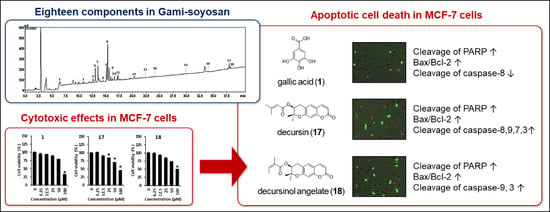
Analysis and Identification of Active Compounds from Gami-Soyosan Toxic to MCF-7 Human Breast Adenocarcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Gami-Soyosan Sample
2.2. Chemicals
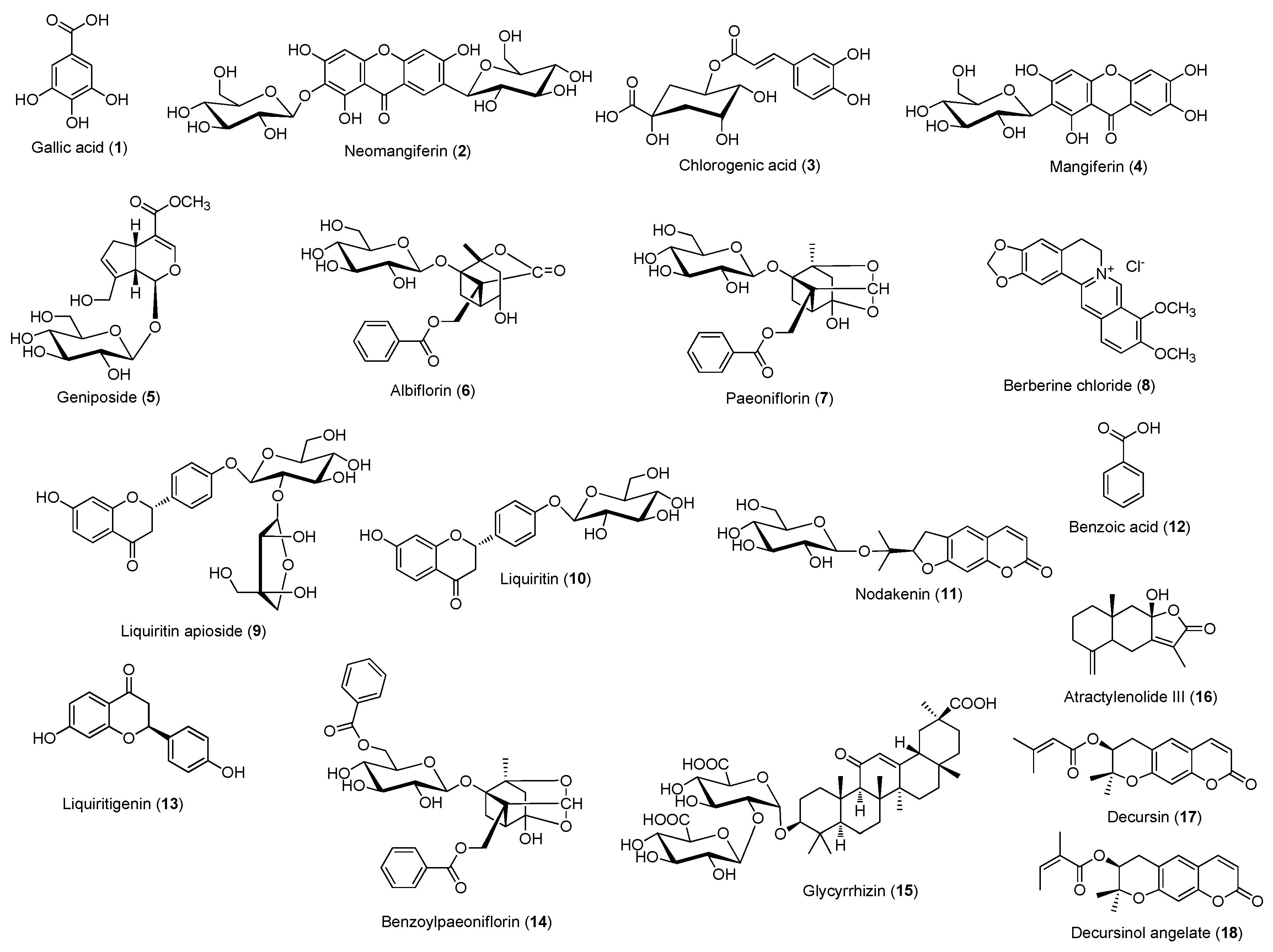
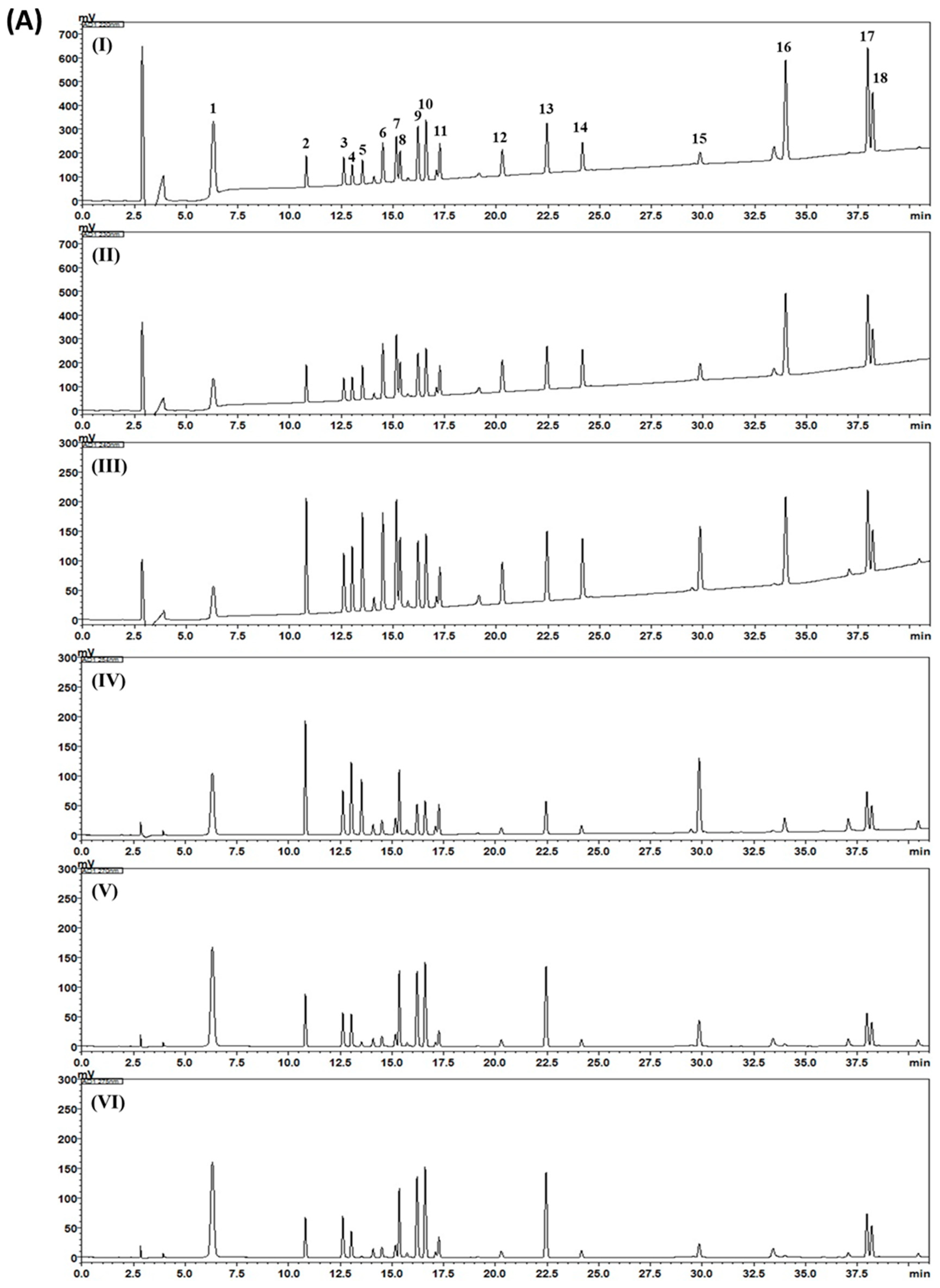
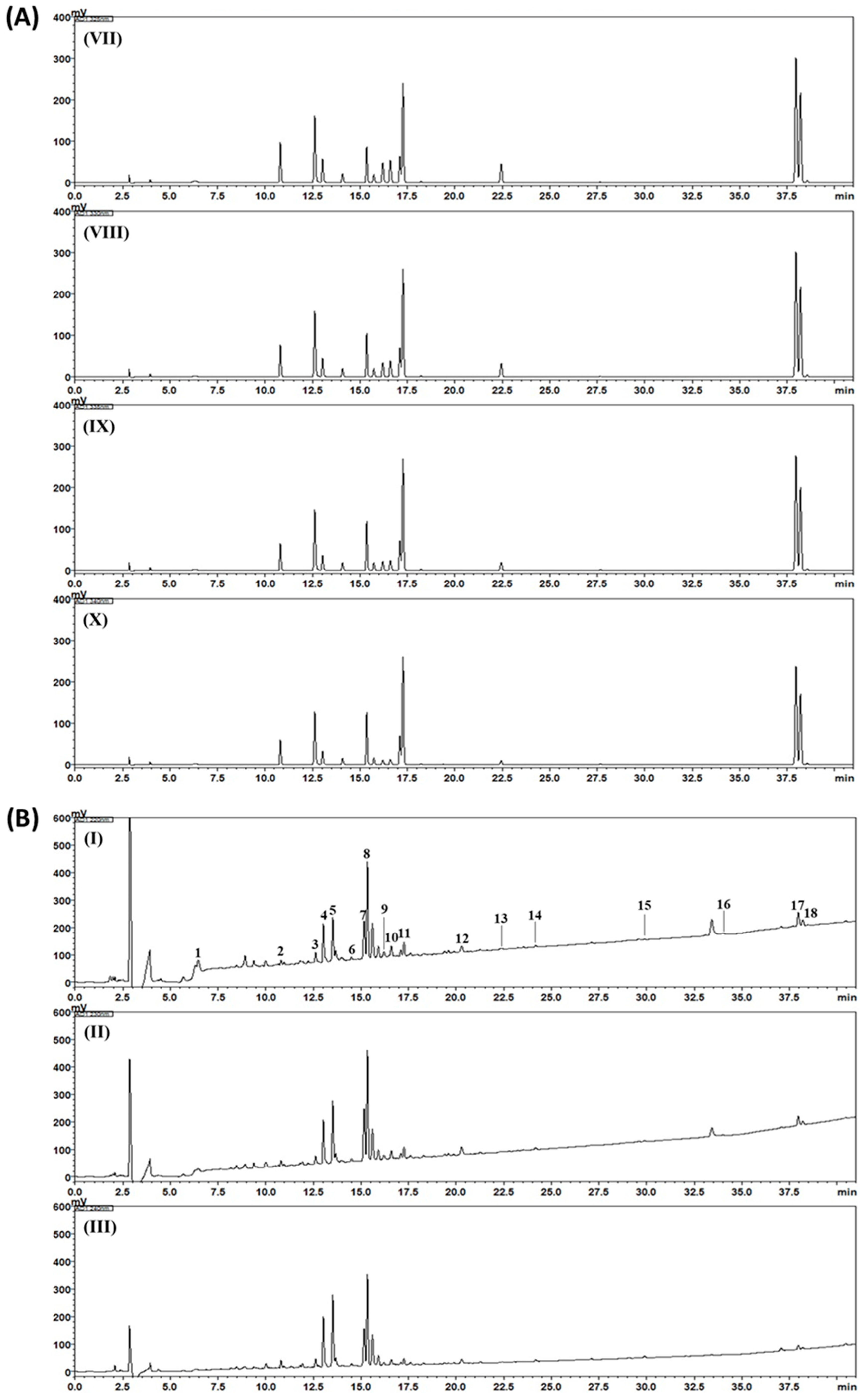
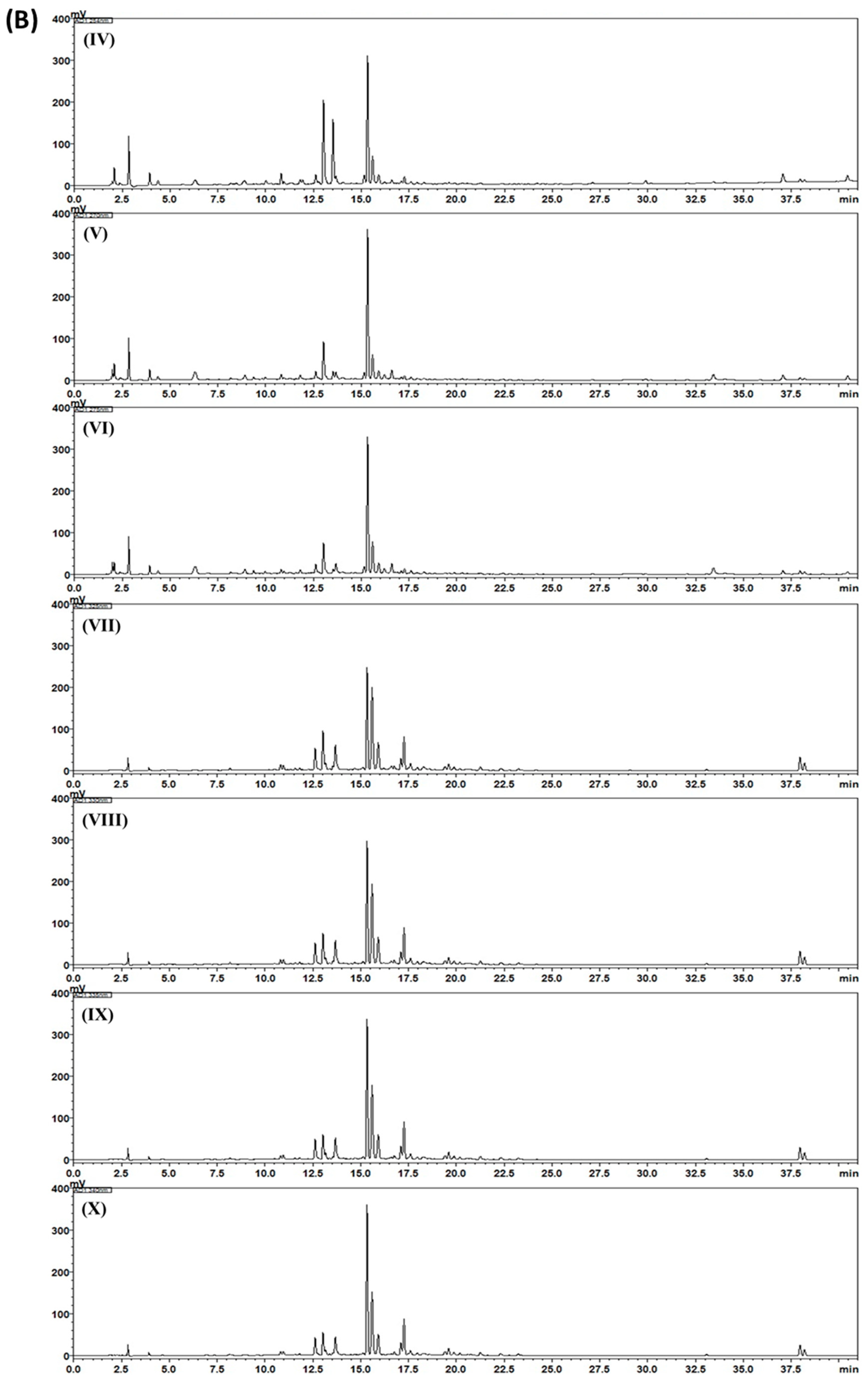
2.3. HPLC Analysis of Gami-Soyosan
2.4. Cells and Cell Culture
2.5. Determination of Cell Viability
2.6. Image-Based Cytometric Assay
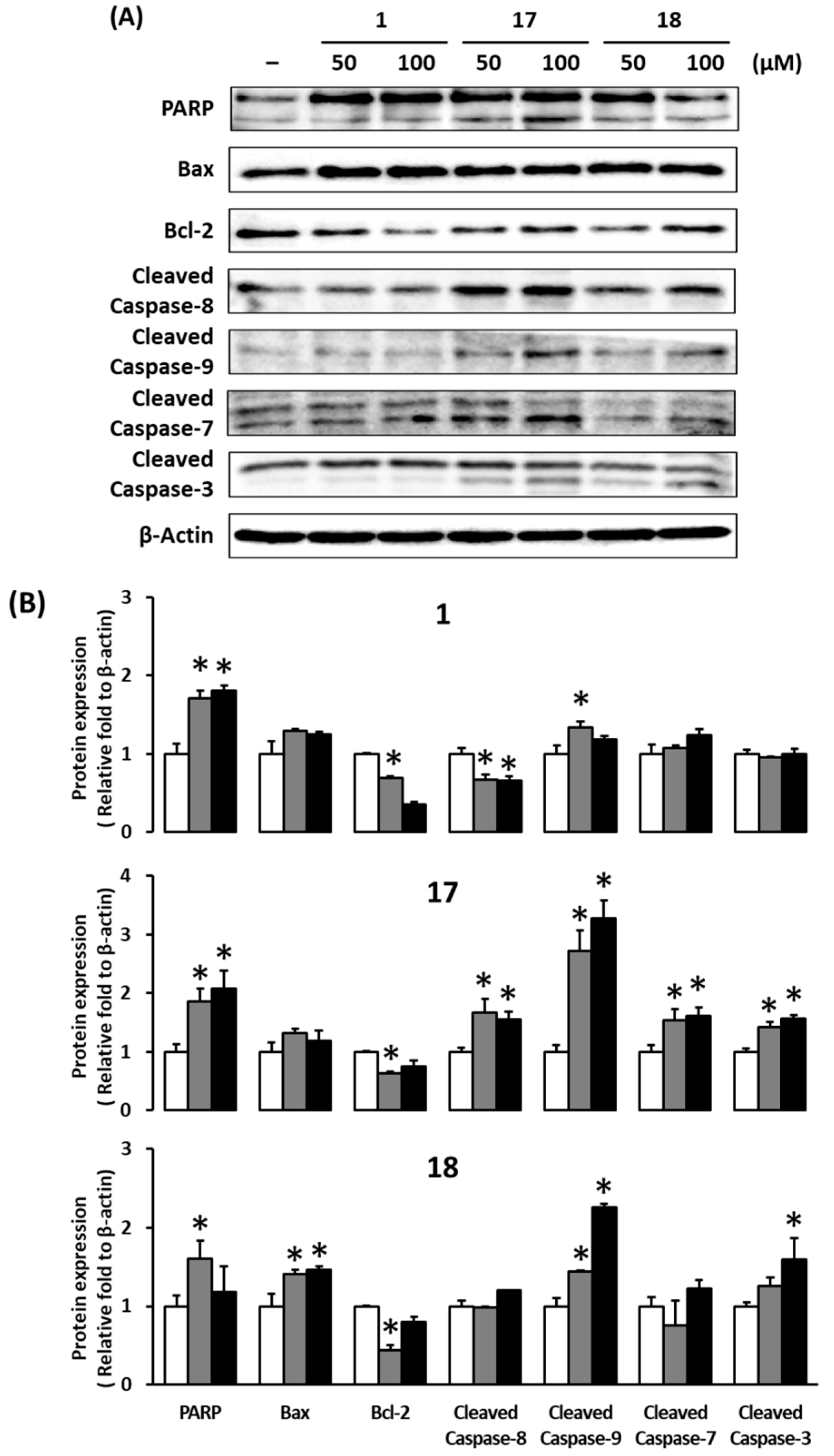
2.7. Western Blotting
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.; Moynihan, J.; Frost, A.; Evans, S. Ectopic breast cancer: Case report and literature review. Surg. Oncol. 1994, 3, 295–304. [Google Scholar] [CrossRef]
- McPherson, K.; Steel, C.; Dixon, J. ABC of breast diseases: Breast cancer—Epidemiology, risk factors, and genetics. BMJ 2000, 321, 624. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Brunello, A.; Maruzzo, M.; Basso, U.; Mm Basso, S. Treatment of estrogen receptor-positive breast cancer. Curr. Med. Chem. 2013, 20, 596–604. [Google Scholar] [CrossRef]
- Woo, J.; Lim, W. Endocrine therapy for breast cancer. Ewha Med. J. 2014, 37, 83–91. [Google Scholar] [CrossRef]
- Eden, J. ENDOCRINE DILEMMA: Managing menopausal symptoms after breast cancer. Eur. J. Endocr. 2016, 174, R71–R77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, M.E.; Schoemaker, M.J.; Wright, L.; McFadden, E.; Griffin, J.; Thomas, D.; Hemming, J.; Wright, K.; Ashworth, A.; Swerdlow, A.J. Menopausal hormone therapy and breast cancer: What is the true size of the increased risk? Br. J. Cancer 2016, 115, 607. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, B.; Quesenberry, C.; Schroeder, D.A.; Friedman, G. Long-term postmenopausal estrogen therapy may be associated with increased risk of breast cancer: A cohort study. Menopause 2018, 25, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Hormone-replacement therapy: Current thinking. Nat. Rev. Endocrinol. 2017, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Je, Y.M.; Yoo, D.Y. Two case report of UL-syndrome (鬱症) Treated with Gamisoyosan (加味逍遙散). J. Haehwa Med. 2011, 19, 187–193. [Google Scholar]
- Kim, T. Pathogenesis and management guideline of dysmenorrhea. Obstet. Gynecol. Sci. 2005, 48, 1613–1620. [Google Scholar]
- Hamilton, M. A scale for rating depression. J. Neurol. Neurosurg. Psychiatr. 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.M.; Cho, J.H.; Lee, C.H.; Jang, J.B.; Lee, K.S. Antioxidant and neuroprotective effects of gamisoyo-san. J. Korean Obstet. Gynecol. 2010, 23, 1–13. [Google Scholar]
- Park, C.S.; Sohn, Y.J. A study on the effects of Gamisoyosan on ovariectomized osteoporosis in rats. J. Korean Obstet. Gynecol. 2008, 21, 1–15. [Google Scholar]
- Chen, J.L.; Chang, C.J.; Wang, J.Y.; Wen, C.S.; Tseng, L.M.; Chang, W.C.; Noomhorm, N.; Liu, H.J.; Chen, W.S.; Chiu, J.H.; et al. In vitro and in vivo effects of Jia-Wei-Xiao-Yao-San in human breast cancer MCF-7 cells treated with tamoxifen. Integr. Cancer Ther. 2014, 13, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.N.; Wu, C.T.; Wang, J.D. Prescription pattern of chinese herbal products for breast cancer in Taiwan: A population-based study. Evid. Based Complement. Altern. Med. 2012, 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, Y.H.; Wu, J.C.; Chen, Y.C.; Yang, S.H.; Chen, J.L.; Chen, T.J. Prescription patterns of chinese herbal products for menopausal syndrome: Analysis of a nationwide prescription database. J. Ethnopharmacol. 2011, 137, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Scheid, V.; Ward, T.; Cha, W.S.; Watanabe, K.; Liao, X. The treatment of menopausal symptoms by traditional east asian medicines: Review and perspectives. Maturitas 2010, 66, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Kim, O.S.; Yoo, S.R.; Seo, C.S.; Kim, Y.; Shin, H.K.; Jeong, S.J. Anti-inflammatory effect and action mechanisms of traditional herbal formula Gamisoyo-san in RAW 264.7 macrophages. BMC Complement. Altern. Med. 2016, 16, 219. [Google Scholar] [CrossRef]
- Debeb, Y.G.; Berihu, B.A. Review on the role of estrogen receptors in breast cancer. Int. J. Pharma Sci. Res. (IJPSR) 2015, 6, 1100–1103. [Google Scholar]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Awolaran, O.T. Cellular mechanisms of oestrogen in breast cancer development. Open Access J. Sci. Technol. 2015, 3, 7. [Google Scholar] [CrossRef]
- Anjum, F.; Razvi, N.; Masood, M. Breast cancer therapy: A mini review. MOJ Drug Des. Dev. Ther. 2017, 1, 00006. [Google Scholar] [CrossRef]
- Butta, A.; MacLennan, K.; Flanders, K.C.; Sacks, N.P.; Smith, I.; McKinna, A.; Dowsett, M.; Wakefield, L.M.; Sporn, M.B.; Baum, M. Induction of transforming growth factor β1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res. 1992, 52, 4261–4264. [Google Scholar] [PubMed]
- Massarweh, S.; Osborne, C.K.; Creighton, C.J.; Qin, L.; Tsimelzon, A.; Huang, S.; Weiss, H.; Rimawi, M.; Schiff, R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008, 68, 826–833. [Google Scholar] [CrossRef]
- Brueggemeier, R.W.; Hackett, J.C.; Diaz-Cruz, E.S. Aromatase inhibitors in the treatment of breast cancer. Endocr. Rev. 2005, 26, 331–345. [Google Scholar] [CrossRef]
- Santen, R.; Harvey, H. Use of aromatase inhibitors in breast carcinoma. Endocr. Relat. Cancer 1999, 6, 75–92. [Google Scholar] [CrossRef]
- Cusack, L.; Brennan, M.; Baber, R.; Boyle, F. Menopausal symptoms in breast cancer survivors: Management update. Br. J. Gen. Pract. 2013, 63, 51–52. [Google Scholar] [CrossRef]
- Sim, T.K.; Jung, I.C.; Lee, S.R. The effect of Gamisoyo-san (Jiaweixiaoyaosan) on serotonin metabolism. J. Orient. Neuropsychiatr. 2011, 22, 37–51. [Google Scholar] [CrossRef]
- Nowak, B.; Matuszewska, A.; Szandruk, M.; Matkowski, A.; Woźniak, D.; Zduniak, K.; Rzeszutko, M.; Landwójtowicz, M.; Jędrzejuk, D.; Piasecki, T.; et al. Effect of long-term administration of mangiferin from Belamcanda chinensis on bone metabolism in ovariectomized rats. J. Funct. Foods 2018, 46, 12–18. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, L.; Tan, Y.; Zhang, Z.; He, F.; Song, C.; Wang, X.; Li, W.; Liu, T.; Hua, Q. ERβ modulation and non-modulation of ERα by administration of geniposide and panax notoginseng saponins in SH-SY5Y cells. J. Tradit. Chinese Med. Sci. 2019, 6, 147–154. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Jiang, L.; Xie, Y.; Xia, Y.; Lv, R.; Dong, L. Paeoniflorin improves menopause depression in ovariectomized rats under chronic unpredictable mild stress. Intern. J. Clin. Exp. Med. 2015, 8, 5103–5111. [Google Scholar]
- Caliceti, C.; Rizzo, P.; Cicero, A.F.G. Potential benefits of berberine in the management of perimenopausal syndrome. Oxid. Med.Cellular Longev. 2015, 2015, 723093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Faried, A.; Kurnia, D.; Faried, L.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Inter. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef]
- Hsu, J.D.; Kao, S.H.; Ou, T.T.; Chen, Y.J.; Li, Y.J.; Wang, C.J. Gallic acid induces G2/M phase arrest of breast cancer cell MCF-7 through stabilization of p27Kip1 attributed to disruption of p27Kip1/Skp2 complex. J. Agric. Food Chem. 2011, 59, 1996–2003. [Google Scholar] [CrossRef]
- Jiang, C.; Guo, J.; Wang, Z.; Xiao, B.; Lee, H.J.; Lee, E.O.; Kim, S.H.; Lu, J. Decursin and decursinol angelate inhibit estrogen-stimulated and estrogen-independent growth and survival of breast cancer cells. Breast Cancer Res. 2007, 9, R77. [Google Scholar] [CrossRef]
- Jung, M.H.; Lee, S.H.; Ahn, E.M.; Lee, Y.M. Decursin and decursinol angelate inhibit VEGF-induced angiogenesis via suppression of the VEGFR-2-signaling pathway. Carcinogenesis 2009, 30, 655–661. [Google Scholar] [CrossRef]
- Burz, C.; Berindan-Neagoe, I.; Balacescu, O.; Irimie, A. Apoptosis in cancer: Key molecular signaling pathways and therapy targets. Acta Oncol. 2009, 48, 811–821. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicolog. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.G.; Ding, X.Z.; Adrian, T.E. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 296, 942–948. [Google Scholar] [CrossRef]
- Leibowitz, B.; Yu, J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol. Ther. 2010, 9, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, A.; Brea-Calvo, G.; Fernández-Ayala, D.; Cordero, M.; Navas, P.; Sanchez-Alcazar, J. Nuclear caspase-3 and capase-7 activation, and poly (ADP-ribose) polymerase cleavage are early events in camptothecin-induced apoptosis. Apoptosis 2006, 11, 131–139. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Zhang, K.; Zhu, L.; Zhou, F. Investigation of gallic acid induced anticancer effect in human breast carcinoma MCF-7 cells. J. Biochem. Mol. Toxicol. 2014, 28, 387–393. [Google Scholar] [CrossRef]
- Moghtaderi, H.; Sepehri, H.; Delphi, L.; Attari, F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. BioImpacts 2018, 8, 185–194. [Google Scholar] [CrossRef]


| Latin Name | Scientific Name | Amount (g) | Origin |
|---|---|---|---|
| Paeoniae Radix | Paeonia lactiflora Pallas | 638.3 | Uiseong, Korea |
| Atractylodis Rhizoma Alba | Atractylodes macrocephala Koidzumi | 638.3 | China |
| Anemarrhenae Rhizoma | Anemarrhena asphodeloides Bunge | 531.9 | Kangjin, Korea |
| Lycii Radicis Cortex | Lycium chinense Miller | 531.9 | China |
| Angelicae Gigantis Radix | Angelica gigas Nakai | 531.9 | Bonghwa, Korea |
| Poria Sclerotium | Poria cocos Wolf | 425.5 | Pyeongchang, Korea |
| Liriope Tuber | Liriope platyphylla Wang et Tang | 425.5 | Miryang, Korea |
| Rehmanniae Radix Crudus | Rehmannia glutinosa Liboschitz var. purpurea Makino | 425.5 | Gunwi, Korea |
| Gardeniae Fructus | Gardenia jasminoides Ellis | 266.0 | Gurye, Korea |
| Phellodendri Cortex | Phellodendron amurense Ruprecht | 266.0 | China |
| Platycodi Radix | Platycodon grandiflorum A. De Candolle | 159.6 | Muju, Korea |
| Glycyrrhizae Radix et Rhizoma | Glycyrrhiza uralensis Fischer | 159.6 | China |
| Total | 5000.0 |
| Chromatographic Parameter | |||
|---|---|---|---|
| Column | SunFire C18 analytical column (250 × 4.6 mm, 5 μm) | ||
| Detector | PDA (220, 230, 240, 254, 270, 275, 325, 330, 335, and 340 nm) | ||
| Flow rate (mL/min) | 1.0 | ||
| Injection volume (μL) | 10.0 | ||
| Column temperature (°C) | 40 | ||
| Mobile phase | A: 0.1% Formic acid in distilled water B: 0.1% Formic acid in acetonitrile | ||
| Gradient elution | Time (min) | A (%) | B (%) |
| 0 | 95 | 5 | |
| 30 | 40 | 60 | |
| 40 | 0 | 100 | |
| 45 | 0 | 100 | |
| 50 | 95 | 5 | |
| Compound | Detection Wavelength (nm) | Linear Range (μg/mL) | Regression Equation a | r2 |
|---|---|---|---|---|
| 1 | 270 | 0.63−40.00 | y = 38259.49x – 10572.16 | 0.9999 |
| 2 | 254 | 0.63−40.00 | y = 32295.16x – 2577.19 | 1.0000 |
| 3 | 325 | 0.63−40.00 | y = 41318.61x – 24432.53 | 0.9996 |
| 4 | 254 | 0.63−40.00 | y = 50951.35x – 13475.92 | 0.9999 |
| 5 | 240 | 0.63−40.00 | y = 16710.90x – 141.25 | 1.0000 |
| 6 | 230 | 0.63−40.00 | y = 10804.67x – 2134.28 | 0.9999 |
| 7 | 230 | 0.63−40.00 | y = 10839.56x + 774.96 | 0.9997 |
| 8 | 340 | 1.56−100.00 | y = 60688.22x – 23759.65 | 1.0000 |
| 9 | 275 | 0.63−40.00 | y = 15209.48x – 2448.89 | 1.0000 |
| 10 | 275 | 0.63−40.00 | y = 17437.96x – 2592.24 | 1.0000 |
| 11 | 335 | 1.56−100.00 | y = 32877.31x – 14561.30 | 1.0000 |
| 12 | 230 | 0.31−20.00 | y = 35372.59x – 5310.55 | 0.9999 |
| 13 | 275 | 0.31−20.00 | y = 33887.88x – 3082.11 | 1.0000 |
| 14 | 230 | 0.31−20.00 | y = 38088.40x – 3804.89 | 0.9999 |
| 15 | 254 | 0.63−40.00 | y = 8282.39x – 1435.76 | 1.0000 |
| 16 | 220 | 0.31−20.00 | y = 59991.32x – 1482.57 | 0.9999 |
| 17 | 330 | 0.63−40.00 | y = 37380.95x – 4157.31 | 1.0000 |
| 18 | 330 | 0.63−40.00 | y = 257615.48x – 2550.42 | 1.0000 |
| Compound | Mean (mg/g) | SD (×10–1) | RSD (%) |
|---|---|---|---|
| 1 | 0.78 | 0.86 | 1.10 |
| 2 | 0.50 | 0.29 | 0.58 |
| 3 | 0.88 | 0.28 | 0.32 |
| 4 | 2.24 | 1.39 | 0.62 |
| 5 | 8.57 | 13.75 | 1.60 |
| 6 | 0.60 | 0.09 | 0.14 |
| 7 | 8.73 | 11.83 | 1.35 |
| 8 | 2.75 | 1.70 | 0.62 |
| 9 | 1.02 | 0.78 | 0.77 |
| 10 | 1.18 | 1.82 | 1.55 |
| 11 | 1.56 | 0.57 | 0.36 |
| 12 | 0.61 | 0.46 | 0.75 |
| 13 | 0.12 | 0.27 | 2.25 |
| 14 | 0.14 | 0.13 | 0.96 |
| 15 | 0.67 | 0.49 | 0.73 |
| 16 | 0.04 | 0.06 | 1.29 |
| 17 | 0.56 | 0.42 | 0.76 |
| 18 | 0.05 | 0.02 | 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, M.-Y.; Seo, C.-S.; Baek, S.-E.; Lee, J.; Shin, M.-S.; Kang, K.S.; Lee, S.; Yoo, J.-E. Analysis and Identification of Active Compounds from Gami-Soyosan Toxic to MCF-7 Human Breast Adenocarcinoma Cells. Biomolecules 2019, 9, 272. https://doi.org/10.3390/biom9070272
Jung M-Y, Seo C-S, Baek S-E, Lee J, Shin M-S, Kang KS, Lee S, Yoo J-E. Analysis and Identification of Active Compounds from Gami-Soyosan Toxic to MCF-7 Human Breast Adenocarcinoma Cells. Biomolecules. 2019; 9(7):272. https://doi.org/10.3390/biom9070272
Chicago/Turabian StyleJung, Mi-Yeon, Chang-Seob Seo, Seon-Eun Baek, Jaemin Lee, Myoung-Sook Shin, Ki Sung Kang, Sullim Lee, and Jeong-Eun Yoo. 2019. "Analysis and Identification of Active Compounds from Gami-Soyosan Toxic to MCF-7 Human Breast Adenocarcinoma Cells" Biomolecules 9, no. 7: 272. https://doi.org/10.3390/biom9070272
APA StyleJung, M.-Y., Seo, C.-S., Baek, S.-E., Lee, J., Shin, M.-S., Kang, K. S., Lee, S., & Yoo, J.-E. (2019). Analysis and Identification of Active Compounds from Gami-Soyosan Toxic to MCF-7 Human Breast Adenocarcinoma Cells. Biomolecules, 9(7), 272. https://doi.org/10.3390/biom9070272