LFB: A Novel Antimicrobial Brevinin-Like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes fujianensi
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Skin Secretions
2.2. Encoding Novel Peptide Biosynthetic Precursors–Shotgun cDNAs Cloning
2.3. Isolation and Structural Analysis of Novel Peptide
2.4. Blast Analysis and Solid-Phase Peptide Synthesis
2.5. Prediction of Secondary Structures of LFB and Its Physicochemical Properties
2.6. Circular Dichroism Spectra of Synthetic Peptide LFB and Detection of Its Antimicrobial Activity Assays
2.7. Tissue Culture of Maintaining Human Cancer Cell Lines
2.8. Studies on Anti-Proliferative Effects of LFB via MTT Assay and Incucyte Live Cell Imaging Systems
2.9. Lactate Dehydrogenase (LDH) Assay
2.10. Phosphatidylserine Exstrophy Detection (Apoptosis Assay)
2.11. Haemolysis Activity Study
2.12. Statistical Analysis
3. Results
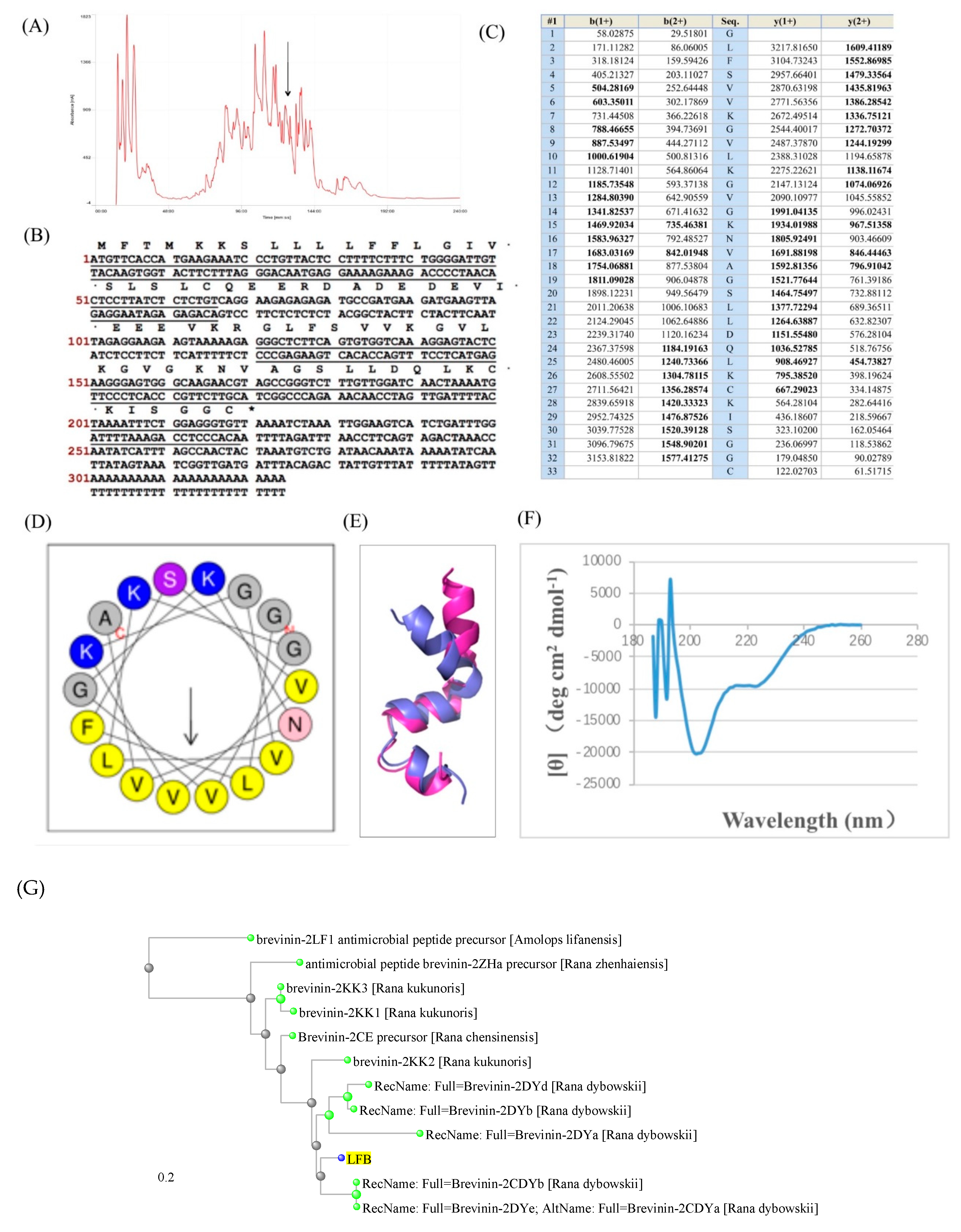
3.1. Isolation and Structural Characterization of the Novel Antimicrobial Peptide
3.2. Molecular Cloning of the Novel Antimicrobial Peptide from the Skin Cdna Library of Limnonectes fujianensis
3.3. Secondary Structures Prediction of Putative Peptide LFB
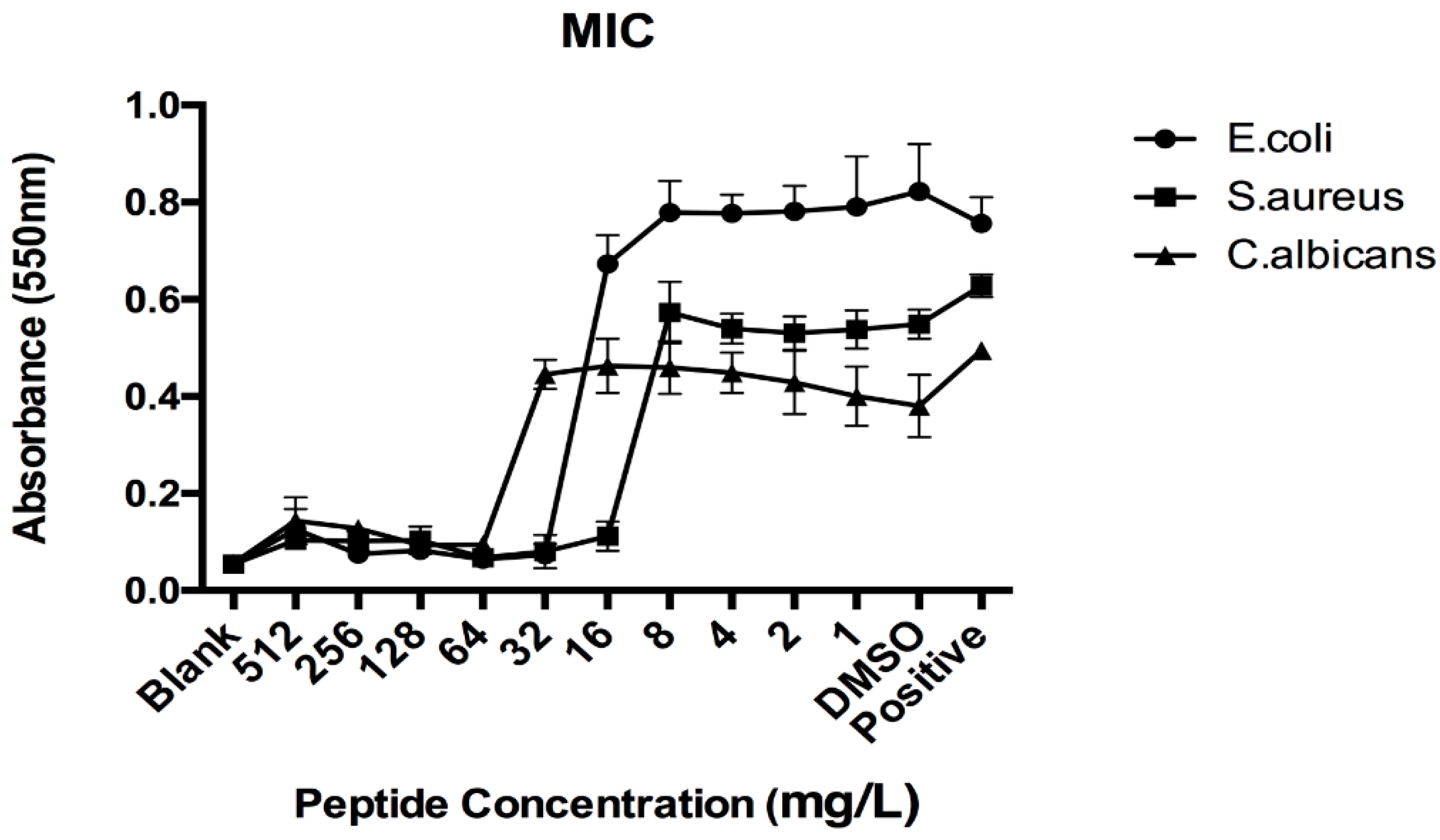
3.4. Antimicrobial Activities of Synthetic LFB
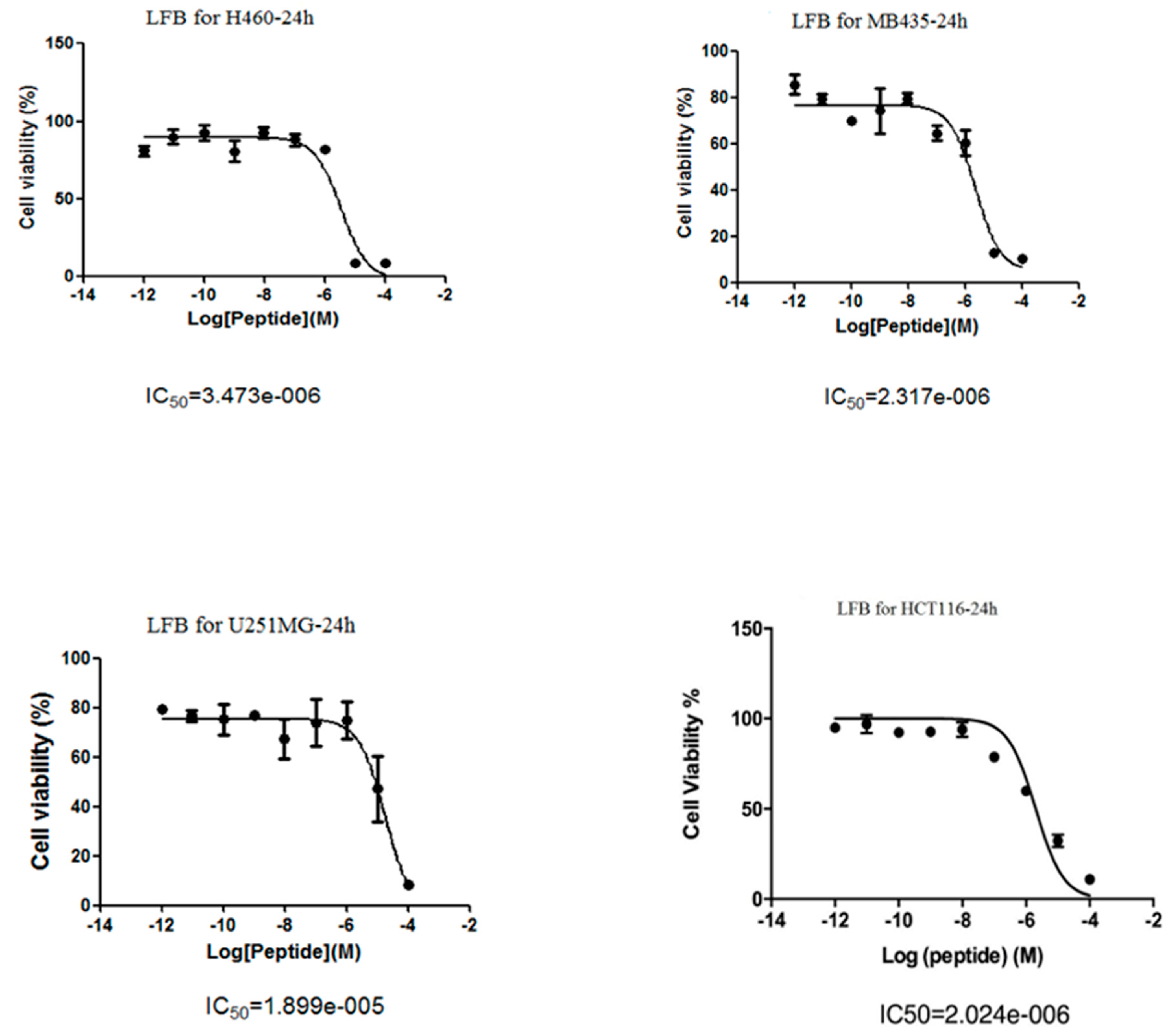
3.5. Anti-Proliferative Effects of LFB on Human Cancer Cells
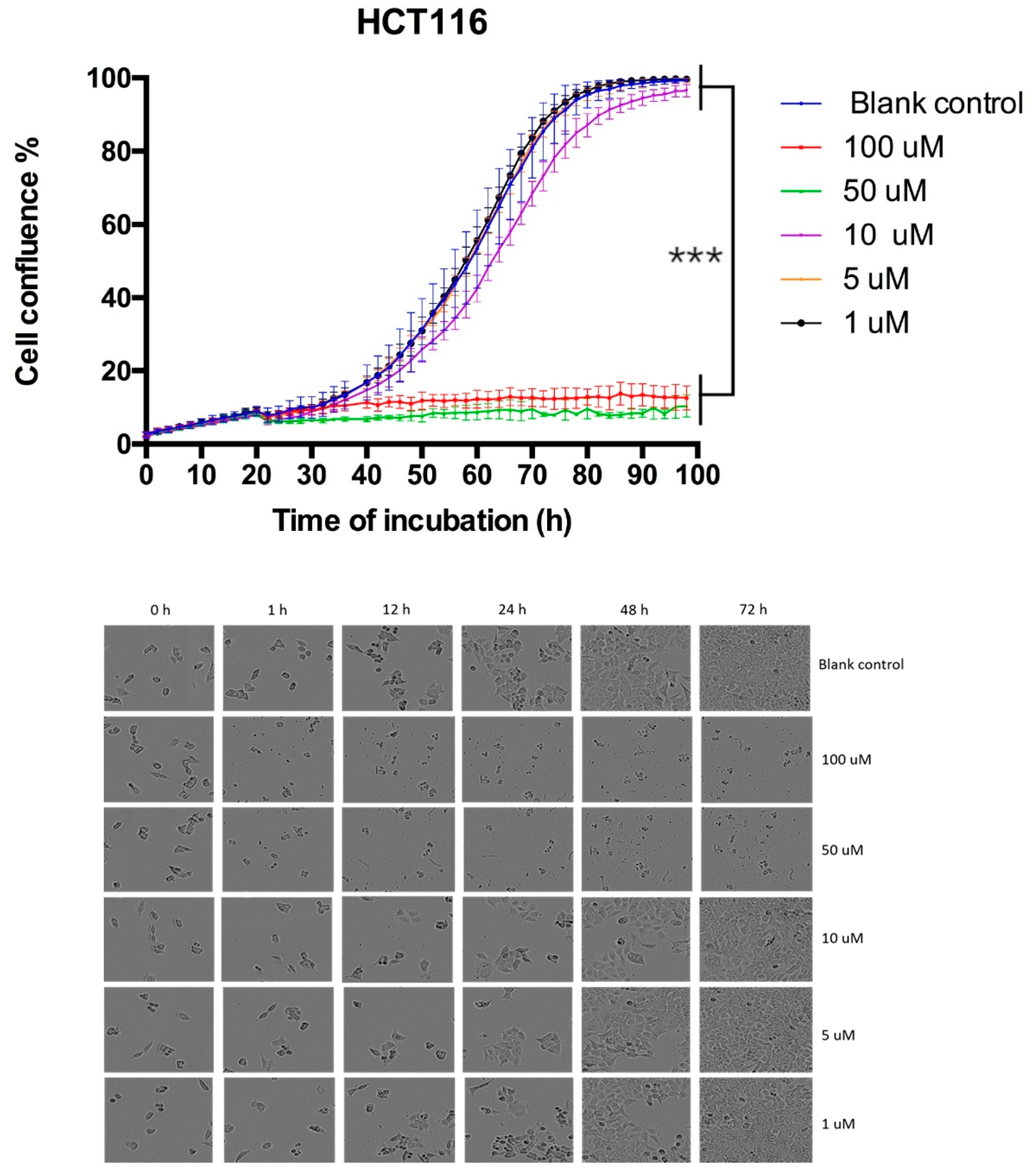
3.6. Proliferation Curve Generated Through Incucyte Live Cell Imaging System
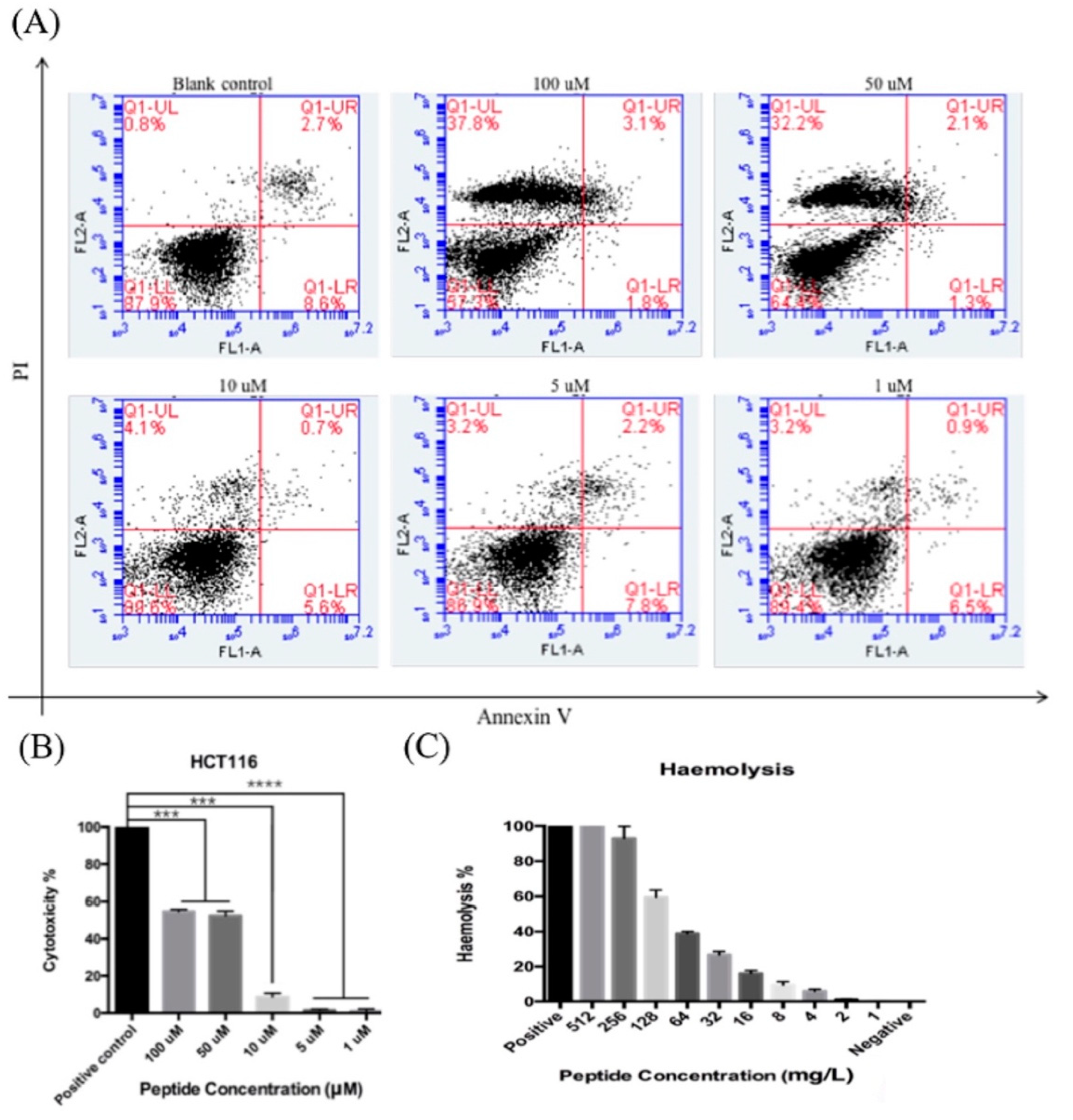
3.7. Apoptosis Assay (Phosphatidylserine Exstrophy Detection)
3.8. LDH Assay of Synthetic Peptide LFB
3.9. Hemolysis Assay of Synthetic Peptide LFB
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hancock, R.; Sahl, H. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Afacan, N.; Yeung, A.; Pena, O.; Hancock, R. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, G. APD: The antimicrobial peptide database. Nucleic Acids Res. 2004, 32, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; Mignogna, G.; Barra, D. Antimicrobial peptides from amphibian skin: What do they tell us? Biopolymers 1998, 47, 435–450. [Google Scholar] [CrossRef]
- Conlon, J. Structural diversity and species distribution of host-defense peptides in frog skin secretions. Cell. Mol. Life Sci. 2011, 68, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hahm, K. Antimicrobial peptides (AMPs): Peptide structure and mode of action. J. Biochem. Mol. Biol. 2005, 38, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P. Multifunctional host defense peptides: Intracellular-targeting antimicrobial peptides. FEBS J. 2009, 276, 6483–6496. [Google Scholar] [CrossRef]
- Yeaman, M.; Yount, N. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Erspamer, V.; Melchiorri, P.; Falconieri, G.; Montecucchi, P.; de Castiglione, R. Phyllomedusa skin: A huge factory and store-house of a variety of active peptides. Peptides 1985, 3, 7–12. [Google Scholar] [CrossRef]
- Amiche, M.; Ladram, A.; Nicolas, P. A consistent nomenclature of antimicrobial peptides isolated from frogs of the subfamily Phyllomedusinae. Peptides 2008, 29, 2074–2082. [Google Scholar] [CrossRef]
- Cantas, L.; Shah, S.; Cavaco, L.; Manaia, C.; Walsh, F.; Popowska, M.; Garelick, H.; Bürgmann, H.; Sørum, H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front. Microbiol. 2013, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Ge, L.; Ma, R.; McCrudden, C.; Chen, T.; Shaw, C.; Kwok, H. Identification and pharmaceutical evaluation of novel frog skin-derived serine proteinase inhibitor peptide—PE-BBI (Pelophylax esculentus Bowman-Birk inhibitor) for the potential treatment of cancer. Sci. Rep. 2018, 8, 14502. [Google Scholar] [CrossRef]
- Gordon, Y.; Romanowski, E.; McDermott, A. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- Rinaldi, A.C. Antimicrobial peptides from amphibian skin: An expanding scenario. Curr. Opin. Chem. Biol. 2002, 6, 799–804. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Reinert, L.K.; O’Leary, C.J.; Houston, L.E.; Woodhams, D.C. Antimicrobial Peptide defenses in amphibian skin. Integr. Comp. Biol. 2005, 45, 137–142. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Borris, R.P. Natural products research: Perspectives from a major pharmaceutical company. J. Ethnopharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.T. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev. Camb. Philos. Soc. 1997, 72, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Fei, L. Atlas of Amphibians of China; Henan Press of Science and Technology: Zhengzhou, China, 1999; p. 202. (In Chinese) [Google Scholar]
- Tyler, M.J.; Stone, D.J.M.; Bowie, J.H. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Methods 1992, 28, 199–200. [Google Scholar] [CrossRef]
- Ge, L.; Lyu, P.; Zhou, M.; Zhang, H.; Wan, Y.; Li, B.; Li, R.; Wang, L.; Chen, T.; Shaw, C. AcT-2: A Novel Myotropic and Antimicrobial Type 2 Tryptophyllin from the Skin Secretion of the Central American Red-Eyed Leaf Frog, Agalychnis callidryas. Sci. World J. 2014, 2014, 158546. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Chen, X.; Ma, C.; Zhou, M.; Xi, X.; Wang, L.; Ding, A.; Duan, J.; Chen, T.; Shaw, C. Balteatide: A novel antimicrobial decapeptide from the skin secretion of the purple-sided leaf frog, Phyllomedusa baltea. Sci. World J. 2014, 2014, 176214. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lyu, P.; Xi, X.; Ge, L.; Mahadevappa, R.; Shaw, C.; Kwok, H. Triggering of cancer cell cycle arrest by a novel scorpion venom-derived peptide—Gonearrestide. J. Cell. Mol. Med. 2018, 22, 4460–4473. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N. Antimicrobial peptides from ranid frogs: Taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim. Biophys. Acta 2004, 1696, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M. The contribution of skin antimicrobial peptides to the system of innate immunity in anurans. Cell Tissue Res. 2011, 343, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Daly, J.W.; Spande, T.F.; Garraffo, H.M. Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J. Nat. Prod. 2005, 68, 1556–1575. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Nouwens, A.; Ge, L.; Frank, N.; Kwok, H.; Fry, B. Habu coagulotoxicity: Clinical implications of the functional diversification of Protobothrops snake venoms upon blood clotting factors. Toxicol. In Vitro 2019, 55, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Debono, J.; Bos, M.; Coimbra, F.; Ge, L.; Frank, N.; Kwok, H.; Fry, B. Basal but divergent: Clinical implications of differential coagulotoxicity in a clade of Asian vipers. Toxicol. In Vitro 2019, 58, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.; Cipriani, V.; Jackson, T.; Arbuckle, K.; Debono, J.; Dashevsky, D.; Panagides, N.; Ikonomopoulou, M.; Koludarov, I.; Li, B.; et al. Proteomic and functional variation within black snake venoms (Elapidae: Pseudechis). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 205, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, F.; Dobson, J.; Zdenek, C.; Brouw, B.; Hamilton, B.; Debono, J.; Masci, P.; Frank, N.; Ge, L.; Kwok, H.; et al. Does size matter? Venom proteomic and functional comparison between night adder species (Viperidae: Causus) with short and long venom glands. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 211, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Ge, L.; Wang, L.; Guo, X.; Zhang, H.; Li, Y.; Zhou, Y.; Zhou, M.; Chen, T.; Shaw, C. Ornithokinin (avian bradykinin) from the skin of the Chinese bamboo odorous frog, Odorrana versabilis. J. Pept. Sci. 2014, 20, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Hou, X.; Ge, L.; Li, R.; Zhou, M.; Wang, H.; Wang, L.; Wei, M.; Chen, T.; Shaw, C. Cationicity-Enhanced Analogues of the Antimicrobial Peptides, AcrAP1 and AcrAP2, from the Venom of the Scorpion, Androctonus crassicauda, Display Potent Growth Modulation Effects on Human Cancer Cell Lines. Int. J. Biol. Sci. 2014, 10, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xi, X.; Ge, L.; Yang, N.; Hou, X.; Ma, J.; Ma, C.; Wu, Y.; Guo, X.; Li, R.; et al. Bradykinin-related peptides (BRPs) from skin secretions of three genera of phyllomedusine leaf frogs and their comparative pharmacological effects on mammalian smooth muscles. Peptides 2013, 52, 122–133. [Google Scholar] [CrossRef]
- Brogden K, A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238. [Google Scholar] [CrossRef]
- Bai, B.; Hou, X.; Wang, L.; Ge, L.; Luo, Y.; Ma, C.; Duan, J.; Chen, T.; Shaw, C. Feleucins: Novel bombinin precursor-encoded nonapeptide amides from the skin secretion of Bombina variegate. BioMed Res. Int. 2014, 2014, 671362. [Google Scholar] [CrossRef]
- Chen, Q.; Cheng, P.; Ma, C.; Xi, X.; Wang, L.; Zhou, M.; Chen, T. Evaluating the Bioactivity of a Novel Broad-Spectrum Antimicrobial Peptide Brevinin-1GHa from the Frog Skin Secretion of Hylarana guentheri and Its Analogues. Toxins 2018, 10, 413. [Google Scholar] [CrossRef]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Erspamer, V.; Erspamer, G.F.; Cei, J.M. Active peptides in the skins of two hundred and thirty American amphibian species. Comp. Biochem. Physiol. C 1986, 85, 125–137. [Google Scholar] [CrossRef]
- Koehn, F.E. High impact technologies for natural products screening. Prog. Drug Res. 2008, 65, 175–210. [Google Scholar] [PubMed]
- Muñoz, A.; López-García, B.; Marcos, J.F. Studies on the mode of action of the antifungal hexapeptide PAF26. Antimicrob. Agents Chemother. 2006, 50, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Hou, X.; Wang, L.; Zhang, Y.; Xi, X.; Wang, H.; Zhou, M.; Duan, J.; Wei, M.; Chen, T.; et al. AaeAP1 and AaeAP2: Novel Antimicrobial Peptides from the Venom of the Scorpion, Androctonus aeneas: Structural Characterisation, Molecular Cloning of Biosynthetic Precursor-Encoding cDNAs and Engineering of Analogues with Enhanced Antimicrobial and Anticancer Activities. Toxins 2015, 7, 219–237. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Lyu, P.; Xie, S.; Qin, H.; Pu, W.; Xu, H.; Chen, T.; Shaw, C.; Ge, L.; Kwok, H.F. LFB: A Novel Antimicrobial Brevinin-Like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes fujianensi. Biomolecules 2019, 9, 242. https://doi.org/10.3390/biom9060242
Li B, Lyu P, Xie S, Qin H, Pu W, Xu H, Chen T, Shaw C, Ge L, Kwok HF. LFB: A Novel Antimicrobial Brevinin-Like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes fujianensi. Biomolecules. 2019; 9(6):242. https://doi.org/10.3390/biom9060242
Chicago/Turabian StyleLi, Bin, Peng Lyu, Shuping Xie, Haixin Qin, Wenyuan Pu, Houxi Xu, Tianbao Chen, Chris Shaw, Lilin Ge, and Hang Fai Kwok. 2019. "LFB: A Novel Antimicrobial Brevinin-Like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes fujianensi" Biomolecules 9, no. 6: 242. https://doi.org/10.3390/biom9060242
APA StyleLi, B., Lyu, P., Xie, S., Qin, H., Pu, W., Xu, H., Chen, T., Shaw, C., Ge, L., & Kwok, H. F. (2019). LFB: A Novel Antimicrobial Brevinin-Like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes fujianensi. Biomolecules, 9(6), 242. https://doi.org/10.3390/biom9060242





_Kwok.png)
