Partial Surface Modification of Low Generation Polyamidoamine Dendrimers: Gaining Insight into their Potential for Improved Carboplatin Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PAMAM G3.0 Dendrimer
2.3. Preparation of Activated mPEG-NPC
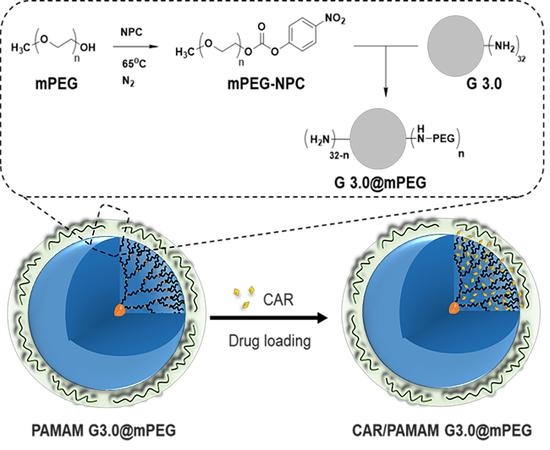
2.4. Preparation of mPEG-Modified PAMAM G3.0 Dendrimer (PAMAM G3.0@mPEG)
2.5. Preparation of CAR/PAMAM G3.0@mPEG
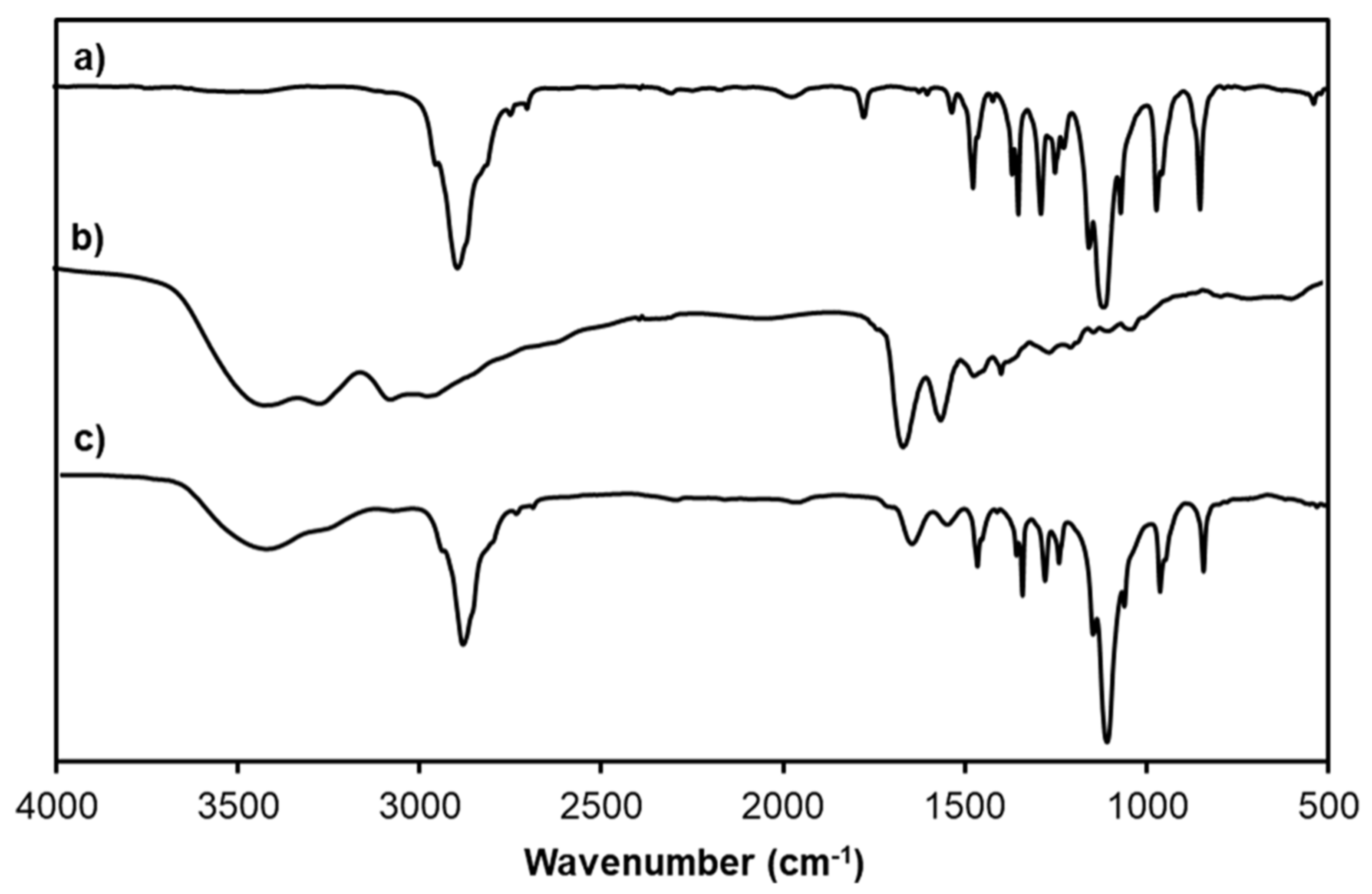
2.6. Characterizations
2.7. CAR Loading Capacity and In Vitro CAR Release
2.8. Cell Viability Assays
3. Results and discussion
3.1. Preparation of PAMAM G3.0@mPEG
3.2. Characterization of CAR/PAMAM G3.0@mPEG
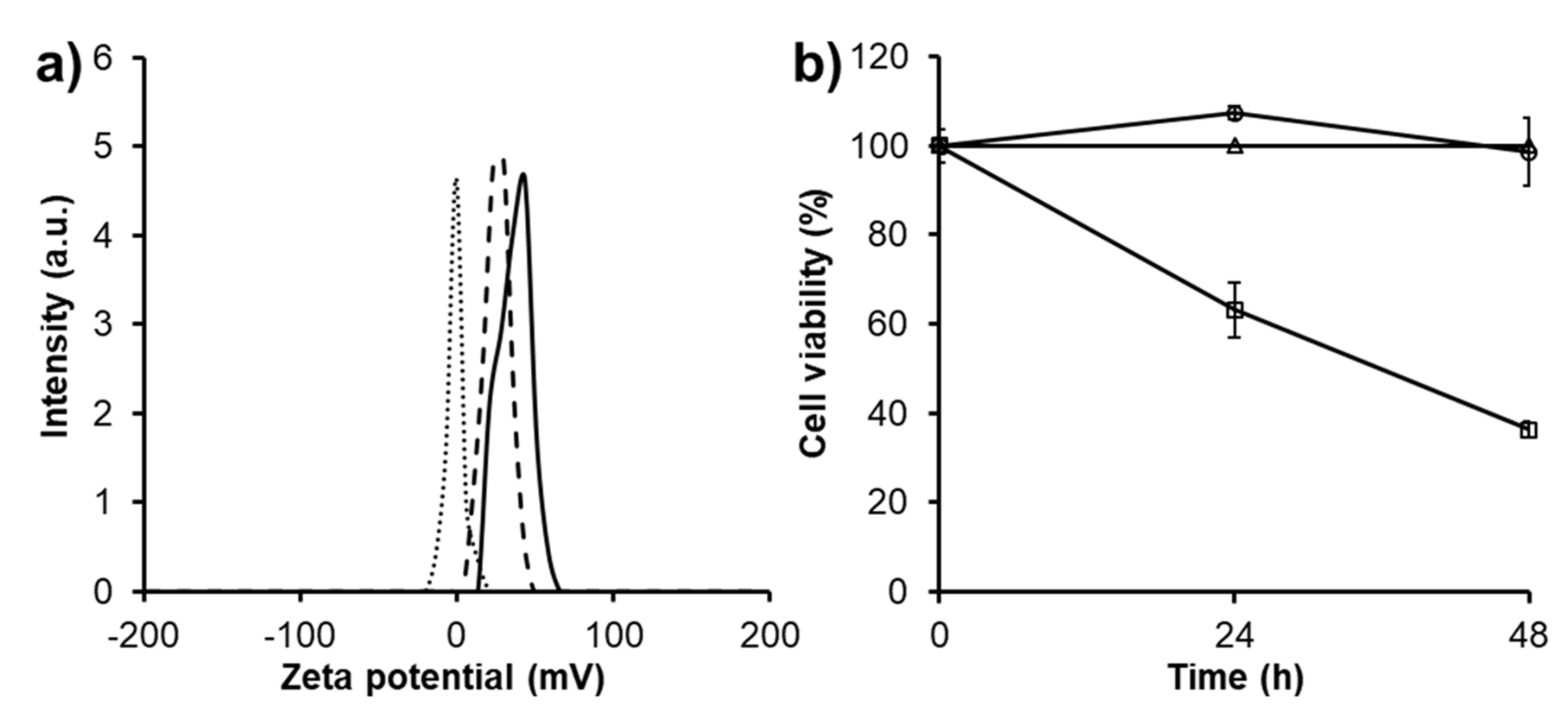
3.3. Charge-Related Cytotoxicity
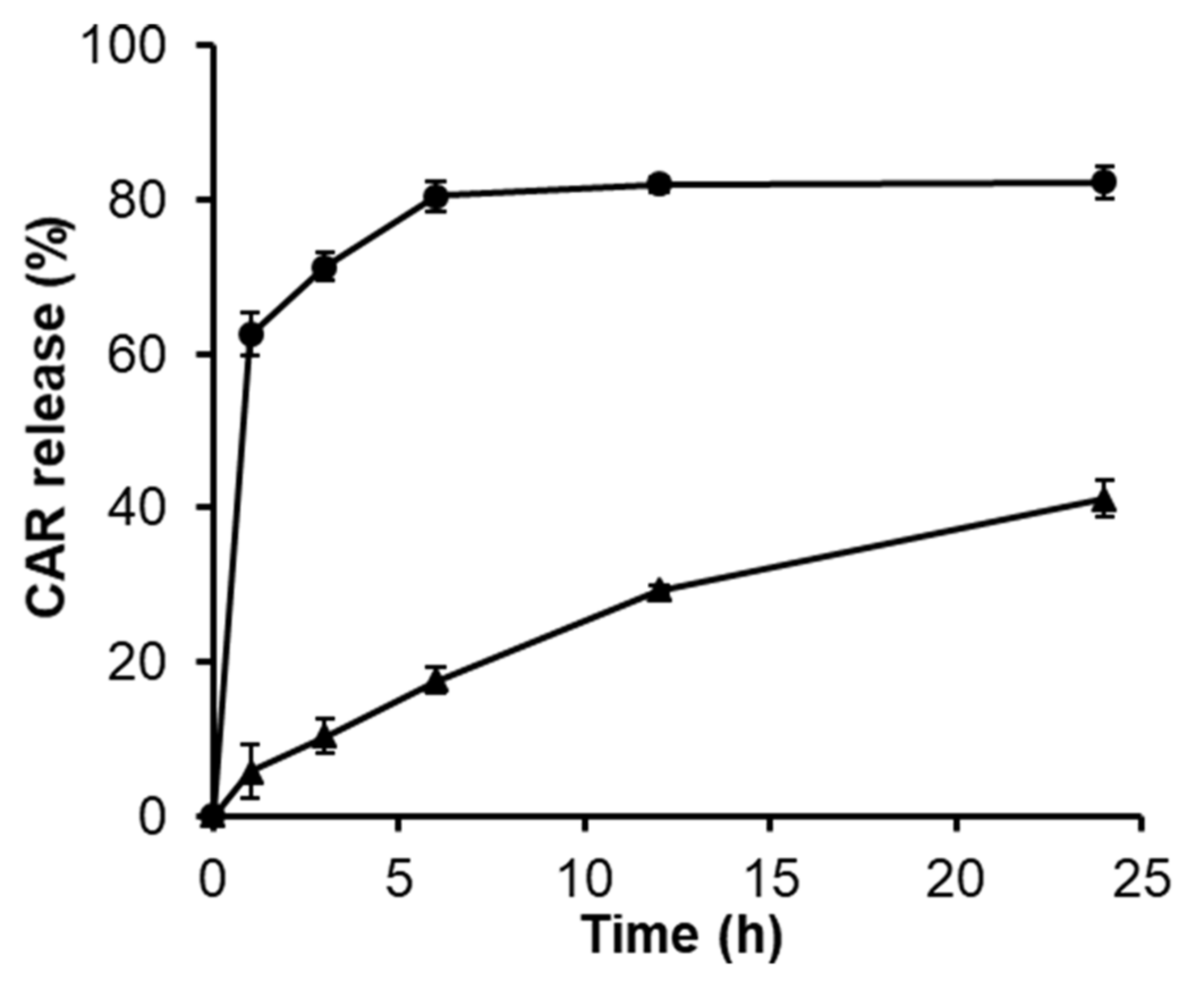
3.4. Release Behavior of CAR
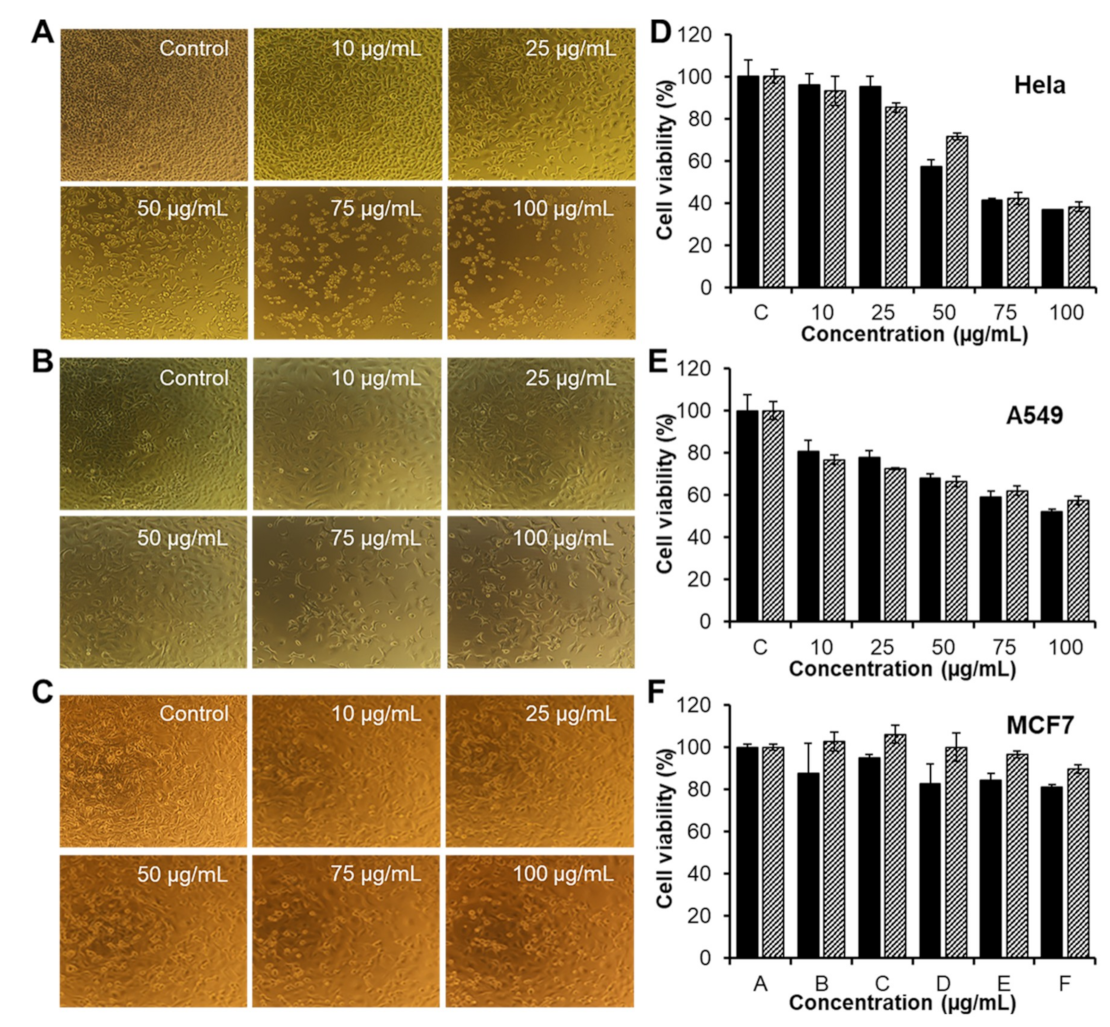
3.5. In Vitro Activities of CAR/PAMAM G3.0@mPEG against Various Types of Cancer Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marques, M.P.M. Platinum and palladium polyamine complexes as anticancer agents: The structural factor. ISRN Spectrosc. 2013, 2013, 1–29. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and improving platinum anticancer drugs–phenanthriplatin. Anticancer Res. 2014, 34, 471–476. [Google Scholar]
- Vu, M.T.; Bach, L.G.; Nguyen, D.C.; Ho, M.N.; Nguyen, N.H.; Tran, N.Q.; Nguyen, D.H.; Nguyen, C.K.; Hoang, T.T.T. Modified carboxyl-terminated PAMAM dendrimers as great cytocompatible nano-based drug delivery system. Int. J. Mol. Sci. 2019, 20, 2016. [Google Scholar] [CrossRef]
- Xiao, H.; Yan, L.; Dempsey, E.M.; Song, W.; Qi, R.; Li, W.; Chen, X. Recent progress in polymer-based platinum drug delivery systems. Progr. Polym. Sci. 2018, 87, 70–106. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Zalba, S.; Garrido, M.J. Liposomes, a promising strategy for clinical application of platinum derivatives. Exp. Opin. Drug Deliv. 2013, 10, 829–844. [Google Scholar] [CrossRef]
- North, S.M.; Banks, T.A. Small Animal Oncology E-Book: An Introduction; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Ebrahimifar, M.; Nili-Ahmadabadi, A.; Akbarzadeh, A.; Shahemabadi, H.E.; Hasanzadegan, M.; Moradi-Sardareh, H.; Madadizadeh, H.; Rezaee-diyan, J. Preparation, characterization and cytotoxic effects of pegylated nanoliposomal containing carboplatin on ovarian cancer cell lines. Indian J. Clin. Biochem. 2017, 32, 230–234. [Google Scholar] [CrossRef]
- Arlt, M.; Haase, D.; Hampel, S.; Oswald, S.; Bachmatiuk, A.; Klingeler, R.; Büchner, B. Delivery of carboplatin by carbon-based nanocontainers mediates increased cancer cell death. Nanotechnology 2010, 21, 335101. [Google Scholar] [CrossRef]
- Alex, A.T.; Joseph, A.; Shavi, G.; Rao, J.V.; Udupa, N. Development and evaluation of carboplatin-loaded PCL nanoparticles for intranasal delivery. Drug Deliv. 2016, 23, 2144–2153. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. The Past, Present, and Future for Dendrons and Dendrimers; Cambridge University Press: Cambridge, UK, 2012; pp. 378–406. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Tang, G.T.; Zhang, L.H.; Kong, S.Y.; Zhu, S.J.; Pei, Y.Y. PEGylated PAMAM dendrimers as a potential drug delivery carrier: In vitro and in vivo comparative evaluation of covalently conjugated drug and noncovalent drug inclusion complex. J. Drug Target. 2010, 18, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, H.; Li, Y.; Zhang, M.; Tomás, H.; Shen, M.; Shi, X. Antitumor efficacy of doxorubicin encapsulated within PEGylated poly (amidoamine) dendrimers. J. Appl. Polym. Sci. 2014, 131, 40358. [Google Scholar] [CrossRef]
- Ho, M.N.; Bach, L.G.; Nguyen, T.H.; Ho, M.H.; Nguyen, D.H.; Nguyen, C.K.; Nguyen, C.H.; Nguyen, N.V.; Thi, T.T.H. PEGylated poly (amidoamine) dendrimers-based drug loading vehicles for delivering carboplatin in treatment of various cancerous cells. J. Nanopart. Res. 2019, 21, 43. [Google Scholar] [CrossRef]
- Ly, T.U.; Tran, N.Q.; Hoang, T.K.D.; Phan, K.N.; Truong, H.N.; Nguyen, C.K. Pegylated dendrimer and its effect in fluorouracil loading and release for enhancing antitumor activity. J. Biomed. Nanotechnol. 2013, 9, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, C.K.; Nguyen, D.H. Redox and pH responsive poly (amidoamine) dendrimer-heparin conjugates via disulfide linkages for letrozole delivery. BioMed. Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Tran, N.Q.; Nguyen, C.K.; Nguyen, T.P. Dendrimer-based nanocarriers demonstrating a high efficiency for loading and releasing anticancer drugs against cancer cells in vitro and in vivo. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045013. [Google Scholar] [CrossRef]
- Nguyen, D.T.D.; Bach, L.G.; Nguyen, T.H.; Ho, M.H.; Ho, M.N.; Nguyen, D.H.; Nguyen, C.K.; Thi, T.T.H. Preparation and characterization of oxaliplatin drug delivery vehicle based on PEGylated half-generation PAMAM dendrimer. J. Polym. Res. 2019, 26, 116. [Google Scholar] [CrossRef]
- Ho, M.N.; Bach, L.G.; Nguyen, D.H.; Nguyen, C.H.; Nguyen, C.K.; Tran, N.Q.; Nguyen, N.V.; Thi, T.T.H. PEGylated PAMAM dendrimers loading oxaliplatin with prolonged release and high payload without burst effect. Biopolymers 2019, e23272. [Google Scholar] [CrossRef]
- Nguyen, H.; Nguyen, N.H.; Tran, N.Q.; Nguyen, C.K. Improved method for preparing cisplatin-dendrimer nanocomplex and its behavior against NCI-H460 lung cancer cell. J. Nanosci. Nanotechnol. 2015, 15, 4106–4110. [Google Scholar] [CrossRef]
- Pan, D.; Guo, C.; Luo, K.; Yi, Q.; Gu, Z. PEGylated dendritic diaminocyclohexyl-platinum (II) conjugates as pH-responsive drug delivery vehicles with enhanced tumor accumulation and antitumor efficacy. Biomaterials 2014, 35, 10080–10092. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Koushik, O.S.; Kumar, V.P. Surface modification of cationic dendrimers eases drug delivery of anticancer drugs. Nanosci. Nanotechnol. 2016, 10, 108. [Google Scholar]
- Kim, Y.; Klutz, A.M.; Jacobson, K.A. Systematic investigation of polyamidoamine dendrimers surface-modified with poly (ethylene glycol) for drug delivery applications: Synthesis, characterization, and evaluation of cytotoxicity. Bioconjug. Chem. 2008, 19, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Lee, J.S.; Choi, J.H.; Park, K.M.; Lee, Y.; Park, K.D. Hierarchical self-assembly of magnetic nanoclusters for theranostics: Tunable size, enhanced magnetic resonance imagability, and controlled and targeted drug delivery. Acta Biomater. 2016, 35, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.Q.; Le, N.H.; Nguyen, D.H.T.; Tran, T.V.; Pham, L.P.T.; Bach, L.G.; Nguyen, T.H.; Nguyen, D.H. Evolution and present scenario of multifunctionalized mesoporous nanosilica platform: A mini review. Mater. Sci. Eng. C 2018, 91, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.K.; Nguyen, T.H.; Bao, B.Q.; Bach, L.G.; Nguyen, D.H. Efficient Self-assembly of mpeg end-capped porous silica as a redox-sensitive nanocarrier for controlled doxorubicin delivery. Int. J. Biomater. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Qiu, L.; Cheng, L.; Hu, Q.; Liu, Y.; Hu, Z.; Cheng, L. Redox and pH dual responsive poly (amidoamine) dendrimer-poly (ethylene glycol) conjugates for intracellular delivery of doxorubicin. Acta Biomater. 2016, 36, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Kurokawa, Y.; Win-Shwe, T.T.; Zeng, Q.; Hirano, S.; Zhang, Z.; Sone, H. Effects of PAMAM dendrimers with various surface functional groups and multiple generations on cytotoxicity and neuronal differentiation using human neural progenitor cells. J. Toxicol. Sci. 2016, 41, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.D.; Kumar, P.V.; Selvam, T.P.a.; Rao, K.S. Prolonged drug delivery system of PEGylated PAMAM dendrimers with a anti-HIV drug. Res. Pharm. 2015, 3, 8–17. [Google Scholar]
- Li, Y.; He, H.; Lu, W.; Jia, X. A poly (amidoamine) dendrimer-based drug carrier for delivering DOX to gliomas cells. RSC Adv. 2017, 7, 15475–15481. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; McKeown, N.B.; D’emanuele, A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharmaceut. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Thanh, V.M.; Nguyen, T.H.; Tran, T.V.; Ngoc, U.T.P.; Ho, M.N.; Nguyen, T.T.; Nguyen, D.H. Low systemic toxicity nanocarriers fabricated from heparin-mPEG and PAMAM dendrimers for controlled drug release. Mater. Sci. Eng. C 2018, 82, 291–298. [Google Scholar] [CrossRef]
- Hoang, D.Q.; Tran, T.V.; Tran, N.Q.; Nguyen, C.K.; Nguyen, T.H.; Truong, M.D.; Nguyen, D.H. Functionalization of Fe3O4 nanoparticles with biodegradable chitosan-grafted-mPEG for paclitaxel delivery. Green Process. Synth. 2016, 5, 459–466. [Google Scholar] [CrossRef]
- Tong, N.A.N.; Nguyen, T.H.; Nguyen, D.H.; Nguyen, C.K.; Tran, N.Q. Preparation of the cationic dendrimer-based hydrogels for controlled heparin release. J. Macromol. Sci. Part A 2015, 52, 830–837. [Google Scholar] [CrossRef]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharmaceut. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef]
- Mori, A.; Klibanov, A.L.; Torchilin, V.P.; Huang, L. Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS Lett. 1991, 284, 263–266. [Google Scholar] [CrossRef]
- Matsushima, A.; Kodera, Y.; Hiroto, M.; Nishimura, H.; Inada, Y. Bioconjugates of proteins and polyethylene glycol: Potent tools in biotechnological processes. J. Mol. Catal. B Enzym. 1996, 2, 1–17. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Giorgi, M.E.; Agusti, R.; de Lederkremer, R.M. Carbohydrate PEGylation, an approach to improve pharmacological potency. Beilstein J. Organic Chem. 2014, 10, 1433–1444. [Google Scholar] [CrossRef]
- Thi, T.N.L.; Nguyen, T.H.; Hoang, D.Q.; Tran, T.V.; Nguyen, N.T.; Nguyen, D.H. Development of new magnetic nanoparticles: Oligochitosan obtained by γ-rays and–coated Fe3O4 nanoparticles. App. Surf. Sci. 2017, 422, 863–868. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Choi, J.H.; Joung, Y.K.; Park, K.D. Disulfide-crosslinked heparin-pluronic nanogels as a redox-sensitive nanocarrier for intracellular protein delivery. J. Bioactive Comp. Polym. 2011, 26, 287–300. [Google Scholar] [CrossRef]
- Tran, D.H.N.; Nguyen, T.H.; Vo, T.N.N.; Pham, L.P.T.; Vo, D.M.H.; Nguyen, C.K.; Bach, L.G.; Nguyen, D.H. Self-assembled poly (ethylene glycol) methyl ether-grafted gelatin nanogels for efficient delivery of curcumin in cancer treatment. J. Appl. Polym. Sci. 2019, 136, 47544. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Tran, D.H.N.; Bach, L.G.; Vu, Q.H.; Nguyen, D.C.; Park, K.D.; Nguyen, D.H. Functional magnetic core-shell system-based iron oxide nanoparticle coated with biocompatible copolymer for anticancer drug delivery. Pharmaceutics 2019, 11, 120. [Google Scholar]
- Tripathy, S.; Das, M.K. Dendrimers and their applications as novel drug delivery carriers. J. Appl. Pharmaceut. Sci. 2013, 3, 142–149. [Google Scholar]
- Nguyen, D.H.; Bae, J.W.; Choi, J.H.; Lee, J.S.; Park, K.D. Bioreducible cross-linked Pluronic micelles: pH-triggered release of doxorubicin and folate-mediated cellular uptake. J. Bioactive Compat. Polym. 2013, 28, 341–354. [Google Scholar] [CrossRef]
- Kang, S.J.; Durairaj, C.; Kompella, U.B.; O’Brien, J.M.; Grossniklaus, H.E. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Archiv. Ophthalmol. 2009, 127, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Domanski, D.M.; Klajnert, B.; Bryszewska, M. Influence of PAMAM dendrimers on human red blood cells. Bioelectrochemistry 2004, 63, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Controll. Release 2000, 65, 133–148. [Google Scholar]
- Zhu, S.; Hong, M.; Tang, G.; Qian, L.; Lin, J.; Jiang, Y.; Pei, Y. Partly PEGylated polyamidoamine dendrimer for tumor-selective targeting of doxorubicin: The effects of PEGylation degree and drug conjugation style. Biomaterials 2010, 31, 1360–1371. [Google Scholar] [CrossRef]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N.K. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharmaceut. 2003, 257, 111–124. [Google Scholar] [CrossRef]
- Yang, H.; Lopina, S.T.; DiPersio, L.P.; Schmidt, S.P. Stealth dendrimers for drug delivery: Correlation between PEGylation, cytocompatibility, and drug payload. J. Mater. Sci. Mater. Med. 2008, 19, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.H.; Bach, L.G.; Nguyen Tran, D.-H.; Cao, V.D.; Nguyen, T.N.Q.; Le, T.T.H.; Tran, T.T.; Thi, T.T.H. Partial Surface Modification of Low Generation Polyamidoamine Dendrimers: Gaining Insight into their Potential for Improved Carboplatin Delivery. Biomolecules 2019, 9, 214. https://doi.org/10.3390/biom9060214
Nguyen DH, Bach LG, Nguyen Tran D-H, Cao VD, Nguyen TNQ, Le TTH, Tran TT, Thi TTH. Partial Surface Modification of Low Generation Polyamidoamine Dendrimers: Gaining Insight into their Potential for Improved Carboplatin Delivery. Biomolecules. 2019; 9(6):214. https://doi.org/10.3390/biom9060214
Chicago/Turabian StyleNguyen, Dai Hai, Long Giang Bach, Diem-Huong Nguyen Tran, Van Du Cao, Thi Nhu Quynh Nguyen, Thi Thu Hong Le, Thach Thao Tran, and Thai Thanh Hoang Thi. 2019. "Partial Surface Modification of Low Generation Polyamidoamine Dendrimers: Gaining Insight into their Potential for Improved Carboplatin Delivery" Biomolecules 9, no. 6: 214. https://doi.org/10.3390/biom9060214
APA StyleNguyen, D. H., Bach, L. G., Nguyen Tran, D.-H., Cao, V. D., Nguyen, T. N. Q., Le, T. T. H., Tran, T. T., & Thi, T. T. H. (2019). Partial Surface Modification of Low Generation Polyamidoamine Dendrimers: Gaining Insight into their Potential for Improved Carboplatin Delivery. Biomolecules, 9(6), 214. https://doi.org/10.3390/biom9060214