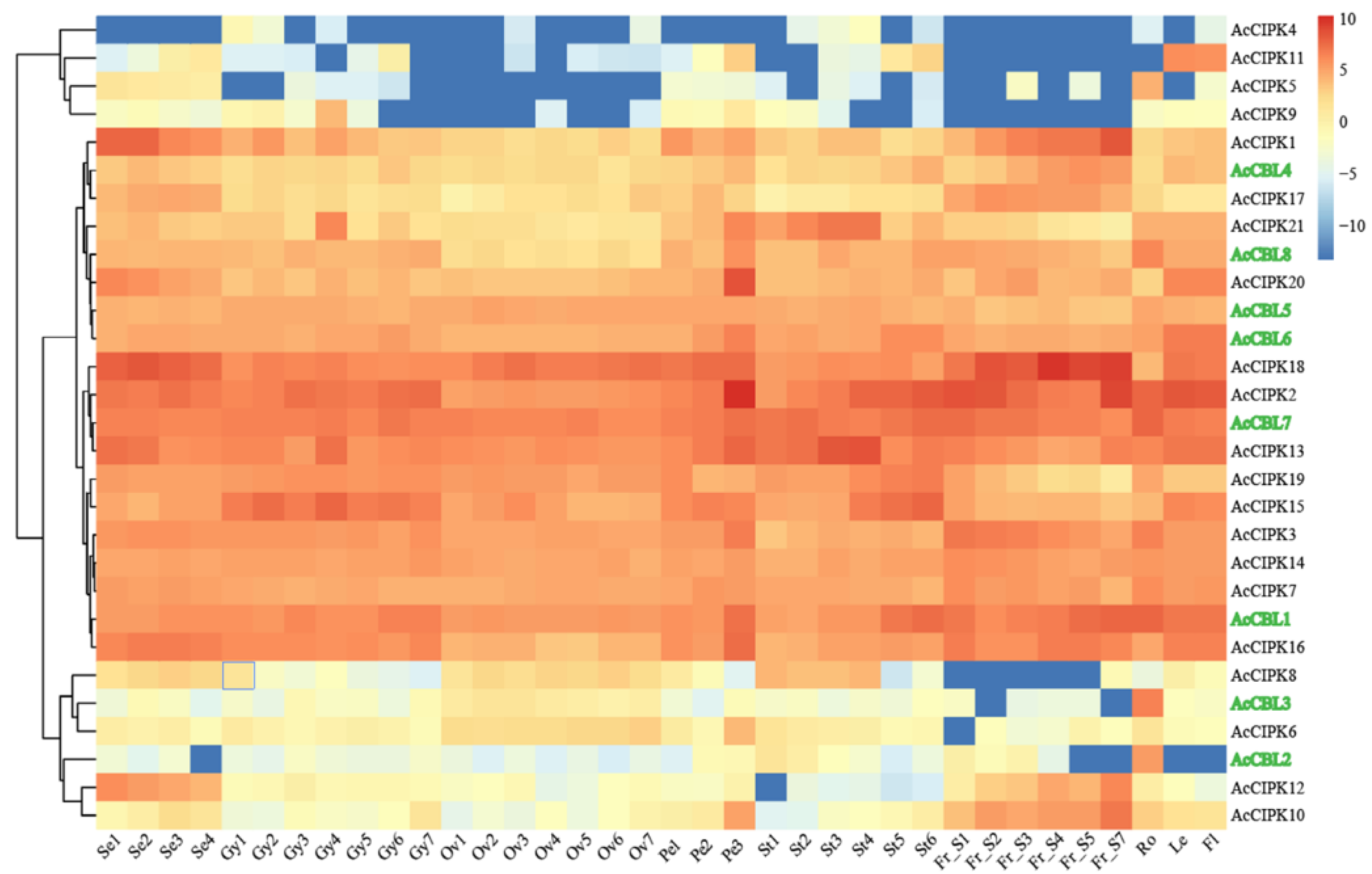
3.4. Expression Profiling of Pineapple CBL-CIPK Genes in Different Developmental Stages
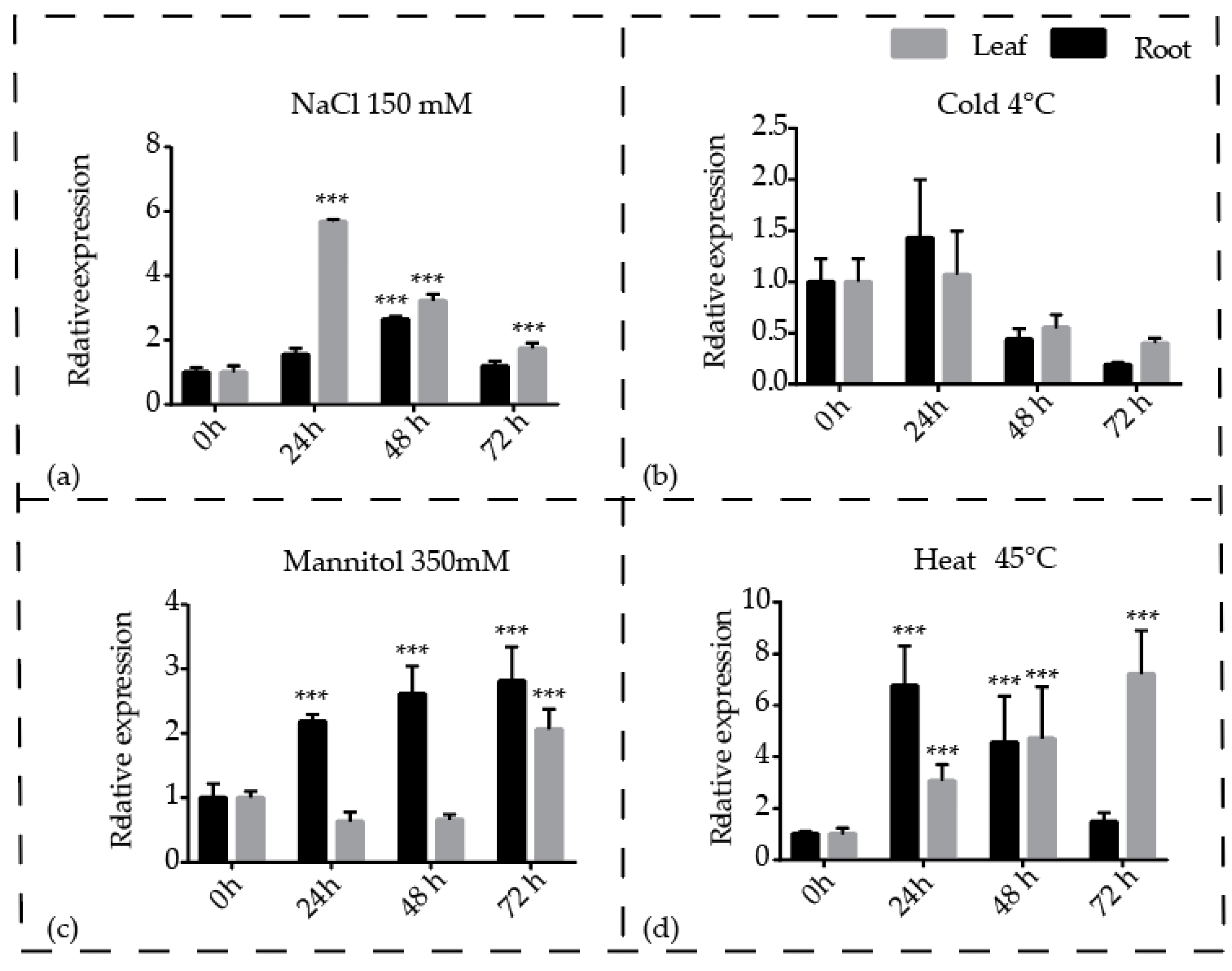
3.5. Expression Profiling of Pineapple CBL1 Gene in Response to Different Treatments
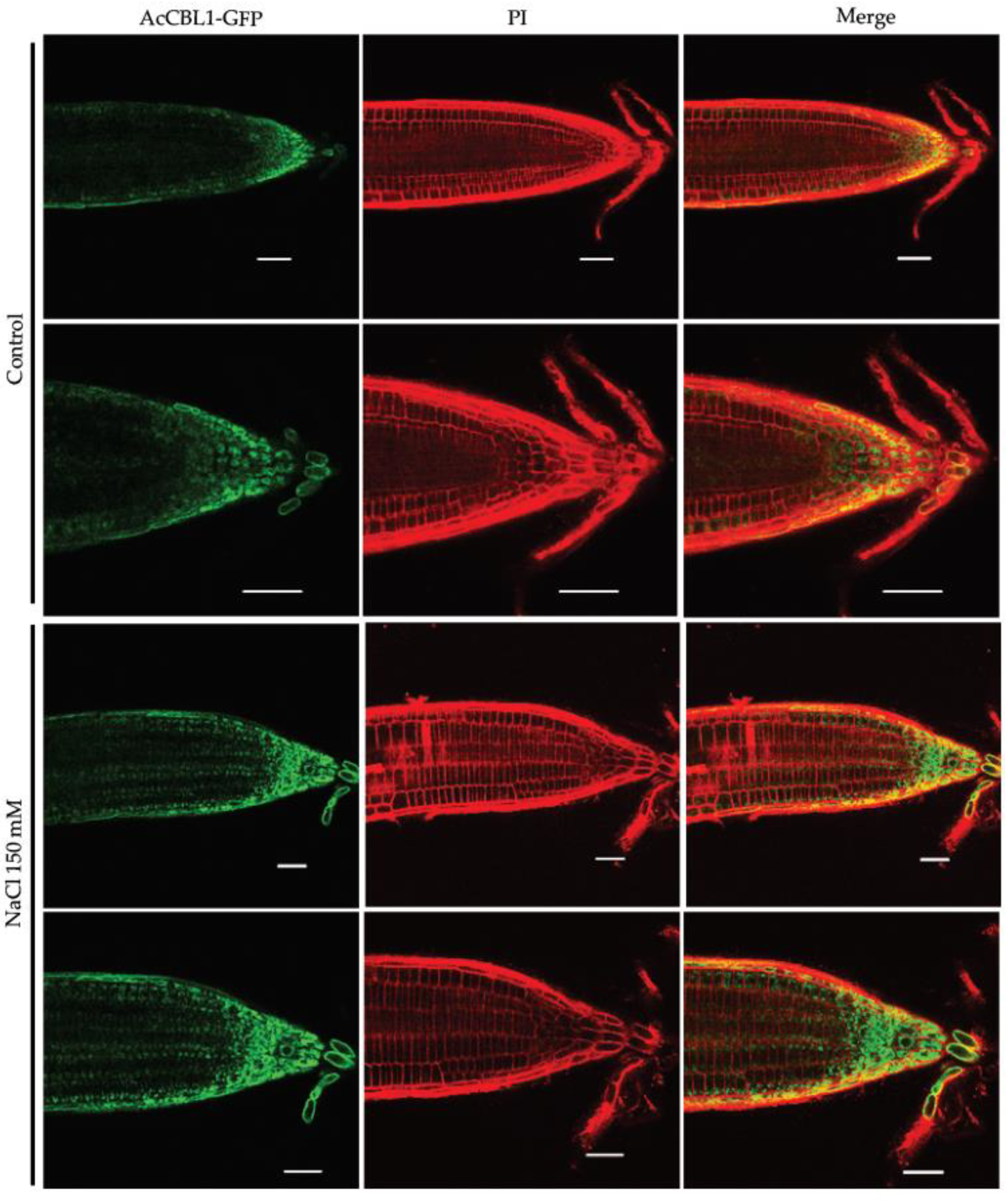
3.6. Salt Stress Increases the Localization of AcCBL1 in Arabidopsis Roots
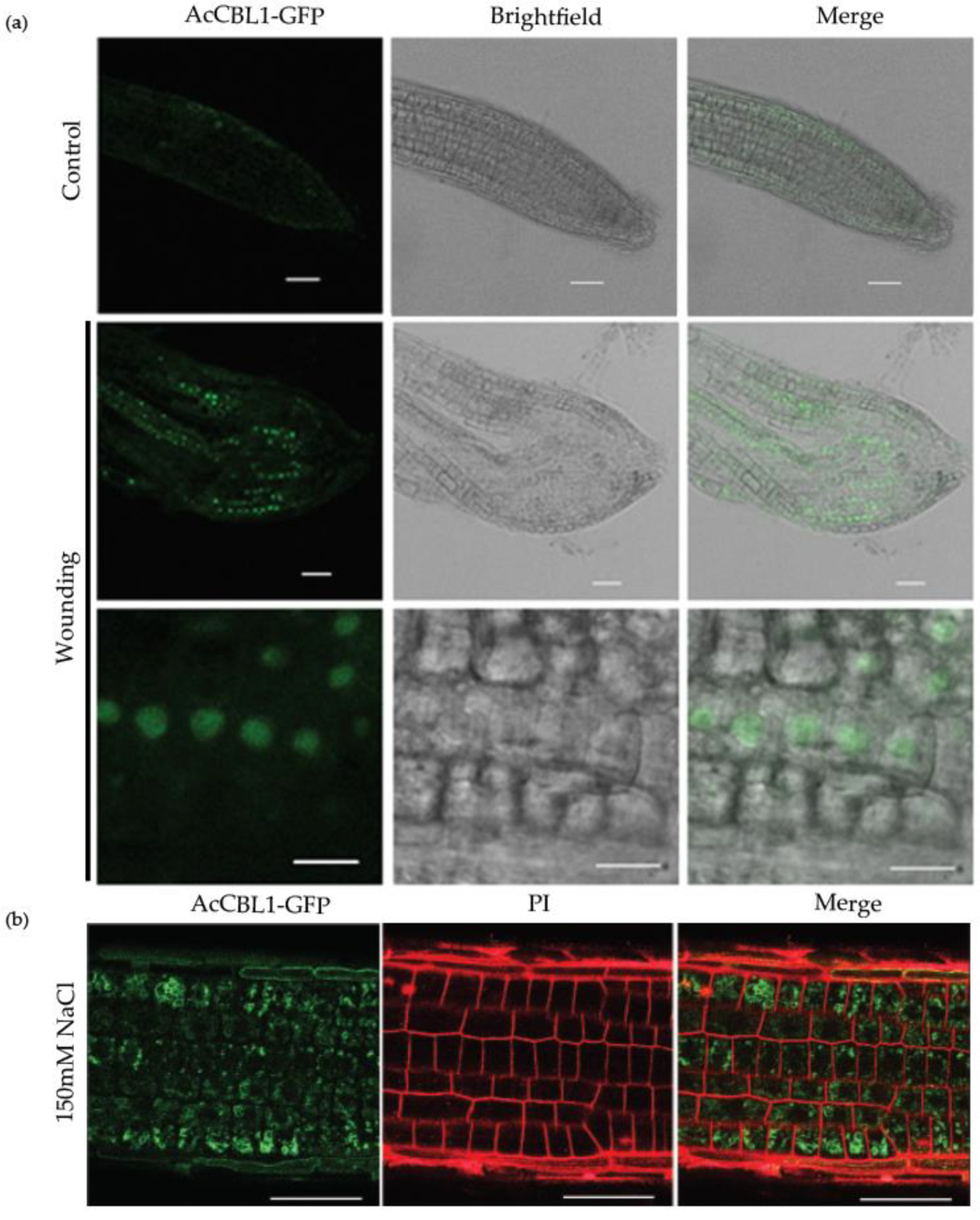
3.7. Injury Induces AcCBL1 Translocation from Cytosol to the Nucleus
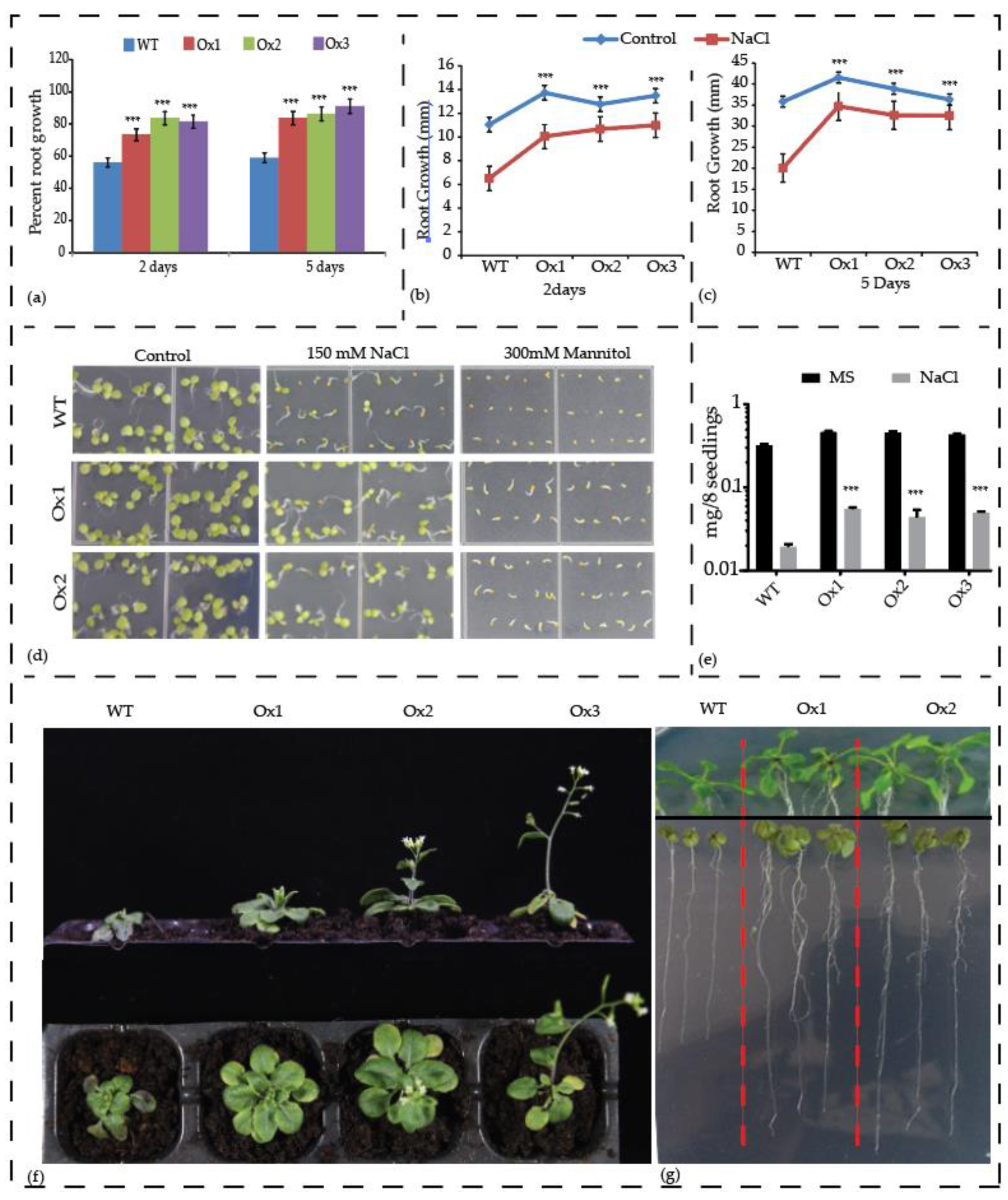
3.8. Overexpression of AcCBL1 Results in Resistance to Salinity, Osmotic, and Biotic Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CBL | Calcineurin B-like |
| CIPK | CBL-interacting protein kinases |
| Ca2+ | Calcium ion |
| ER | Endoplasmic reticulum |
| CaM | Calmodulin |
| CDPKs | Calcium-dependent protein kinases |
| SOS | Salt overlay sensitive |
| UTR | Untranslated region |
| CDS | Coding sequence |
| MEME | Multiple Em for Motif Elicitation |
| LG | Linkage groups |
| DAB | 3,3-diaminobenzidine |
References
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Navazio, L.; Szabo, I. The contribution of organelles to plant intracellular Calcium signalling. J. Exp. Bot. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Behera, S.; Zhaolong, X.; Luoni, L.; Bonza, M.C.; Doccula, F.G.; De Michelis, M.I.; Morris, R.J.; Schwarzlander, M.; Costa, A. Cellular Ca(2+) Signals Generate Defined pH Signatures in Plants. Plant Cell. 2018, 30, 2704–2719. [Google Scholar] [CrossRef]
- Meena, M.K.; Prajapati, R.; Krishna, D.; Divakaran, K.; Pandey, Y.; Reichelt, M.; Mathew, M.K.; Boland, W.; Mithofer, A.; Vadassery, J. The Ca2+ Channel CNGC19 Regulates Arabidopsis defense against Spodoptera Herbivory. Plant Cell. 2019. [Google Scholar] [CrossRef]
- Van Loon, L.C. The Intelligent Behavior of Plants. Trend. Plant Sci. 2016, 21, 286–294. [Google Scholar] [CrossRef]
- De Vriese, K.; Himschoot, E.; Dünser, K.; Nguyen, L.; Drozdzecki, A.; Costa, A.; Nowack, M.K.; Kleine-Vehn, J.; Audenaert, D.; Beeckman, T.; et al. Identification of Novel Inhibitors of Auxin-Induced Ca2+ Signaling via a Plant-Based Chemical Screen. Plant Physiol. 2019, 180, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Blatt, M.R.; Grabov, A. Signal redundancy, gates and integration in the control of ion channels for stomatal movement. J. Exp. Bot. 1997, 48, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Michard, E.; Simon, A.A.; Tavares, B.; Wudick, M.M.; Feijo, J.A. Signaling with Ions: The Keystone for Apical Cell Growth and Morphogenesis in Pollen Tubes. Plant Physiol. 2017, 173, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.W.; Zielinski, R.E.; Huber, S.C. Revisiting paradigms of Ca(2+) signaling protein kinase regulation in plants. Biochem. J. 2018, 475, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.P.; Thuleau, P.; Mazars, C. Calcium Sensors as Key Hubs in Plant Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Xu, Q.; Harter, K.; Gruissem, W.; Luan, S. Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc. Natl. Acad. Sci. USA 1999, 96, 4718–4723. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. A calcium sensor homolog required for plant salt tolerance. Science 1998, 280, 1943–1945. [Google Scholar] [CrossRef]
- Albrecht, V.; Ritz, O.; Linder, S.; Harter, K.; Kudla, J. The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J. 2001, 20, 1051–1063. [Google Scholar] [CrossRef]
- Kolukisaoglu, U.; Weinl, S.; Blazevic, D.; Batistic, O.; Kudla, J. Calcium sensors and their interacting protein kinases: Genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004, 134, 43–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Y.; Jahan, N.; Chen, G.; Ren, D.; Guo, L. Sensing of Abiotic Stress and Ionic Stress Responses in Plants. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Kumar, V.; Han, X.; Kim, S.H.; Jeong, J.H.; Kim, C.M.; Gao, Y.; Han, C.D. CBL-INTERACTING PROTEIN KINASE 9 regulates ammonium-dependent root growth downstream of IDD10 in rice (Oryza sativa). Ann. Bot. 2019. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Ma, L.; Yang, Z.; Dong, Q.; Li, Q.; Ni, X.; Kudla, J.; Song, C.-P.; Guo, Y. The Ca2+ sensor SCaBP3/CBL7 Fine Tunes Arabidopsis Alkali Tolerance and Modulats Plasma Membrane H+-ATPase Activity. Plant Cell 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Xu, Z.S.; He, G.Y.; Yang, G.X.; Chen, M.; Li, L.C.; Ma, Y. The voltage-dependent anion channel 1 (AtVDAC1) negatively regulates plant cold responses during germination and seedling development in Arabidopsis and interacts with calcium sensor CBL1. Int. J. Mol. Sci. 2013, 14, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ding, S.; Zhang, H.; Du, H.; An, L. CIPK7 is involved in cold response by interacting with CBL1 in Arabidopsis thaliana. Plant Sci. 2011, 181, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ligaba-Osena, A.; Fei, Z.; Liu, J.; Xu, Y.; Shaff, J.; Lee, S.C.; Luan, S.; Kudla, J.; Kochian, L.; Pineros, M. Loss-of-function mutation of the calcium sensor CBL1 increases aluminum sensitivity in Arabidopsis. New Phytol. 2017, 214, 830–841. [Google Scholar] [CrossRef]
- Lan, W.Z.; Lee, S.C.; Che, Y.F.; Jiang, Y.Q.; Luan, S. Mechanistic analysis of AKT1 regulation by the CBL-CIPK-PP2CA interactions. Mol. Plant 2011, 4, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Xu, Z.S.; Chen, Y.; He, G.Y.; Yang, G.X.; Chen, M.; Li, L.C.; Ma, Y.Z. A novel role for Arabidopsis CBL1 in affecting plant responses to glucose and gibberellin during germination and seedling development. PLoS ONE 2013, 8, e56412. [Google Scholar] [CrossRef]
- Mahs, A.; Steinhorst, L.; Han, J.P.; Shen, L.K.; Wang, Y.; Kudla, J. The calcineurin B-like Ca2+ sensors CBL1 and CBL9 function in pollen germination and pollen tube growth in Arabidopsis. Mol. Plant 2013, 6, 1149–1162. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Pandey, G.K.; Grant, J.J.; Batistic, O.; Li, L.; Kim, B.G.; Lee, S.C.; Kudla, J.; Luan, S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007, 52, 223–239. [Google Scholar] [CrossRef]
- Eckert, C.; Offenborn, J.N.; Heinz, T.; Armarego-Marriott, T.; Schultke, S.; Zhang, C.; Hillmer, S.; Heilmann, M.; Schumacher, K.; Bock, R.; et al. The vacuolar calcium sensors CBL2 and CBL3 affect seed size and embryonic development in Arabidopsis thaliana. Plant J. 2014, 78, 146–156. [Google Scholar] [CrossRef]
- Liu, L.L.; Ren, H.M.; Chen, L.Q.; Wang, Y.; Wu, W.H. A protein kinase, calcineurin B-like protein-interacting protein Kinase9, interacts with calcium sensor calcineurin B-like Protein3 and regulates potassium homeostasis under low-potassium stress in Arabidopsis. Plant Physiol. 2013, 161, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Pascaud, F.; Eckert, C.; Gajdanowicz, P.; Hashimoto, K.; Corratge-Faillie, C.; Offenborn, J.N.; Lacombe, B.; Dreyer, I.; Thibaud, J.B.; et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011, 21, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yang, Y.; Quan, R.; Mendoza, I.; Wu, Y.; Du, W.; Zhao, S.; Schumaker, K.S.; Pardo, J.M.; Guo, Y. Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell. 2009, 21, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Waadt, R.; Cheong, Y.H.; Pandey, G.K.; Dominguez-Solis, J.R.; Schultke, S.; Lee, S.C.; Kudla, J.; Luan, S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007, 52, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Ming, R. Genomic analyses of the CAM plant pineapple. J. Exp. Bot. 2014, 65, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef]
- Chen, X.; Gu, Z.; Xin, D.; Hao, L.; Liu, C.; Huang, J.; Ma, B.; Zhang, H. Identification and characterization of putative CIPK genes in maize. J. Genet. Genom. 2011, 38, 77–87. [Google Scholar] [CrossRef]
- Li, J.; Jiang, M.M.; Ren, L.; Liu, Y.; Chen, H.Y. Identification and characterization of CBL and CIPK gene families in eggplant (Solanum melongena L.). Mol. Genet. Genom. 2016, 291, 1769–1781. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, J.; Dong, C.; Cheng, Z.M. The CBL and CIPK Gene Family in Grapevine (Vitis vinifera): Genome-Wide Analysis and Expression Profiles in Response to Various Abiotic Stresses. Front. Plant Sci. 2017, 8, 978. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, W.; Xia, X. Calcineurin B-Like family in Populus: Comparative genome analysis and expression pattern under cold, drought and salt stress treatment. Plant Growth Regul. 2008, 56, 129–140. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Q.; Chen, Q.; Xiang, N.; Yang, Y.; Yang, Y. Genome-Wide Identification and Functional Analysis of the Calcineurin B-like Protein and Calcineurin B-like Protein-Interacting Protein Kinase Gene Families in Turnip (Brassica rapa var. rapa). Front. Plant Sci. 2017, 8, 1191. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Wai, C.M.; Guyot, R. Pineapple Genome: A Reference for Monocots and CAM Photosynthesis. Trends Genet. 2016, 32, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, S.; Hu, B.; Li, W.; Ali, H.; Jia, H.; Zhao, L.; Ojolo, S.P.; Azam, S.M.; Xiong, J.; Yan, M.; et al. Simple protoplast isolation system for gene expression and protein interaction studies in pineapple (Ananas comosus L.). Plant Method. 2018, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, Y.; Zhao, L.; Hou, Z.; Yan, M.; Hu, B.; Liu, Y.; Azam, S.M.; Zhang, Z.; Rahman, Z.U.; et al. Genome-Wide Identification and Expression Profiling of ATP-Binding Cassette (ABC) Transporter Gene Family in Pineapple (Ananas comosus (L.) Merr.) Reveal the Role of AcABCG38 in Pollen Development. Front. Plant Sci. 2017, 8, 2150. [Google Scholar] [CrossRef] [PubMed]
- Baskin, T.I.; Wilson, J.E. Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 1997, 113, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. Auxin: A regulator of cold stress response. Physiol. Plant 2013, 147, 28–35. [Google Scholar] [CrossRef]
- Daudi, A.; O’Brien, J.A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio Protoc. 2012, 2. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Yadav, A.K.; Jha, S.K.; Sanyal, S.K.; Luan, S.; Pandey, G.K. Arabidopsis calcineurin B-like proteins differentially regulate phosphorylation activity of CBL-interacting protein kinase 9. Biochem. J. 2018, 475, 2621–2636. [Google Scholar] [CrossRef]
- Manik, S.M.; Shi, S.; Mao, J.; Dong, L.; Su, Y.; Wang, Q.; Liu, H. The Calcium Sensor CBL-CIPK Is Involved in Plant’s Response to Abiotic Stresses. Int. J. Genom. 2015, 2015, 493191. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, T.; Chen, C.; Luan, A.; Long, J.; Li, C.; Ding, Y.; He, Y. Genome-wide organization and expression profiling of the R2R3-MYB transcription factor family in pineapple (Ananas comosus). BMC Genom. 2017, 18, 503. [Google Scholar] [CrossRef] [PubMed]
- Tak, H.; Negi, S.; Ganapathi, T.R. Banana NAC transcription factor MusaNAC042 is positively associated with drought and salinity tolerance. Protoplasma 2017, 254, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Dong, B.; Song, Z.; Meng, D.; Fu, Y. Genome-Wide Identification and Characterization of CIPK Family and Analysis Responses to Various Stresses in Apple (Malus domestica). Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Boudet, N.; Aubourg, S.; Toffano-Nioche, C.; Kreis, M.; Lecharny, A. Evolution of intron/exon structure of DEAD helicase family genes in Arabidopsis, Caenorhabditis, and Drosophila. Genome Res. 2001, 11, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The Evolution of Calcium-Based Signalling in Plants. Curr. Biol. 2017, 27, R667–R679. [Google Scholar] [CrossRef]
- Lyzenga, W.J.; Sullivan, V.; Liu, H.; Stone, S.L. The Kinase Activity of Calcineurin B-like Interacting Protein Kinase 26 (CIPK26) Influences Its Own Stability and that of the ABA-regulated Ubiquitin Ligase, Keep on Going (KEG). Front. Plant Sci. 2017, 8, 502. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef]

| (a) Characteristics of Pineapple CBL Gene | |||||
|---|---|---|---|---|---|
| Name | Transcript ID | Chromosome Location | Length | MW | pI |
| AcCBL1 | Aco000875 | LG02:16012605-16018403 | 226 | 25,958.74 | 4.81 |
| AcCBL2 | Aco004447 | LG05:3255687-3258668 | 213 | 24,478.92 | 4.81 |
| AcCBL3 | Aco005668 | LG11:12099528-12103047 | 216 | 24,888.39 | 4.86 |
| AcCBL4 | Aco005877 | LG16:10709202-10717927 | 359 | 40,867.51 | 4.68 |
| AcCBL5 | Aco011645 | LG08:12541062-12545693 | 234 | 26,797.47 | 4.72 |
| AcCBL6 | Aco012888 | LG03:15510233-15516379 | 193 | 21,928.83 | 4.52 |
| AcCBL7 | Aco015301 | LG20:10385809-10391109 | 224 | 25,763.52 | 4.81 |
| AcCBL8 | Aco024403 | LG12:9915463-9927546 | 252 | 28,939.19 | 6.16 |
| (b) Characteristics of Pineapple CIPK Genes | |||||
| AcCIPK1 | Aco001621 | LG18:9219002-9220612 | 409 | 43,926.73 | 9.35 |
| AcCIPK2 | Aco001625 | LG18:9192567-9193895 | 442 | 50,063.84 | 9.26 |
| AcCIPK3 | Aco002215 | LG04:3262495-3268163 | 450 | 50,565.19 | 8.78 |
| AcCIPK4 | Aco004322 | LG05:2160825-2162243 | 472 | 52,996.59 | 6.69 |
| AcCIPK5 | Aco004323 | LG05:2169056-2170823 | 487 | 54,823.09 | 8.75 |
| AcCIPK6 | Aco005225 | LG07:3071294-3072667 | 405 | 42,654.61 | 9.99 |
| AcCIPK7 | Aco006700 | LG01:22600010-22610245 | 385 | 44,053.77 | 7.12 |
| AcCIPK8 | Aco007507 | LG12:11547144-11558347 | 382 | 43,701.04 | 6.87 |
| AcCIPK9 | Aco008201 | LG19:8409312-8411200 | 424 | 45,918.02 | 8.61 |
| AcCIPK10 | Aco010015 | LG10:2187779-2189581 | 478 | 54,071.92 | 6.51 |
| AcCIPK11 | Aco010017 | LG10:2198749-2200149 | 466 | 52,913.08 | 9.06 |
| AcCIPK12 | Aco011132 | LG04:13486171-13487538 | 455 | 49,351.15 | 6.94 |
| AcCIPK13 | Aco012533 | LG13:1862440-1863744 | 434 | 49,113.68 | 9.01 |
| AcCIPK14 | Aco014260 | LG05:45295-60064 | 422 | 47,747.21 | 7.71 |
| AcCIPK15 | Aco015008 | LG04:11703020-11710396 | 449 | 50,875.82 | 8.01 |
| AcCIPK16 | Aco015525 | LG03:12649272-12654172 | 396 | 45,072.72 | 8.99 |
| AcCIPK17 | Aco016931 | LG02:2099383-2101473 | 451 | 50,452.21 | 8.09 |
| AcCIPK18 | Aco016932 | LG02:2036912-2038291 | 459 | 50,213.68 | 9.08 |
| AcCIPK19 | Aco019253 | LG13:3471010-3478477 | 441 | 50,378.76 | 7.2 |
| AcCIPK20 | Aco021653 | LG16:7092847-7095892 | 506 | 55,836.96 | 6.63 |
| AcCIPK21 | Aco022003 | LG04:312075-316545 | 444 | 49,867.09 | 6.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, M.; Fakher, B.; Jakada, B.H.; Zhao, L.; Cao, S.; Cheng, Y.; Qin, Y. Genome-Wide Identification and Expression Profiling of CBL-CIPK Gene Family in Pineapple (Ananas comosus) and the Role of AcCBL1 in Abiotic and Biotic Stress Response. Biomolecules 2019, 9, 293. https://doi.org/10.3390/biom9070293
Aslam M, Fakher B, Jakada BH, Zhao L, Cao S, Cheng Y, Qin Y. Genome-Wide Identification and Expression Profiling of CBL-CIPK Gene Family in Pineapple (Ananas comosus) and the Role of AcCBL1 in Abiotic and Biotic Stress Response. Biomolecules. 2019; 9(7):293. https://doi.org/10.3390/biom9070293
Chicago/Turabian StyleAslam, Mohammad, Beenish Fakher, Bello Hassan Jakada, Lihua Zhao, Shijiang Cao, Yan Cheng, and Yuan Qin. 2019. "Genome-Wide Identification and Expression Profiling of CBL-CIPK Gene Family in Pineapple (Ananas comosus) and the Role of AcCBL1 in Abiotic and Biotic Stress Response" Biomolecules 9, no. 7: 293. https://doi.org/10.3390/biom9070293
APA StyleAslam, M., Fakher, B., Jakada, B. H., Zhao, L., Cao, S., Cheng, Y., & Qin, Y. (2019). Genome-Wide Identification and Expression Profiling of CBL-CIPK Gene Family in Pineapple (Ananas comosus) and the Role of AcCBL1 in Abiotic and Biotic Stress Response. Biomolecules, 9(7), 293. https://doi.org/10.3390/biom9070293







