Ameliorative Effect of Beta vulgaris Root Extract on Chlorpyrifos-Induced Oxidative Stress, Inflammation and Liver Injury in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of RBR Extract
2.2. Determination of Total Phenolics, Flavonoids and Radical Scavenging Activity of RBR
2.3. Experimental Animals and Treatments
2.4. Preparation of Tissue Homogenate and Assay of Protein Content
2.5. Biochemical Parameters
2.5.1. Determination of Liver Function Markers
2.5.2. Determination of Oxidative Stress Biomarkers and Antioxidants
2.5.3. Determination of Pro-Inflammatory Cytokines
2.5.4. Determination of Apoptosis Markers
2.6. Gene Expression Analysis
2.7. Histology and Immunohistochemistry
2.8. Statistical Analysis
3. Results
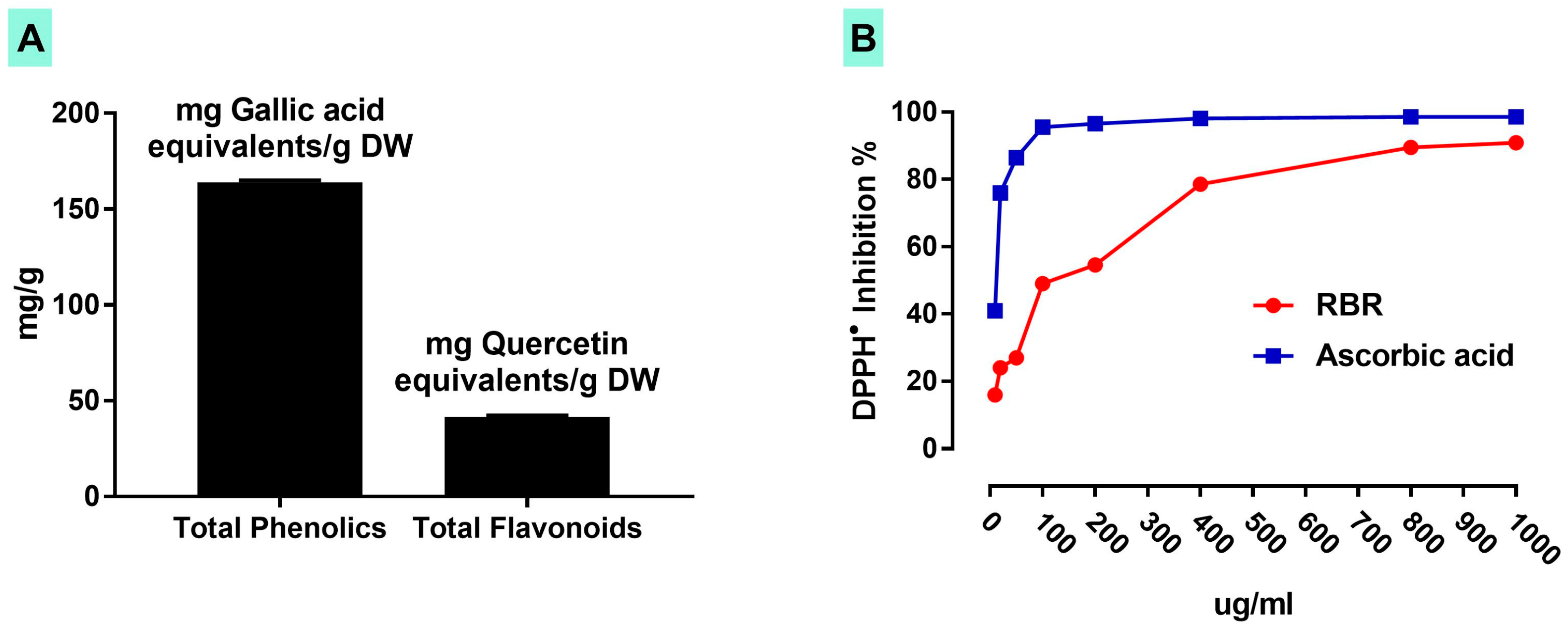
3.1. Total Phenolics, Flavonoids Content and DPPH Radical Scavenging Activity of RBR
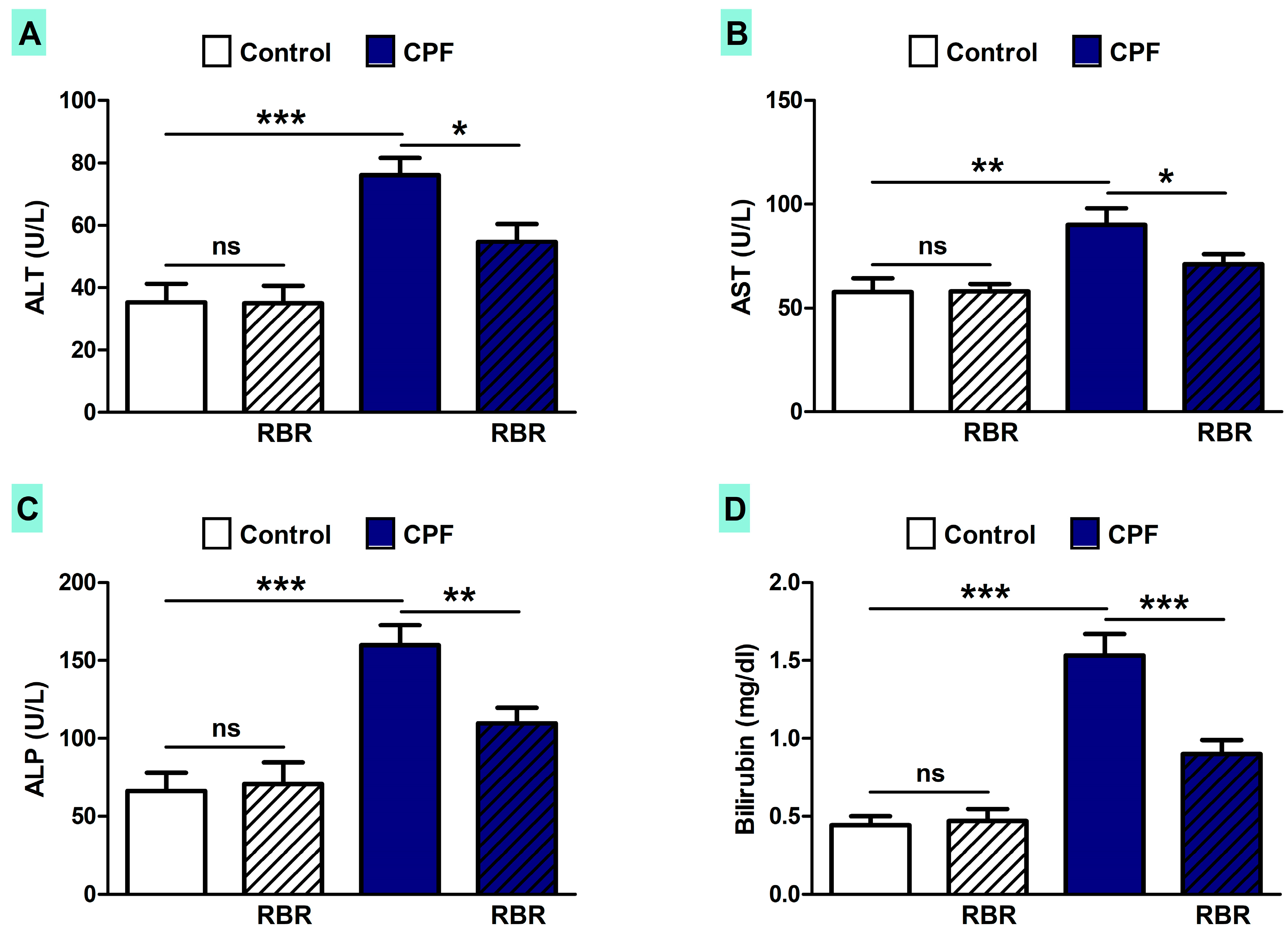
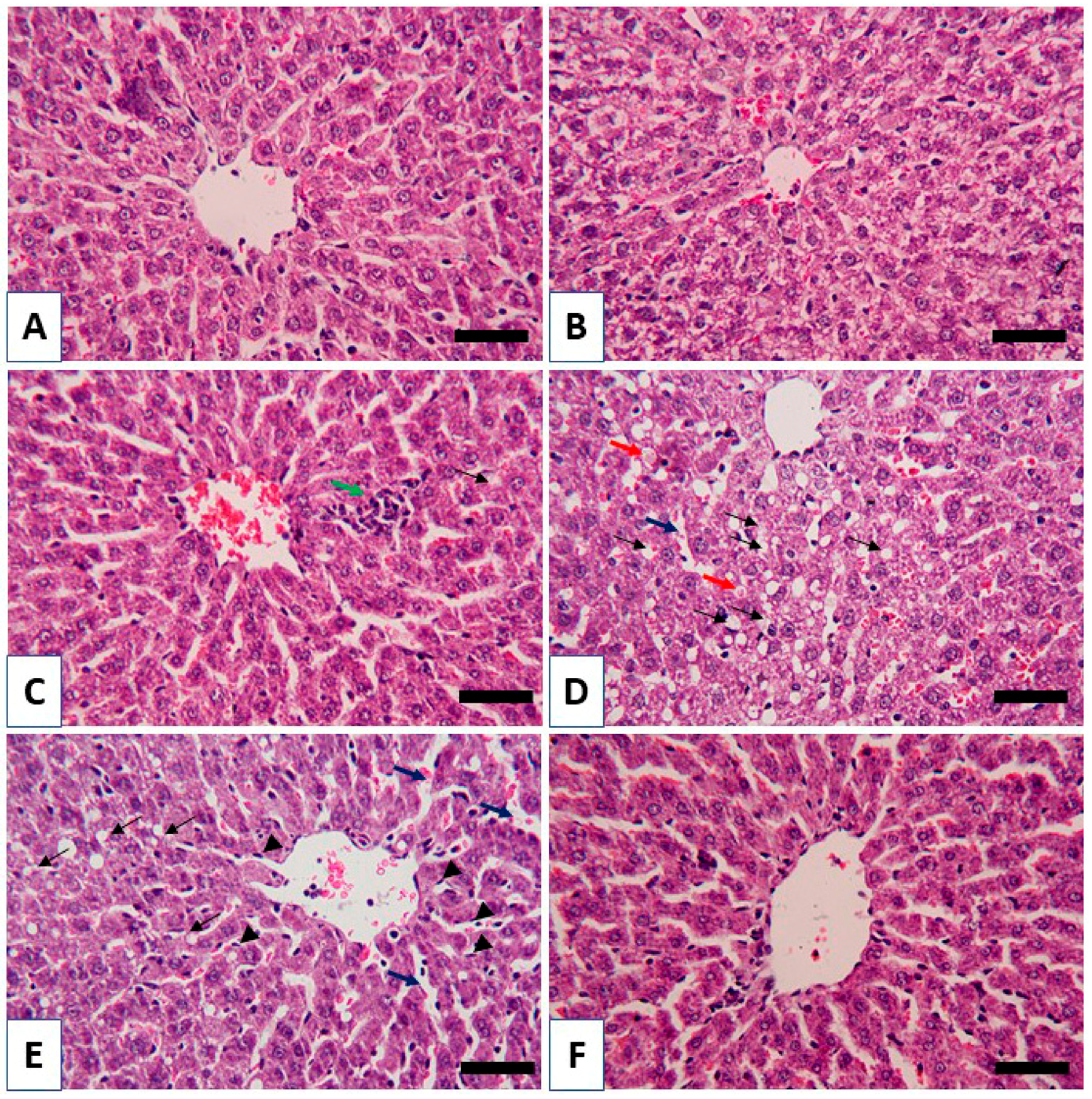
3.2. RBR Prevents Liver Injury in CPF-Induced Rats
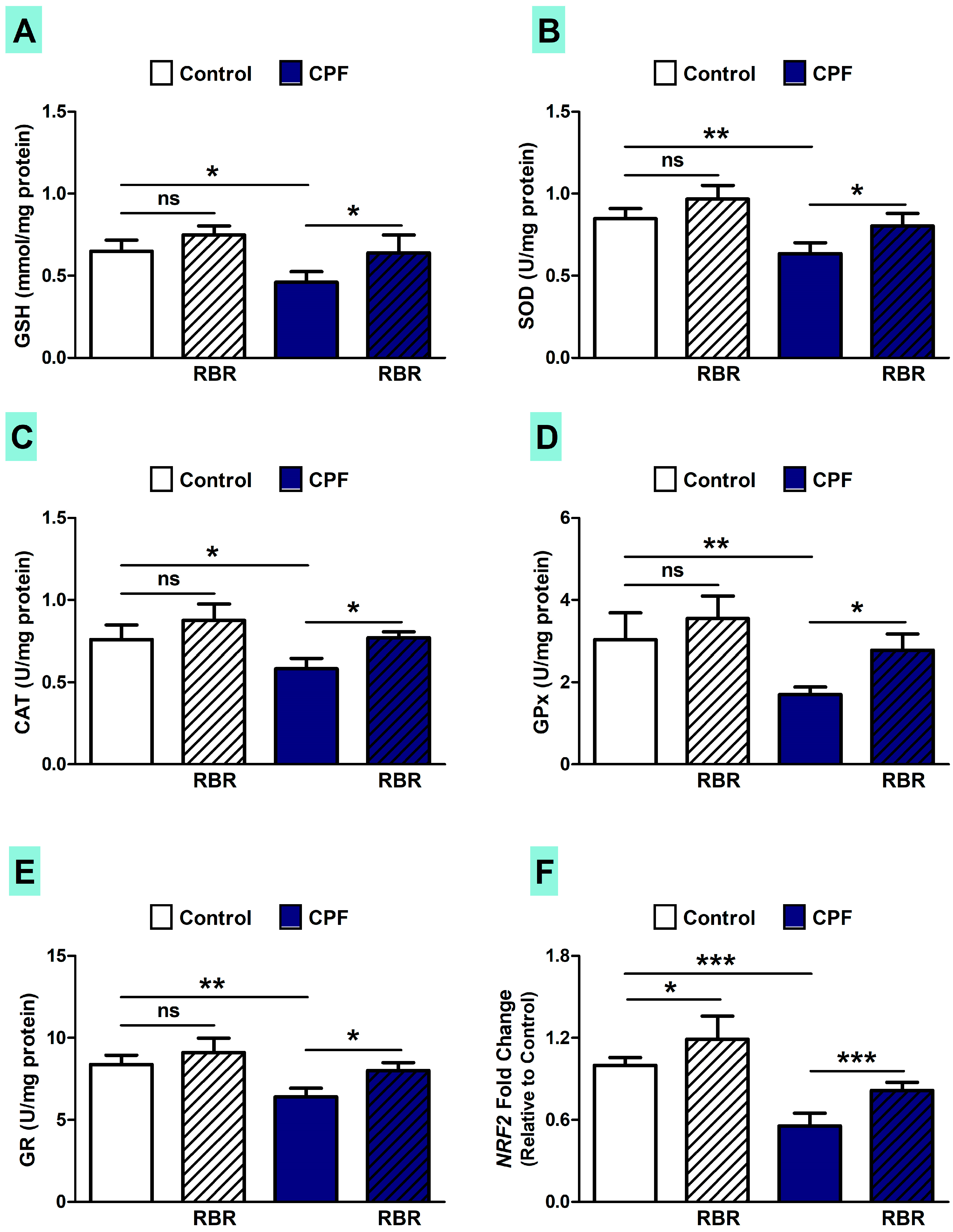
3.3. RBR Ameliorates Oxidative/Nitrative Stress and Improves Redox Homeostasis in CPF-Induced Rats
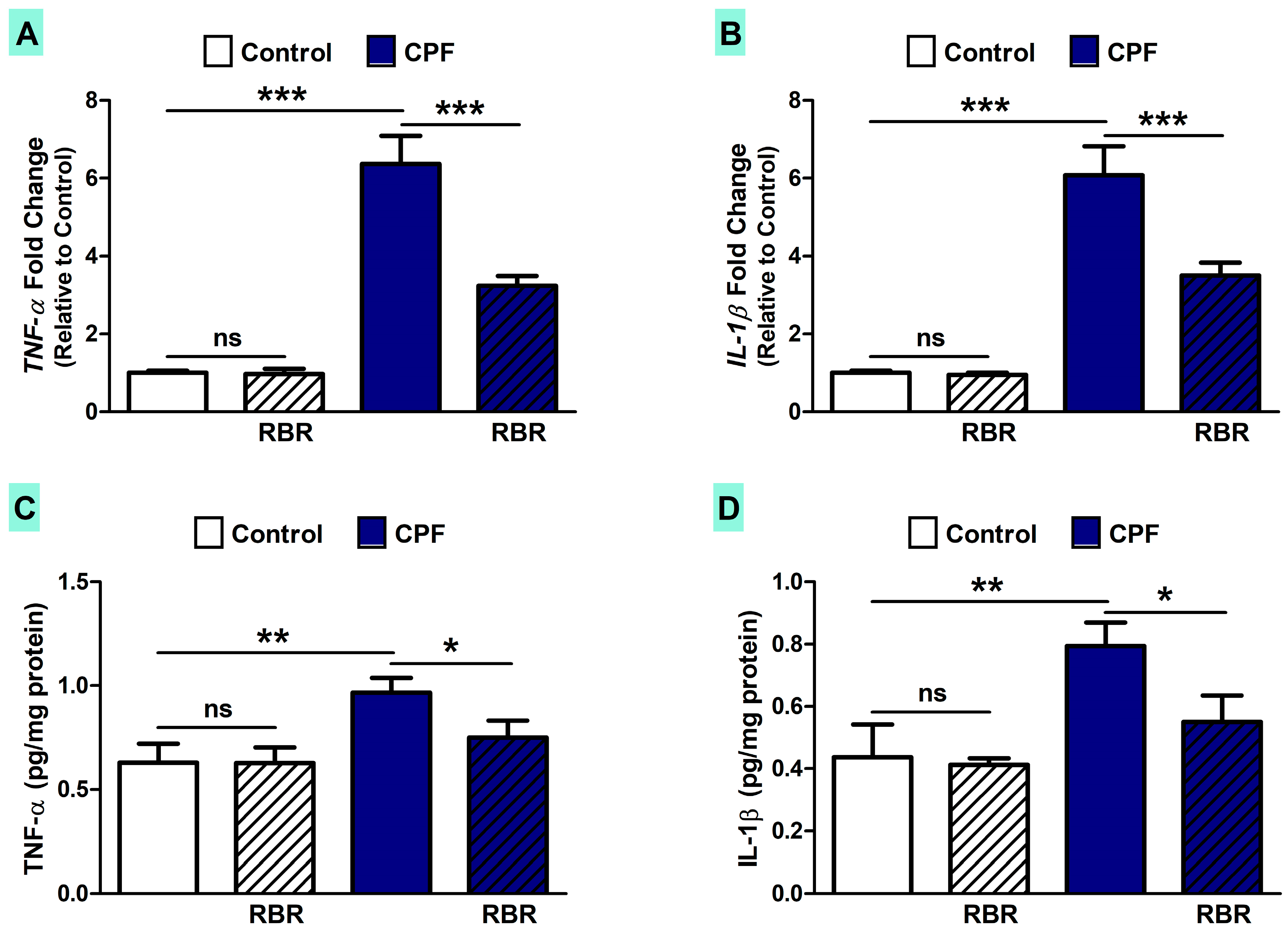
3.4. RBR Attenuates Inflammation in CPF-Induced Rats
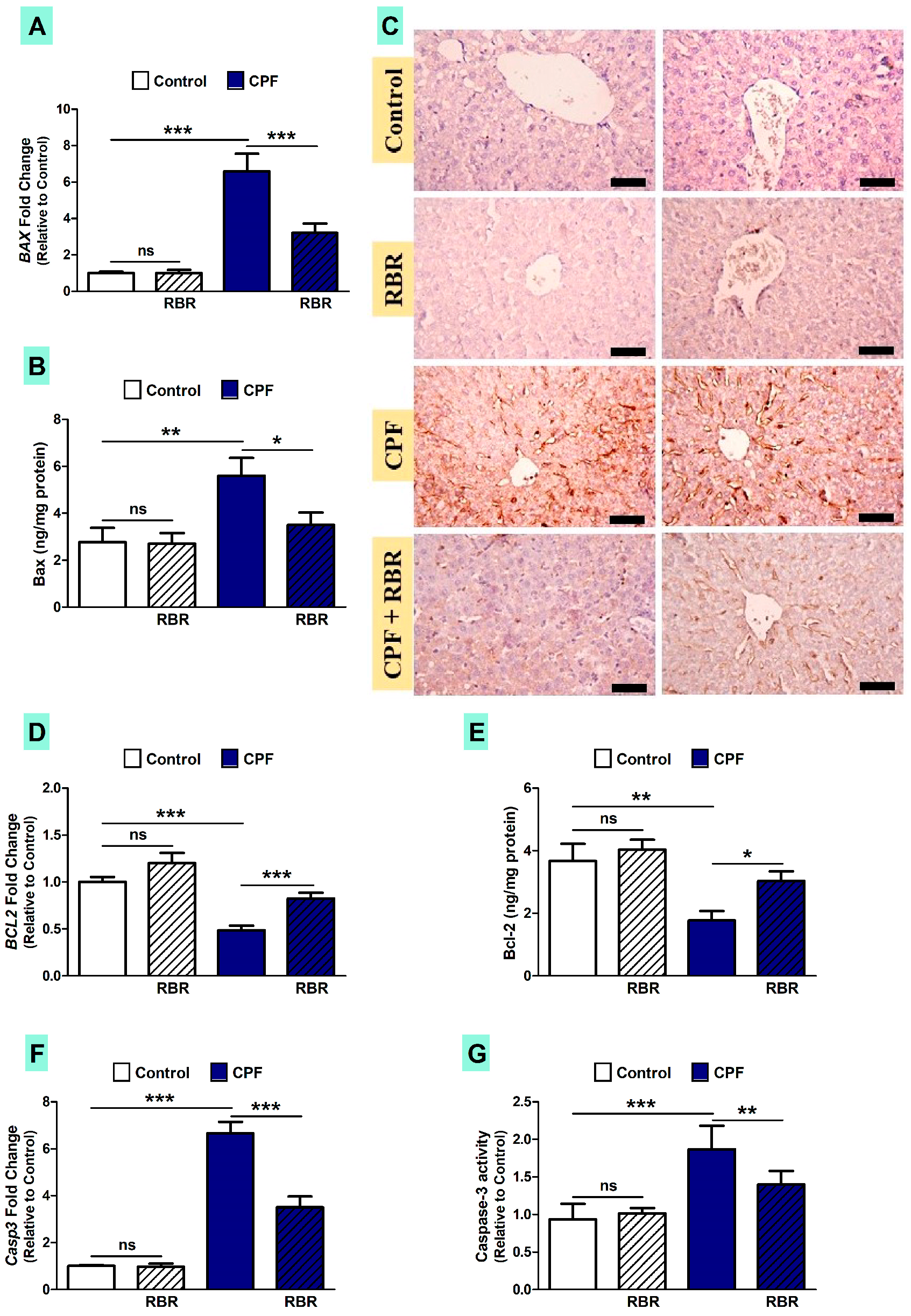
3.5. RBR Mitigates Apoptosis in CPF-Induced Rats
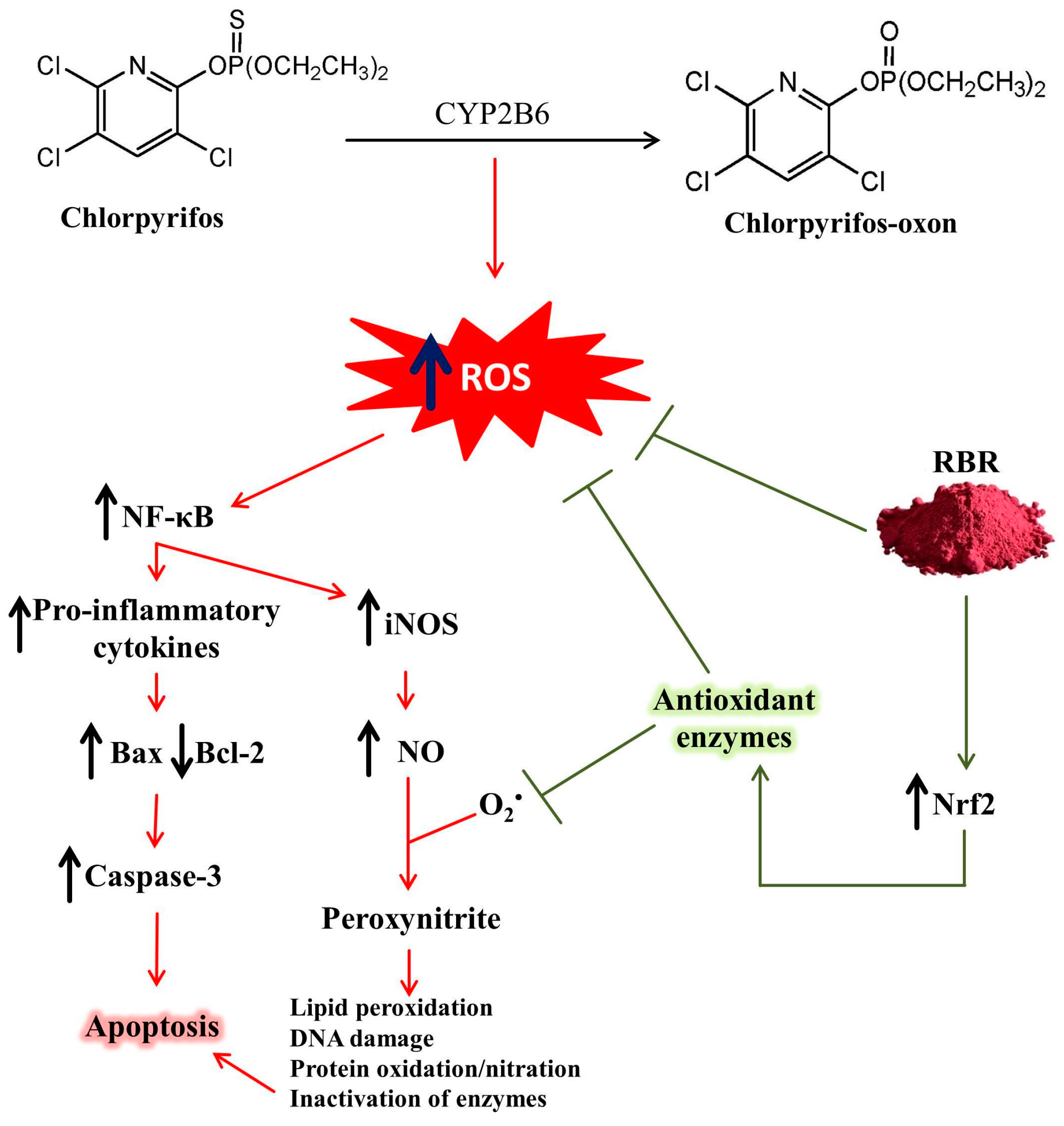
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suratman, S.; Edwards, J.W.; Babina, K. Organophosphate pesticides exposure among farmworkers: Pathways and risk of adverse health effects. Rev. Environ. Health 2015, 30, 65–79. [Google Scholar] [PubMed]
- Peter, J.V.; Sudarsan, T.I.; Moran, J.L. Clinical features of organophosphate poisoning: A review of different classification systems and approaches. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2014, 18, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Nurulain, S.M. Different approaches to acute organophosphorus poison treatment. Jpma. J. Pak. Med Assoc. 2012, 62, 712–717. [Google Scholar] [PubMed]
- Uzun, F.G.; Kalender, Y. Chlorpyrifos induced hepatotoxic and hematologic changes in rats: The role of quercetin and catechin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 55, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, E.M.; Afroz, R.; Chowdhury, M.; Gan, S.H.; Karim, N.; Islam, M.N.; Khalil, M.I. A model of chlorpyrifos distribution and its biochemical effects on the liver and kidneys of rats. Hum. Exp. Toxicol. 2016, 35, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M.; Mohamed, W.R.; Omar, H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3beta-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018, 152, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.A.; Owoeye, O.; Ajayi, B.O.; Awogbindin, I.O.; Rocha, J.B.T.; Farombi, E.O. Diphenyl diselenide abrogates chlorpyrifos-induced hypothalamic-pituitary-testicular axis impairment in rats. Biochem. Biophys. Res. Commun. 2018, 503, 171–176. [Google Scholar] [CrossRef]
- Gomez-Gimenez, B.; Felipo, V.; Cabrera-Pastor, A.; Agusti, A.; Hernandez-Rabaza, V.; Llansola, M. Developmental exposure to pesticides alters motor activity and coordination in rats: Sex differences and underlying mechanisms. Neurotox. Res. 2018, 33, 247–258. [Google Scholar] [CrossRef]
- Shou, L.; Bei, Y.; Song, Y.; Wang, L.; Ai, L.; Yan, Q.; He, W. Nrf2 mediates the protective effect of edaravone after chlorpyrifos-induced nervous system toxicity. Environ. Toxicol. 2019, 34, 626–633. [Google Scholar] [CrossRef]
- Zafiropoulos, A.; Tsarouhas, K.; Tsitsimpikou, C.; Fragkiadaki, P.; Germanakis, I.; Tsardi, M.; Maravgakis, G.; Goutzourelas, N.; Vasilaki, F.; Kouretas, D.; et al. Cardiotoxicity in rabbits after a low-level exposure to diazinon, propoxur, and chlorpyrifos. Hum. Exp. Toxicol. 2014, 33, 1241–1252. [Google Scholar] [CrossRef]
- Aroonvilairat, S.; Tangjarukij, C.; Sornprachum, T.; Chaisuriya, P.; Siwadune, T.; Ratanabanangkoon, K. Effects of topical exposure to a mixture of chlorpyrifos, cypermethrin and captan on the hematological and immunological systems in male Wistar rats. Environ. Toxicol. Pharmacol. 2018, 59, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lu, Y.; Zhao, Y.; Ren, H. Hepatotoxicity and nephrotoxicity induced by the chlorpyrifos and chlorpyrifos-methyl metabolite, 3,5,6-trichloro-2-pyridinol, in orally exposed mice. Sci. Total Environ. 2016, 544, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.R.; Mehany, A.B.M.; Hussein, R.M. Alpha lipoic acid protects against chlorpyrifos-induced toxicity in Wistar rats via modulating the apoptotic pathway. Environ. Toxicol. Pharmacol. 2018, 59, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.F.; Stoner, G.D. Red beetroot and betalains as cancer chemopreventative agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef] [PubMed]
- Lorizola, I.M.; Furlan, C.P.B.; Portovedo, M.; Milanski, M.; Botelho, P.B.; Bezerra, R.M.N.; Sumere, B.R.; Rostagno, M.A.; Capitani, C.D. Beet stalks and leaves (Beta vulgaris L.) protect against high-fat diet-induced oxidative damage in the liver in mice. Nutrients 2018, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Beltagi, H.S.; Mohamed, H.I.; Megahed, B.M.H.; Gamal, M.; Safwat, G. Evaluation of some chemical constituents, antioxidant, antibacterial and anticancer activities of beta Vulgaris L. Root. Fresenius Environ. Bull. 2018, 27, 6369–6378. [Google Scholar]
- Ninfali, P.; Antonini, E.; Frati, A.; Scarpa, E.S. C-glycosyl flavonoids from beta vulgaris cicla and betalains from beta vulgaris rubra: antioxidant, anticancer and antiinflammatory activities—A review. Phytother. Res. Ptr. 2017, 31, 871–884. [Google Scholar] [CrossRef]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef]
- Nade, V.S.; Kawale, L.A.; Zambre, S.S.; Kapure, A.B. Neuroprotective potential of Beta vulgaris L. in Parkinson’s disease. Indian J. Pharmacol. 2015, 47, 403–408. [Google Scholar] [CrossRef]
- Cho, J.; Bing, S.J.; Kim, A.; Lee, N.H.; Byeon, S.H.; Kim, G.O.; Jee, Y. Beetroot (Beta vulgaris) rescues mice from gamma-ray irradiation by accelerating hematopoiesis and curtailing immunosuppression. Pharm. Biol. 2017, 55, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wielgomas, B.; Krechniak, J. Effect of α-cypermethrin and chlorpyrifos in a 28-day study on free radical parameters and cholinesterase activity in Wistar rats. Pol. J. Environ. Stud. 2007, 16, 91–95. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–675. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Belfield, A.; Goldberg, D.M. Normal ranges and diagnostic value of serum 5’nucleotidase and alkaline phosphatase activities in infancy. Arc.h Dis. Child. 1971, 46, 842–846. [Google Scholar] [CrossRef]
- Schmidt, M.; Eisenburg, J. Serum bilirubin determination in newborn infants. A new micromethod for the determination of serum of plasma bilirubin in newborn infants. Fortschr. Med 1975, 93, 1461–1466. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988, 34, 497–500. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Factor, V.M.; Kiss, A.; Woitach, J.T.; Wirth, P.J.; Thorgeirsson, S.S. Disruption of redox homeostasis in the transforming growth factor-alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J. Biol. Chem. 1998, 273, 15846–15853. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Twab, S.M.; Hozayen, W.G.; Hussein, O.E.; Mahmoud, A.M. 18beta-Glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren Fail 2016, 38, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Mahmoud, A.M. Gamma-glutamylcysteine ethyl ester protects against cyclophosphamide-induced liver injury and hematologic alterations via upregulation of PPARgamma and attenuation of oxidative stress, inflammation, and apoptosis. Oxid Med. Cell Longev. 2016, 2016, 4016209. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Abd El-Twab, S.M. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARgamma and Nrf2: Protective effect of 18beta-glycyrrhetinic acid. Chem. Biol. Interact. 2017, 270, 59–72. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mahmoud, A.M. Sitagliptin attenuates cardiomyopathy by modulating the JAK/STAT signaling pathway in experimental diabetic rats. Drug Des. Dev. Ther. 2016, 10, 2095–2107. [Google Scholar]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The role of Nrf2 in cardiovascular function and disease. Oxid Med. Cell Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hozayen, W.G.; Ramadan, S.M. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARgamma, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother. 2017, 94, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Elgebaly, H.A.; Mosa, N.M.; Allach, M.; El-Massry, K.F.; El-Ghorab, A.H.; Al Hroob, A.M.; Mahmoud, A.M. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2018, 98, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of Nrf2 and PPARgamma up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ahmed, O.M.; Galaly, S.R. Thymoquinone and curcumin attenuate gentamicin-induced renal oxidative stress, inflammation and apoptosis in rats. Excli. J. 2014, 13, 98–110. [Google Scholar]
- Mansour, S.A.; Mossa, A.-T.H. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol. 2010, 96, 14–23. [Google Scholar] [CrossRef]
- Ma, P.; Wu, Y.; Zeng, Q.; Gan, Y.; Chen, J.; Ye, X.; Yang, X. Oxidative damage induced by chlorpyrifos in the hepatic and renal tissue of Kunming mice and the antioxidant role of vitamin E. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 58, 177–183. [Google Scholar] [CrossRef]
- Olumese, F.; Oboh, H. Hepatoprotective effect of beetroot juice on liver injury in male Sprague-Dawley rats. Ann. Trop. Pathol. 2018, 9, 83–88. [Google Scholar]
- Egeonu, S.; Ihentuge, C.; Okechukwu, H.; Anibeze, C.; Akpuaka, F. Protective effect of beta vulgaris on carbon tetrachloride induced hepatotoxicity in adult wistar rat. FASEB J. 2018, 32, 511–516. [Google Scholar]
- Darwiche, W.; Delanaud, S.; Dupont, S.; Ghamlouch, H.; Ramadan, W.; Joumaa, W.; Bach, V.; Gay-Quéheillard, J. Impact of prenatal and postnatal exposure to the pesticide chlorpyrifos on the contraction of rat ileal muscle strips: Involvement of an inducible nitric oxide synthase-dependent pathway. Neurogastroenterol. Motil. 2017, 29, e12918. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Al Dera, H.S. 18β-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: Potential role of PPARγ and Nrf2 upregulation. Genes Nutr. 2015, 10, 41. [Google Scholar] [CrossRef]
- El-Sayed, N.M.; Ahmed, A.A.M.; Selim, M.A.A. Cytotoxic effect of chlorpyrifos is associated with activation of Nrf-2/HO-1 system and inflammatory response in tongue of male Wistar rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 12072–12082. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, B.; Saraf, M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-beta pathway. J. Ethnopharmacol. 2017, 198, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Indumathi, D.; Sujithra, K.; Srinivasan, S.; Vinothkumar, V. Betanin exhibits significant potential as an antihyperglycemic and attenuating the glycoprotein components in streptozotocin-nicotinamide-induced experimental rats. Toxicol. Mech. Methods 2018, 28, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 73, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Kamel, E.M.; Mahmoud, A.M.; Ahmed, S.A.; Lamsabhi, A.M. A phytochemical and computational study on flavonoids isolated from Trifolium resupinatum L. and their novel hepatoprotective activity. Food Funct. 2016, 7, 2094–2106. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; Jones, A.M.; Wilkinson, J.A.; Romero, M.; Duarte, J.; Alexander, M.Y. A novel role for small molecule glycomimetics in the protection against lipid-induced endothelial dysfunction: Involvement of Akt/eNOS and Nrf2/ARE signaling. Biochim. Biophys. Acta 2017, 1861, 3311–3322. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; McCarthy, E.M.; Moreno-Martinez, D.; Langford-Smith, A.; Romero, M.; Duarte, J.; Alexander, M.Y. Endothelial microparticles prevent lipid-induced endothelial damage via Akt/eNOS signaling and reduced oxidative stress. FASEB J. 2017, 31, 4636–4648. [Google Scholar] [CrossRef]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum. Nutr. (Dordr. Neth.) 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M. Influence of rutin on biochemical alterations in hyperammonemia in rats. Exp. Toxicol. Pathol. 2012, 64, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dai, H.; Zhang, C.; Tian, J.; Deng, Y.; Zhao, M.; Zhao, M.; Bing, G.; Zhao, L. Evaluation of the effects of chlorpyrifos combined with lipopolysaccharide stress on neuroinflammation and spatial memory in neonatal rats. Toxicology 2018, 410, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Abolaji, A.O.; Ojo, M.; Afolabi, T.T.; Arowoogun, M.D.; Nwawolor, D.; Farombi, E.O. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. Biol. Interact. 2017, 270, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, A.Y.; Jeong, S.-H.; Park, K.-H.; Paik, M.-K.; Cho, N.-J.; Kim, J.-E.; Cho, M.-H. Chlorpyrifos induces NLRP3 inflammasome and pyroptosis/apoptosis via mitochondrial oxidative stress in human keratinocyte HaCaT cells. Toxicology 2015, 338, 37–46. [Google Scholar] [CrossRef]
- El Gamal, A.A.; AlSaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; Al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat. Inflamm. 2014, 2014, 983952. [Google Scholar] [CrossRef] [PubMed]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jiminez, R.; Michalowski, T.; Wybraniec, S. Influence of betalin-rich extracts on reduction of discomfort associated with osteoarthritis. New Med. 2010, 1, 12–17. [Google Scholar]
- Pan, H.; Wang, H.; Wang, X.; Zhu, L.; Mao, L. The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat. Inflamm. 2012, 2012, 217580. [Google Scholar] [CrossRef]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Chen, R.; Cui, Y.; Zhang, X.; Zhang, Y.; Chen, M.; Zhou, T.; Lan, X.; Dong, W.; Pan, C. Chlorpyrifos Induction of Testicular-Cell Apoptosis through Generation of Reactive Oxygen Species and Phosphorylation of AMPK. J. Agric. Food Chem. 2018, 66, 12455–12470. [Google Scholar] [CrossRef]


| Gene | Genbank Accession Number | Sequence (5′-3′) |
|---|---|---|
| BAX | NM_017059.2 | F: GGGCCTTTTTGCTACAGGGT R: TTCTTGGTGGATGCGTCCTG |
| BCL2 | NM_016993 | F: ACTCTTCAGGGATGGGGTGA R: TGACATCTCCCTGTTGACGC |
| NOS2 | NM_012611.3 | F: GTTCCTCAGGCTTGGGTCTT R: TGGGGGAACACAGTAATGGC |
| TNFα | NM_012675.3 | F: GGCTTTCGGAACTCACTGGA R: CCCGTAGGGCGATTACAGTC |
| Il1β | NM_031512.2 | F: GACTTCACCATGGAACCCGT R: GGAGACTGCCCATTCTCGAC |
| Nfe2l2 | NM_031789.2 | F: TTGTAGATGACCATGAGTCGC R: ACTTCCAGGGGCACTGTCTA |
| Casp3 | NM_012922.2 | F: GAGCTTGGAACGCGAAGAAA R: TAACCGGGTGCGGTAGAGTA |
| Gapdh | NM_017008.4 | F: AGTGCCAGCCTCGTCTCATA R: GATGGTGATGGGTTTCCCGT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albasher, G.; Almeer, R.; Al-Otibi, F.O.; Al-Kubaisi, N.; Mahmoud, A.M. Ameliorative Effect of Beta vulgaris Root Extract on Chlorpyrifos-Induced Oxidative Stress, Inflammation and Liver Injury in Rats. Biomolecules 2019, 9, 261. https://doi.org/10.3390/biom9070261
Albasher G, Almeer R, Al-Otibi FO, Al-Kubaisi N, Mahmoud AM. Ameliorative Effect of Beta vulgaris Root Extract on Chlorpyrifos-Induced Oxidative Stress, Inflammation and Liver Injury in Rats. Biomolecules. 2019; 9(7):261. https://doi.org/10.3390/biom9070261
Chicago/Turabian StyleAlbasher, Gadah, Rafa Almeer, Fatimah O. Al-Otibi, Noorah Al-Kubaisi, and Ayman M. Mahmoud. 2019. "Ameliorative Effect of Beta vulgaris Root Extract on Chlorpyrifos-Induced Oxidative Stress, Inflammation and Liver Injury in Rats" Biomolecules 9, no. 7: 261. https://doi.org/10.3390/biom9070261
APA StyleAlbasher, G., Almeer, R., Al-Otibi, F. O., Al-Kubaisi, N., & Mahmoud, A. M. (2019). Ameliorative Effect of Beta vulgaris Root Extract on Chlorpyrifos-Induced Oxidative Stress, Inflammation and Liver Injury in Rats. Biomolecules, 9(7), 261. https://doi.org/10.3390/biom9070261






