Extraction of Cathepsin D-Like Protease from Neon Flying Squid (Ommastrephes bartramii) Viscera and Application in Antioxidant Hydrolysate Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
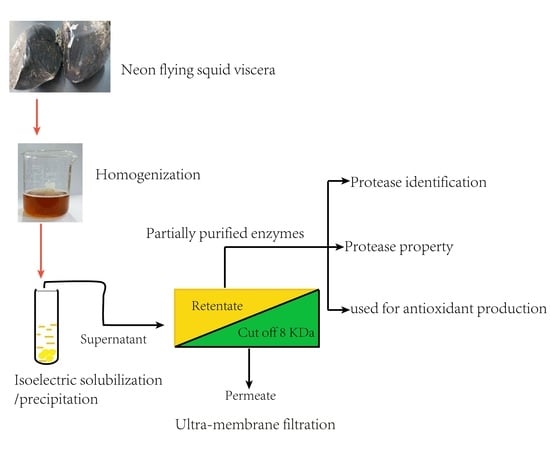
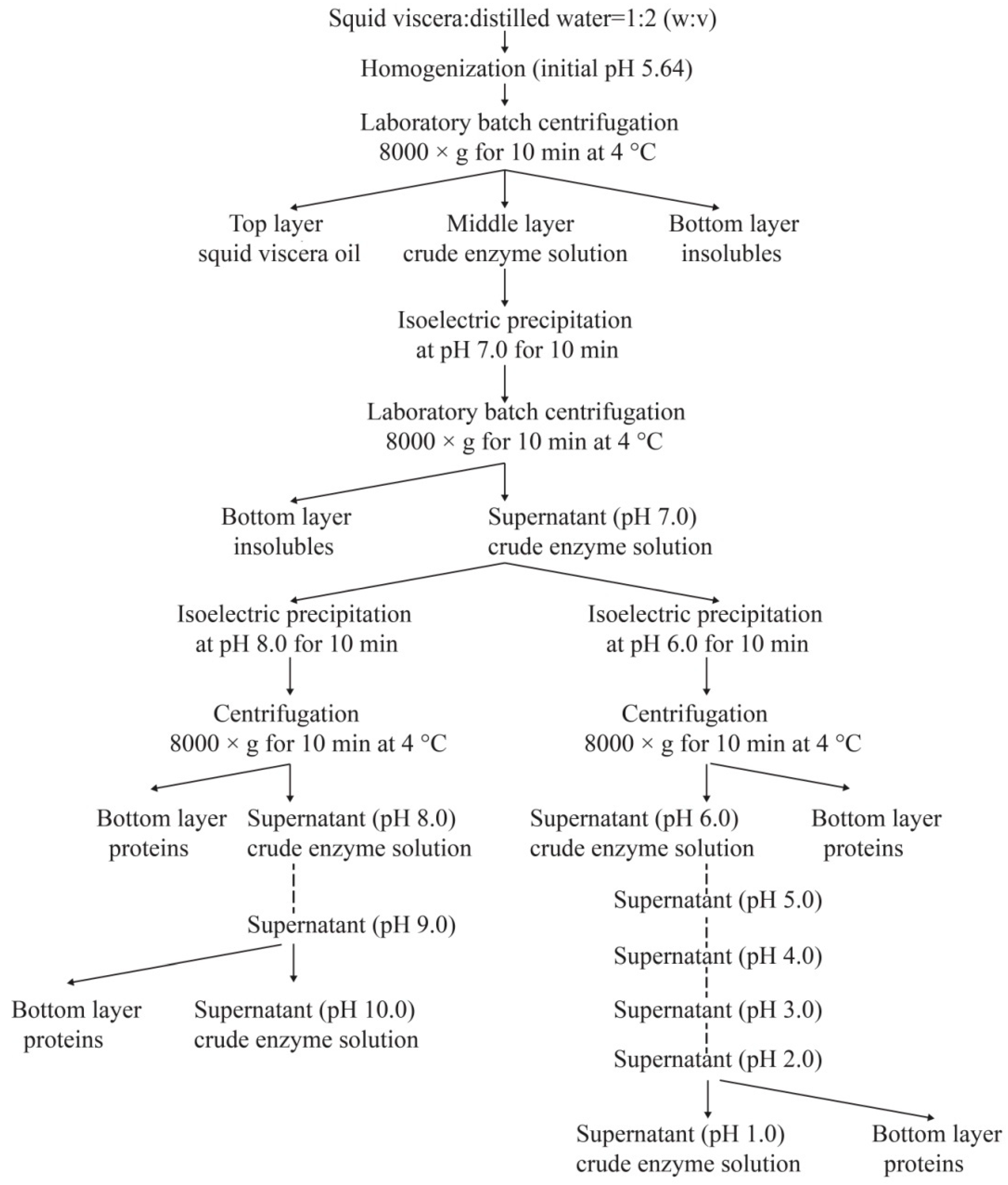
2.2. Extraction of Crude Enzymes
2.3. Protease Activity and Specific Activity Assays
2.4. Purification of Crude Enzymes
2.5. Identification of Enzyme by UPLC-ESI-MS/MS
2.6. Protease Features and Stability of SVCE3(f)
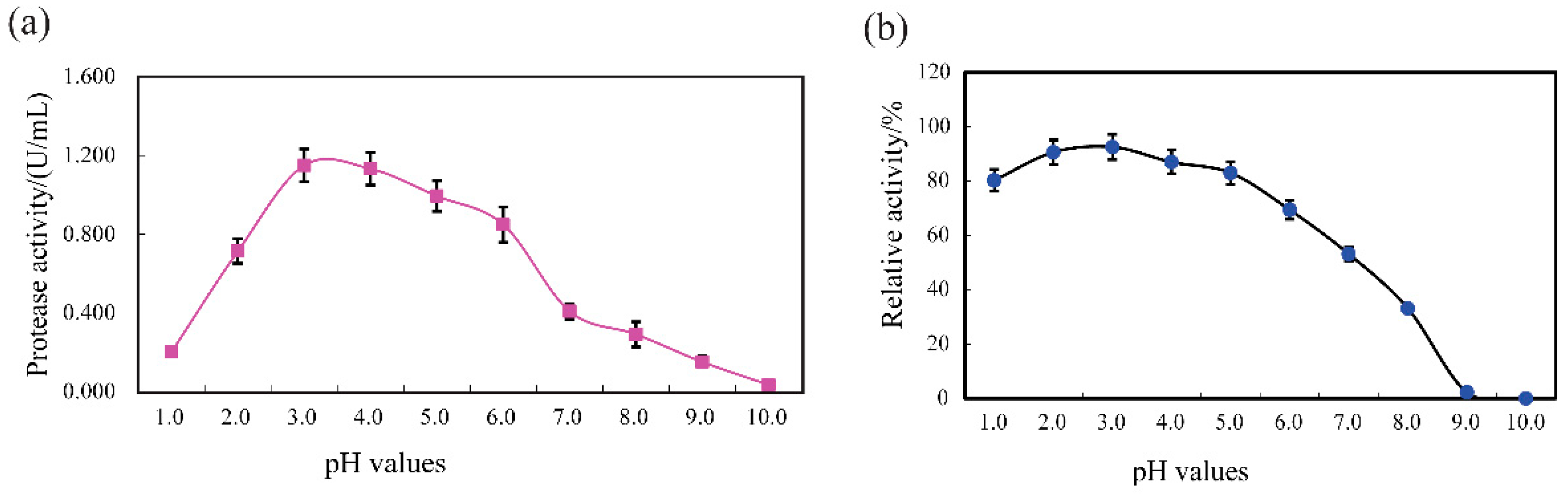
2.6.1. pH Range and Stability
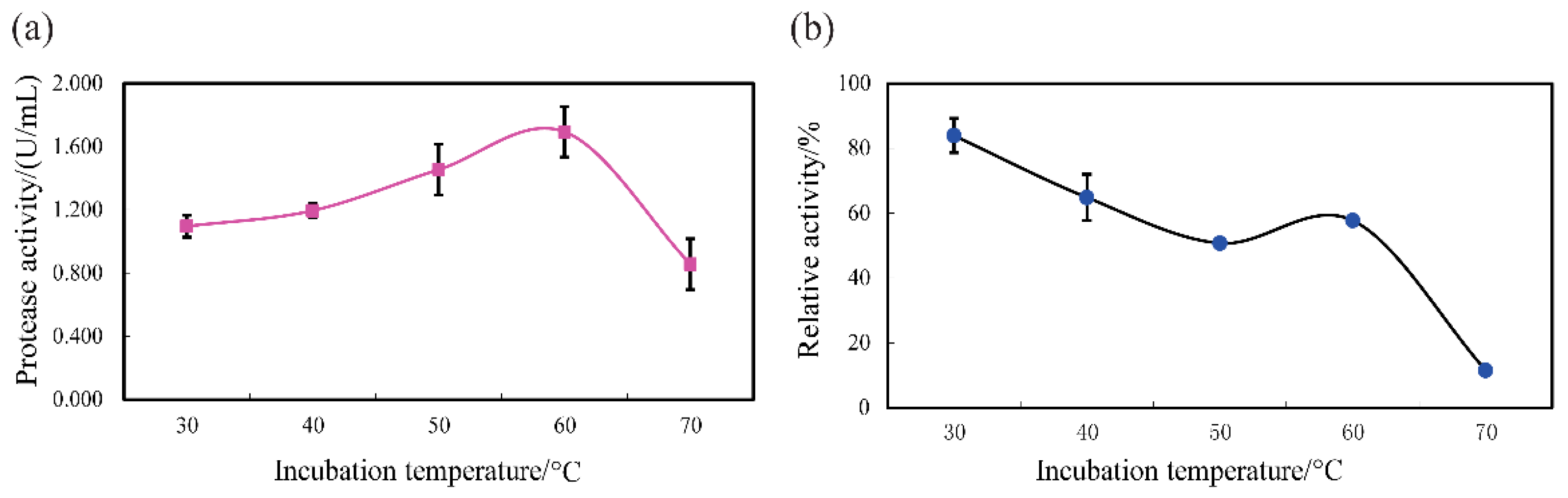
2.6.2. Temperature Tolerance Profile and Stability
2.7. Preparation of Antioxidant Half-Fin Anchovy Hydrolysates using SVCE3(f)
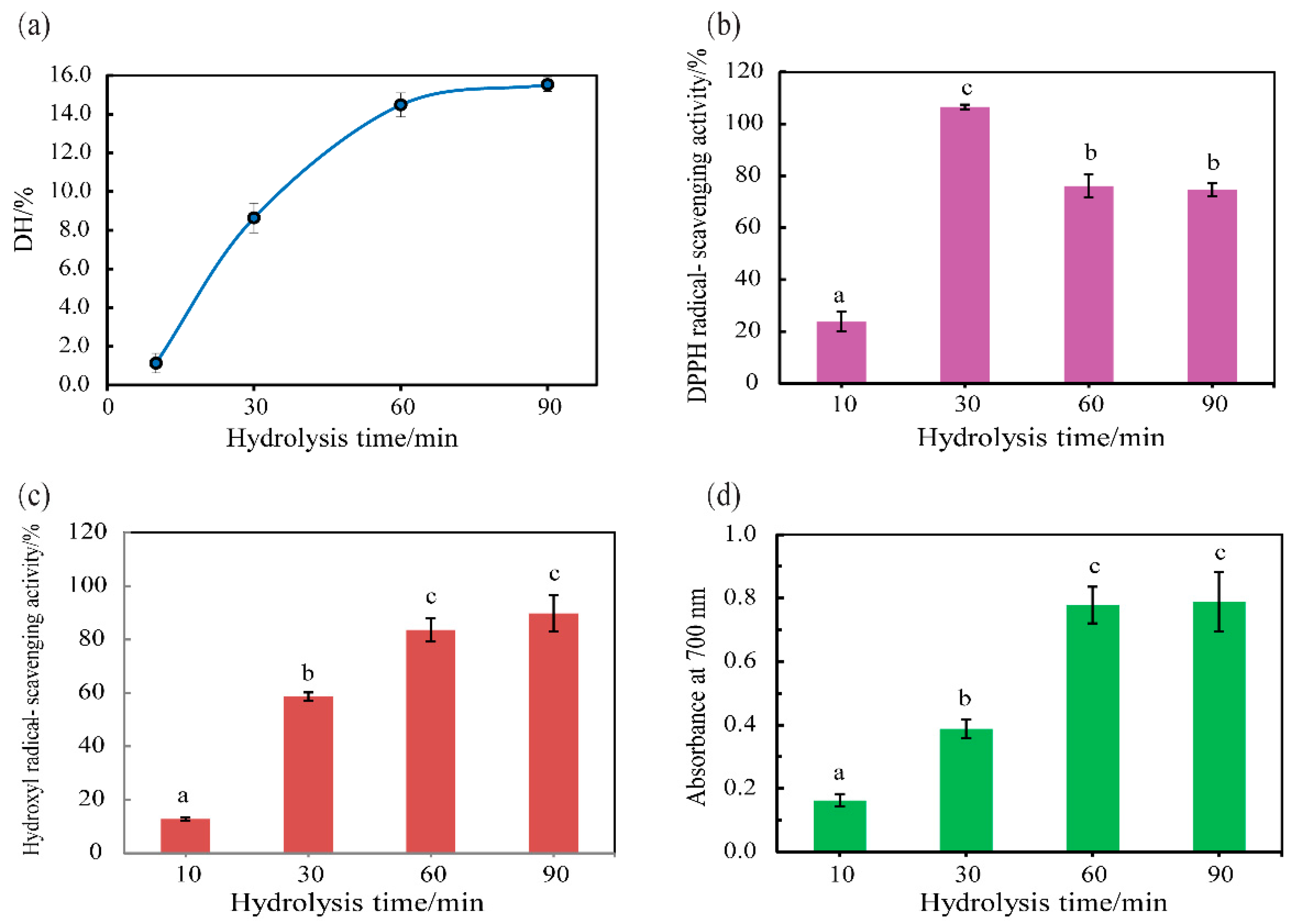
2.8. DH Assay
2.9. In Vitro Antioxidant Activity of HAHp-SEs
2.9.1. DPPH Radical Scavenging Activity
2.9.2. Reducing Power
2.9.3. Hydroxyl Radical Scavenging Activity
2.10. Statistical Analysis
3. Results and Discussion
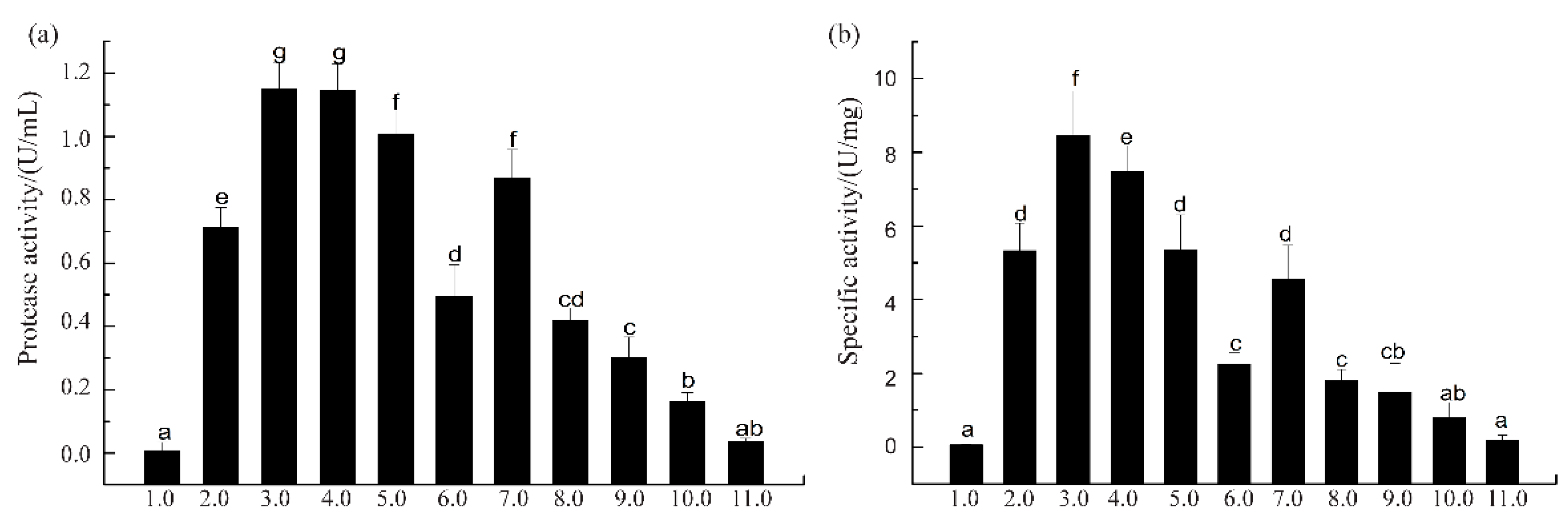
3.1. Protease Activity and Specific Activity of SVCEs
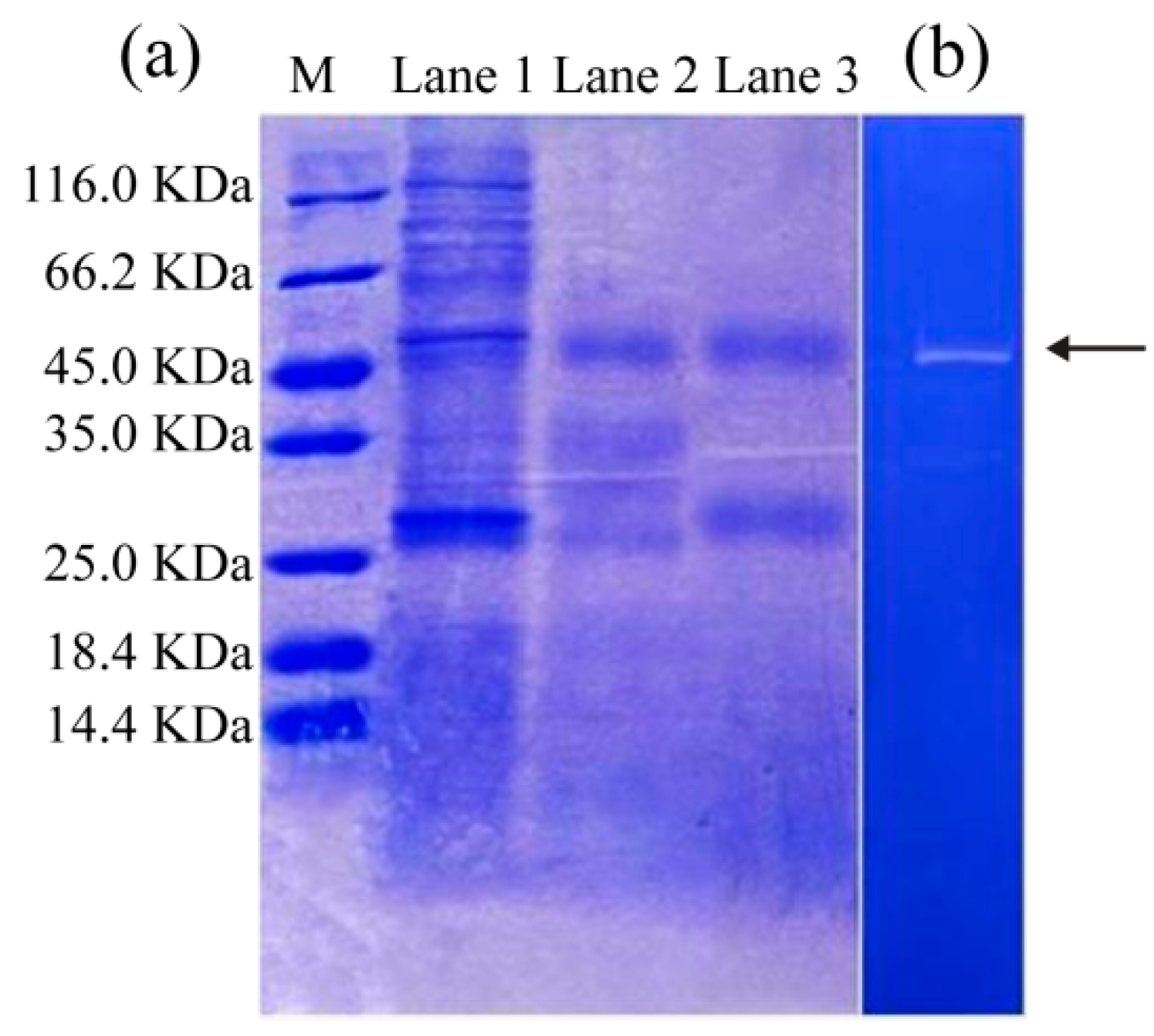
3.2. SDS-PAGE and Zymography Analysis
3.3. Identification of Protease in SVCE3(f) by UPLC-ESI-MS/MS
3.4. Protease Features and Stability of SVCE3(f)
3.4.1. pH Profile and Stability
3.4.2. Temperature Tolerance and Stability
3.5. Hydrolysis of Half-Fin Anchovy with SVCE3(f)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Liu, B.; Chen, Y. A review of the development of Chinese distant-water squid jigging fisheries. Fish. Res. 2008, 89, 211–221. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Tian, S.; Liu, B.; Qian, W. An assessment of the west winter–spring cohort of neon flying squid (Ommastrephes bartramii) in the Northwest Pacific Ocean. Fish. Res. 2008, 92, 221–230. [Google Scholar] [CrossRef]
- Balti, R.; Barkia, A.; Bougatef, A.; Ktari, N.; Nasri, M. A heat-stable trypsin from the hepatopancreas of the cuttlefish (Sepia officinalis): Purification and characterisation. Food Chem. 2009, 113, 146–154. [Google Scholar] [CrossRef]
- Cardenas-Lopez, J.C.; Haard, N.F. Cysteine proteinase activity in jumbo squid (Dosidicus Gigas) hepatopancreas extracts. J. Food Biochem. 2005, 29, 1745–1753. [Google Scholar] [CrossRef]
- Hameed, K.S.; Haard, N.F. Isolation and characterization of cathepsin C from Atlantic short finned squid Illex illecebrosus. Comp. Biochem. 1985, 82, 241–246. [Google Scholar] [CrossRef]
- Idalis, O.; Gloria, Y.; Ofelia, R.; Marina, E. Aminopeptidase from jumbo squid (Dosidicus gigas) hepatopancreas: Purification, characterisation, and casein hydrolysis. Int. J. Food Sci. Tech. 2010, 45, 387–394. [Google Scholar]
- Komai, T.; Kawabataa, C.; Amanob, M.; Leec, B.R.; Ichishima, E. Todarepsin, a new Cathepsin D from hepatopancreas of Japanese common squid (Todarodes pacificus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 137, 373–382. [Google Scholar] [CrossRef]
- Tian, Y.; Umezawa, E.; Duan, R.; Konno, K. Three types of proteinases in Japanese common squid Todarodes pacificus hepatopancreas as studied by using carp myofibrils as substrate. Fish. Res. 2010, 76, 365–373. [Google Scholar] [CrossRef][Green Version]
- Sukarno; Takahashi, K.; Hatano, M.; Sakurai, Y. Lipase from neon flying squid hepatopancreas: Purification and properties. Food Chem. 1996, 57, 515–521. [Google Scholar] [CrossRef]
- Balti, R.; Bougherra, F.; Bougatef, A.; Hayet, B.K.; Nedjar-Arroume, N.; Dhulster, P.; Guillochon, D.; Nasr, M. Chymotrypsin from the hepatopancreas of cuttlefish (Sepia officinalis) with high activity in the hydrolysis of long chain peptide substrates: Purification and biochemical characterisation. Food Chem. 2012, 130, 475–484. [Google Scholar] [CrossRef]
- Shahidi, F.; Kami, Y.V.A.J. Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci. Tech. 2001, 12, 435–464. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Vanabun, A.; Benjakul, S. Recovery of proteases from the viscera of farmed giant catfish (Pangasianodon gigas) by three-phase partitioning. Process. Biochem. 2012, 47, 2566–2569. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ahn, H.M.; Kishimura, H.; Chun, B.S. Comparative study of digestive enzymes of squid (Todarodes pacificus) viscera after supercritical carbon dioxide and organic solvent extraction. Biotechnol. Bioproc. E 2009, 14, 338–344. [Google Scholar] [CrossRef]
- Matak, K.E.; Tahergorabi, R.; Jaczynski, J. A review: Protein isolates recovered by isoelectric solubilization/precipitation processing from muscle food by-products as a component of nutraceutical foods. Food Res. Int. 2015, 77, 697–703. [Google Scholar] [CrossRef]
- Shi, L.; Beamer, S.K.; Yin, T.; Matak, K.E.; Yang, H.; Jaczynski, J. Mass balance for isoelectric solubilization/precipitation of carp, chicken, menhaden, and krill. LWT Food Sci. Technol. 2017, 81, 26–34. [Google Scholar] [CrossRef]
- Zhao, X.; Xing, T.; Wang, P.; Xu, X.L.; Zhou, G.H. Oxidative stability of isoelectric solubilization/precipitation-isolated PSE like chicken protein. Food Chem. 2019, 283, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.C.; Kaneko, K.; Okazaki, E.; Cao, M.J.; Ohwaki, H.; Weng, W.Y.; Osako, K. Comparative study of proteins recovered from whole North Pacific krill Euphausia pacifica by acidic and alkaline treatment during isoelectric solubilization/precipitation. Fish. Res. 2013, 79, 537–546. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Liang, Y. Effect of pH-shift processing and surimi processing on Atlantic croaker (Micropogonias undulates) muscle proteins. J. Food Sci. 2006, 71, C304–C312. [Google Scholar] [CrossRef]
- Gildberg, A.; Simpson, B.K.; Haard, N.F. Uses of enzymes from marine organisms. In Seafood Enzymes; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 619–640. [Google Scholar]
- Ktari, N.; Bkhairia, I.; Jridi, M.; Hamza, I.; Riadh, B.S.; Nasri, M. Digestive acid protease from zebra blenny (Salaria basilisca): Characteristics and application in gelatin extraction. Food Res. Int. 2014, 57, 218–224. [Google Scholar] [CrossRef]
- Espósito, T.S.; Amaral, I.P.G.; Buarque, D.S.; Oliveira, G.B.; Carvalho, L.B., Jr.; Bezerra, R.S. Fish processing waste as a source of alkaline proteases for laundry detergent. Food Chem. 2009, 112, 125–130. [Google Scholar] [CrossRef]
- Hamdi, M.; Hammami, A.; Hajji, S.; Jridi, M.; Nasri, M.; Nasri, R. Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. Int. J. Biol. Macromol. 2017, 101, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ktari, N.; Jridi, M.; Bkhairia, I.; Sayari, N.; Salah, R.B.; Nasri, M. Functionalities and antioxidant properties of protein hydrolysates from muscle of zebra blenny (Salaria basilisca) obtained with different crude protease extracts. Food Res. Int. 2012, 49, 747–756. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.B.; Ruan, G.Q.; Luo, H.Y. Isolation and identification of antioxidative peptides from peptic hydrolysates of half-fin anchovy (Setipinna taty). LWT Food Sci. Technol. 2015, 60, 221–229. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Khaled, H.B.; Ghorbel-Bellaaj, O.; Hmidet, N.; Jellouli, K.; Ali, N.E.H.; Ghorbel, S.; Nasri, M. A novel aspartic protease from the viscera of Sardinelle (Sardinella aurita): Purification and characterization. Food Chem. 2011, 128, 847–853. [Google Scholar] [CrossRef]
- Cao, W.H.; Zhang, C.H.; Hong, P.Z.; Ji, H.W. Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem. 2008, 109, 176–183. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Zhu, B.W.; Qiao, L.; Wu, H.T.; Li, D.M.; Yang, J.F.; Murata, Y. In vitro antioxidant activity of enzymatic hydrolysates prepared from abalone (Haliotis discus hannai Ino) viscera. Food Bioprod. Process. 2012, 90, 148–154. [Google Scholar] [CrossRef]
- AOAC. International Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Song, R.; Wei, R.; Zhang, B.; Yang, Z.; Wang, D. Antioxidant and antiproliferative activities of heated sterilized pepsin hydrolysate derived from half-fin anchovy (Setipinna taty). Mar. Drugs 2011, 9, 1142–1156. [Google Scholar] [CrossRef]
- de Avellar, I.G.J.; Magalhães, M.M.M.; Silva, A.B.; Souza, L.L.; Leitão, A.C.; Hermes-Lima, M. Reevaluating the role of 1,10-phenanthroline in oxidative reactions involving ferrous ions and DNA damage. BBA Gen. Subj. 2004, 1675, 46–53. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Albers, E.; Undeland, I. pH-shift processing of Nannochloropsis oculata microalgal biomass to obtain a protein-enriched food or feed ingredient. Algal Res. 2015, 11, 95–102. [Google Scholar] [CrossRef]
- Cardenas-Lopez, J.L.; Haard, N.F. Identification of a cysteine proteinase from Jumbo squid (Dosidicus gigas) hepatopancreas as Cathepsin L. Food Chem. 2009, 112, 442–447. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Wu, J.; Chang, R.; Lan, X.; Wei, K.; Jia, X. Effects of cations on the “salt in” of myofibrillar proteins. Food Hydrocolloid. 2016, 58, 179–183. [Google Scholar] [CrossRef]
- Rojo, L.; Sotelo-Mundo, R.; García-Carreño, F.; Gráf, L. Isolation, biochemical characterization, and molecular modeling of American lobster digestive cathepsin D1. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Merino, V.; Siva Kumar, N. Isolation, affinity purification and biochemical characterization of a lysosomal Cathepsin D from the deuterostome Asterias rubens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 161, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yonezawa, S.; Yoshizaki, N. Vitellogenesis-related from Xenopus laevis: Purification and properties in comparison with liver Cathepsin D. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 113, 835–840. [Google Scholar] [CrossRef]
- Venugopal, A.; Kumar, N.S. Biochemical characterization of Cathepsin D from the mussel Lamellidens corrianus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 169, 25–30. [Google Scholar] [CrossRef]
- Gildberg, A. Purification and characterization of Cathepsin D from the digestive gland of the pelagic squid Todarodes sagittatus. J. Sci. Food Agric. 1987, 39, 85–94. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, J.; Ji, X.; Zhou, F.; Fu, Y.; Chen, W.; Zeng, Y.; Li, T.; Wang, H. Molecular cloning, characterization and expression of cathepsin D from grass carp (Ctenopharyngodon idella). Fish Shellfish. Immun. 2012, 33, 1207–1214. [Google Scholar] [CrossRef]
- Nielsen, L.B.; Nielsen, H.K. Purification and characterization of cathepsin D from herring muscle (Clupea harengus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 128, 351–363. [Google Scholar] [CrossRef]
- Negi, M.; Tsuboi, R.; Matsui, T.; Ogawa, H. Isolation and characterization of proteinase from Candida albicans: Substrate specificity. J. Invest. Dermatol. 1984, 83, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, P.; Fusek, M. Two crystal structures for cathepsin D: The lysosomal targeting signal and active site. EMBO J. 1993, 12, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Benes, P.; Vetvicka, V.; Fusek, M. Cathepsin D—Many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 2008, 68, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Mc Sweeney, P.L.H.; Fox, P.F.; Olson, N.F. Proteolysis of bovine caseins by cathepsin D: Preliminary observations and comparison with chymosin. Int. Dairy J. 1995, 5, 321–336. [Google Scholar] [CrossRef]
- Rojo, L.; Muhlia- Almazán, A.; Saborowski, R.; García Carreño, F.L. Aspartic cathepsin D endopeptidase contributes to extracellular digestion in clawed lobsters Homarus americanus and Homarus gammarus. Mar. Biotechnol. 2010, 12, 696–707. [Google Scholar] [CrossRef]
- Simpson, B.K. Digestive proteinases from marine animals. In Seafood Enzymes; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 531–540. [Google Scholar]
- Takahashi, T.; Tang, J. Cathepsin D from porcine and bovine spleen. Methods Enzymol. 1981, 80, 565–581. [Google Scholar]
- Whitaker, J.R. Principles of Enzymology for the Food Sciences, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Wang, P.A.; Stenvik, J.; Larsen, R.; Maehre, H.; Olsen, R.L. Cathepsin D from Atlantic cod (Gadus morhua L) liver. Isolation and comparative studies. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 147, 504–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
| Purification Steps | Protease Activity (U/mL) | Protein Content (mg/mL) | Specific Activity (U/mg) | Purification Fold | Protein Recovery (%) |
|---|---|---|---|---|---|
| SVCE | 0.49 ± 0.06 | 0.22 ± 0.04 | 2.24 ± 0.18 | 1 | 100 |
| SVCE3 | 1.15 ± 0.07 | 0.14 ± 0.04 | 8.25 ± 0.74 | 3.68 ± 0.33 | 63.64 ± 4.55 |
| SVCE3(f) | 1.06 ± 0.09 | 0.12 ± 0.03 | 8.92 ± 0.81 | 3.98 ± 0.36 | 54.55 ± 9.09 |
| Protein Database | Protein Number | Protein | Protein Score | Molecular Weight (Da) | Coverage Ratio | Amino Acid before N-terminus | Sequence | Amino Acid before C-terminus |
|---|---|---|---|---|---|---|---|---|
| Teuthida | gi|46309251 | Cathepsin D [Todarodes pacificus] | 1044 | 42,748 | 38.5 | K | LNEAIGGR | A |
| R | KGYWQIK | M | ||||||
| K | YYTVFDLGK | N | ||||||
| K | LPNVDFVLGGK | T | ||||||
| K | EGGELILGGSDPK | H | ||||||
| K | LSDIACLLHNK | Y | ||||||
| K | VTPVFYQIISQK | L | ||||||
| K | LVDQPVFSFYLNR | D | ||||||
| K | VVFDTGSSNLWVPSK | K | ||||||
| R | ALPGGEYMVDCASIPK | L | ||||||
| K | FDGILGMAYDTISVDK | V | ||||||
| K | VVFDTGSSNLWVPSKK | C | ||||||
| K | AQTFAEATNQPGLVFVAAK | F | ||||||
| K | FDGILGMAYDTISVDKVTPVFYQIISQK | L | ||||||
| gi|315439548 | Actin [Heterololigo bleekeri] | 237 | 42,111 | 12.5 | R | GYSFTTTAER | E | |
| K | SYELPDGQVITIGNER | F | ||||||
| K | DLYANTVLSGGTTMFPGIADR | M | ||||||
| R | GYSFTTTAER | E | ||||||
| K | IWHHTFYNELR | V | ||||||
| gi|223016073 | Actin [T. pacificus] | 73 | 42,023 | 9.8 | K | SYELPDGQVITIGNER | F | |
| gi|51860817 | Beta actin [Doryteuthis pealeii] | 73 | 41,962 | 9.9 | R | GYSFTTTAER | E | |
| K | IWHHTFYNELR | V | ||||||
| K | SYELPDGQVITIGNER | F | ||||||
| gi|1911573 | Enolase [D. pealeii] | 53 | 47,738 | 3.5 | K | VNQIGSVTESIQACK | M | |
| Coleoidea | gi|46309251 | Cathepsin D [T. pacificus] | 829 | 42,748 | 36.7 | K | LSDIACLLHNK | Y |
| K | VTPVFYQIISQK | L | ||||||
| K | LVDQPVFSFYLNR | D | ||||||
| K | VVFDTGSSNLWVPSK | K | ||||||
| R | ALPGGEYMVDCASIPK | L | ||||||
| K | FDGILGMAYDTISVDK | V | ||||||
| K | VVFDTGSSNLWVPSKK | C | ||||||
| K | AQTFAEATNQPGLVFVAAK | F | ||||||
| K | FDGILGMAYDTISVDKVTPVFYQIISQK | L | ||||||
| gi|918295374 | Hypothetical protein OCBIM_22000256mg [Octopus bimaculoides] | 238 | 42,082 | 11.2 | R | GYSFTTTAER | E | |
| K | IWHHTFYNELR | V | ||||||
| K | DLYANTVLSGGTTMYPGIADR | M | ||||||
| gi|315439550 | Actin [S. esculenta] | 208 | 41,996 | 15.4 | R | GYSFTTTAER | E | |
| K | IWHHTFYNELR | V | ||||||
| K | SYELPDGQVITIGNER | F | ||||||
| K | DLYANTVLSGGTTMFPGIADR | M | ||||||
| gi|344944062 | Beta-actin [Sepiella maindroni] | 208 | 42,238 | 15.4 | R | GYSFTTTAER | E | |
| K | IWHHTFYNELR | V | ||||||
| K | SYELPDGQVITIGNER | F | ||||||
| K | DLYANTVLSGGTTMFPGIADR | M | ||||||
| gi|385145402 | Actin I [Sepia officinalis] | 208 | 42,093 | 15.4 | R | GYSFTTTAER | E | |
| K | IWHHTFYNELR | V | ||||||
| K | SYELPDGQVITIGNER | F | ||||||
| K | DLYANTVLSGGTTMFPGIADR | M | ||||||
| gi|918321201 | Hypothetical protein OCBIM_22013417mg [O. bimaculoides] | 208 | 42,093 | 15.4 | R | GYSFTTTAER | E | |
| K | IWHHTFYNELR | V | ||||||
| K | SYELPDGQVITIGNER | F | ||||||
| K | DLYANTVLSGGTTMFPGIADR | M | ||||||
| gi|918314023 | Hypothetical protein OCBIM_22023116mg, partial [O. bimaculoides] | 179 | 48,773 | 10.8 | K | ESTLHLVLR | L | |
| K | ESTLHLVLR | L | ||||||
| R | TLSDYNIQK | E | ||||||
| K | IQDKEGIPPDQQR | L | ||||||
| K | TITLEVEPSDTIENVK | A | ||||||
| gi|918337215 | Hypothetical protein OCBIM_22021358mg [O. bimaculoides] | 67 | 44,939 | 2.6 | K | NTVGWYECHR | C | |
| gi|918336596 | Hypothetical protein OCBIM_22025091mg, partial [O. bimaculoides] | 44 | 45,263 | 5.7 | K | APDSDGGTPLTK | Y | |
| K | VSYDYVDLEWK | A | ||||||
| K | VSYDYVDLEWKAPDSDGGTPLTK | Y | ||||||
| gi|918315574 | Hypothetical protein OCBIM_22020904mg [O. bimaculoides] | 37 | 43,900 | 2.1 | K | GIIQLLEK | C | |
| gi|918321870 | Hypothetical protein OCBIM_22012740mg [O. bimaculoides] | 24 | 43,267 | 2.7 | R | TGATVVDVIR | R | |
| gi|918297782 | Hypothetical protein OCBIM_22038295mg [O. bimaculoides] | 23 | 44,186 | 2.3 | K | IGTPVMLLR | N |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Wei, R.; Song, R. Extraction of Cathepsin D-Like Protease from Neon Flying Squid (Ommastrephes bartramii) Viscera and Application in Antioxidant Hydrolysate Production. Biomolecules 2019, 9, 228. https://doi.org/10.3390/biom9060228
Zhang K, Wei R, Song R. Extraction of Cathepsin D-Like Protease from Neon Flying Squid (Ommastrephes bartramii) Viscera and Application in Antioxidant Hydrolysate Production. Biomolecules. 2019; 9(6):228. https://doi.org/10.3390/biom9060228
Chicago/Turabian StyleZhang, Kaiqiang, Rongbian Wei, and Ru Song. 2019. "Extraction of Cathepsin D-Like Protease from Neon Flying Squid (Ommastrephes bartramii) Viscera and Application in Antioxidant Hydrolysate Production" Biomolecules 9, no. 6: 228. https://doi.org/10.3390/biom9060228
APA StyleZhang, K., Wei, R., & Song, R. (2019). Extraction of Cathepsin D-Like Protease from Neon Flying Squid (Ommastrephes bartramii) Viscera and Application in Antioxidant Hydrolysate Production. Biomolecules, 9(6), 228. https://doi.org/10.3390/biom9060228