Abstract
This paper aimed to evaluate the effect of herbal mixture (Mentha spicata, Zingiber officinale, Cinnamomum zeylanicum, and Citrus sinensis) only and along with clomiphene citrate (CC) compared to CC on serum antioxidants, glycemic status, menstrual regulation, and rate of pregnancy. This single-blind randomized clinical trial was carried out on 60 infertile participants with polycystic ovary syndrome (PCOS) willing to be pregnant. They were randomly allocated into group 1 (n = 20) who received routine dose of CC pills (50–150 mg) for three menstrual cycles from the fifth day of menstruation for five days; group 2 (n = 20) who consumed herbal mixture daily (700 mg); and group 3 (n = 20) who used up herbal mixture along with CC for 3 months. Catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), malondialdehyde (MDA), fasting blood sugar (FBS), insulin, and homeostatic model assessment for insulin resistance (HOMA-IR) were measured in their blood samples. No statistically significant differences were observed between the three groups in terms of socio-demographic characteristics. After intervention, however, the levels of CAT in group 2 (adjusted mean difference (aMD): = 9.0; confidence interval (CI) 95% = 1.1–16.9) and group 3 (aMD = 12.2; CI 95% = 4.5–19.9), GPx in group 2 (aMD = 986.1; CI 95% = 141.1–1831.1) and group 3 (aMD = 1781.2; CI 95% = 960.7–2601.8), and SOD in group 2 (aMD = 55.1; CI 95% = 26.0–84.2) increased. While FBS in group 3 (aMD = −8.7; CI 95% = −14.7 to −2.7), insulin in group 2 (aMD = −5.6; CI 95% = −10.8 to −0.4), and HOMA-IR in group 2 (aMD = −1.3; CI 95% = −2.4 to −0.2) significantly decreased compared to the group 1. To summarize, herbal mixture supplements along with CC have beneficial effects on serum antioxidant levels, as well as glycemic biomarkers of infertile PCOS, menstrual regulation, and pregnancy rate.
1. Introduction
Polycystic ovary syndrome (PCOS) is recognized as a leading cause of infertility with the incidence of 6–26% among women at child bearing age [1]. Clinical manifestations of PCOS as a heterogeneous endocrine disorder are anovulation, hyperandrogenism, amenorrhea or oligomenorrhea, hirsutism, acne, obesity, and dyslipidemia [2]. Polycystic ovary syndrome has been associated with oxidative stress (OS) and metabolic factors, for instance insulin resistance, obesity, and diabetes [3,4]. Oxidative stress can affect female fertility by influencing ovulation, fertilization, embryo development, and implantation [5]. Although reactive oxygen species (ROS) and reactive nitrogen species (RNS) as examples of OS play physiological roles in cellular signaling pathways at low concentrations, they may damage cellular functions in excess levels [6]. Reactive oxygen species are derived from molecular oxygen, and include oxygen ions, free radicals (chemical species with unpaired electrons), and peroxides [3]. Oxidative stress is dramatically increased in PCOS patients, when oxidant/antioxidant status is measured by circulating serum markers, including catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) [3]. However, OS is also correlated with insulin resistance by impairing glucose uptake in muscle and adipose tissue [7]. In vivo and in vitro studies have demonstrated that an excess level of OS results in impaired insulin secretion and insulin resistance [8]. However, antioxidant treatment may ameliorate insulin sensitivity in insulin resistant patients [9]. But whether high levels of OS in PCOS patients derive from PCOS or other potential complications still remains undetermined [10].
Of the most prevalent medications to treat PCOS is clomiphene citrate (CC), long-term consumption of which causes endometrial thickness [11]. Since CC has a structural similarity to estrogen compounds, it binds to estrogen receptors and suppresses the endometrial E2 which plays an important role in endometrial growth and maturation [12]. Therefore, use of an herbal agent with antioxidant and polyphenolic properties without significant side effects helps to treat PCOS as a disease of antioxidant deficiency [13].
Cinnamomum zeylanicum nees (Cinnamon) from the Lauraceae family has been known for its antioxidant and anti-inflammatory properties [14]. Cinnamon extract can be used as an antioxidant due to its phenolic content particularly cinnamaldehyde, which improves level of SOD, GPx, CAT, and reduces MDA concentration, as well as increase rate of pregnancy [15,16]. Furthermore, it decreases insulin and blood glucose markedly [17]. Citrus Sinensis (L.) Osbeck from Rutaceae family mainly contains hesperidin, polymethoxylated flavonoids (PMF), and terpenoids (limonene and linalool), and these phenolic bioactive compounds indicate considerable cytoprotective effects against OS [18]. Moreover, hesperidin in C. sinensis increases the levels of antioxidants including SOD and CAT, and decreases the MDA level [19].
Major components of Zingiber officinale Roscoe (ginger) from Zingiberaceae family are zingiberene, camphene, and p-cineole, which show antioxidant, anti-cancer, anti-clotting, and anti-inflammatory properties [20,21]. Moreover, it significantly reduces serum levels of fasting blood sugar (FBS) and insulin [22]. In vitro and in vivo studies have confirmed that ginger enhances the levels of SOD, CAT, and GPX, as well as increasing the antioxidant capacity in blood [23]. Mentha spicata (spearmint) from Lamiaceae family, widely spread in the temperate zone, has anti-inflammatory, anti-diabetic, and anticancer features [24]. Some studies have confirmed spearmint contains different volatile compounds such as p-Cymene, isopiperitone, menthone, and β-linalool, and various phenolic phytoconstituents are not only considered an antioxidant source, but they also reduce glucose and OS levels [25,26].
Although aforesaid studies have illustrated the potency of each herb, there are hypotheses that the mixture of these herbs may have more potent antioxidant efficacy. However, to the best of our knowledge, no study has subjected its effect on PCOS. Therefore, the aims of this study were to compare the efficacy of four herbal mixtures with CC on serum antioxidants (CAT, GPX, SOD, MDA) and glycemic biomarkers (insulin, insulin resistance, and FBS) as primary outcomes in PCOS patients, and to determine total phenolic content (TPC), total flavonoid content (TFC), free radical scavenging activity, ferric reducing antioxidant potential (FRAP), and phytochemical analysis of herbal mixture as secondary outcome.
2. Materials and Methods
2.1. Medicinal Plant and Capsule Preparation
The dried plant samples consisting of the leaves of M. spicata, rhizomes of Z. officinale, bark of C. zeylanicum, and peels of C. sinensis were provided from the Herbal Medicine Market and identified at the Department of Pharmacognosy, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran. The different plant samples were thoroughly powdered and sieved. The powders were mixed with 5 (250 mg): 4 (200 mg): 3 (150 mg): 2 (100 mg) weight ratios of spearmint, ginger, cinnamon, and C. sinensis, respectively. Finally, obtained powder was used for preparation of 700 mg capsules.
2.2. Standardization of the Herbal Mixture
2.2.1. Preparation of Methanolic Extract
The methanolic extract was obtained from 72 g of herbal mixture powder using the maceration method with 700 mL methanol (MeOH) during three consecutive days. The obtained extract was filtered and dried using a rotary evaporator at 45 °C. The yielded methanolic extract was 12.04 g.
2.2.2. Free Radical Scavenging Assay
Free radical scavenging activity of methanolic extract was measured using DPPH (2, 2-diphenyl-1-picrylhydrazyl) method. The extract was dissolved in methanol to prepare a stock solution with the concentration of 10 mg/10 mL. Serial dilutions (0.5, 0.25, 0.125, 0.625, 0.313, and 0.156 mg/mL) were made to reduce the concentrations. Two mL of diluted solution was mixed with 2 mL of 0.08 mg/mL DPPH solution and was allowed to stay for 30 min for any reaction. The ultraviolet (UV)-visible absorbance was set at 517 nm by Thermo Fisher spectrophotometer (Walthman, MA, USA). The reduction percentage was recorded against the extract concentration to compute RC50 values (the concentration of extract that provides 50% loss of DPPH activity). Quercetin was used as the positive control and the experiment was performed in triplicate [27].
2.2.3. Ferric Reducing Antioxidant Potential
Total antioxidant activity was determined by the ferric reducing antioxidant potential (FRAP) following the method of Benzie and Strain with some modifications. This method is based on the reduction of Fe (III)-TPTZ (ferric 2,4,6-tripyridyl-s triazine complex) by antioxidants to Fe (II)-TPTZ (ferrous form). Light blue reagent clarifies the presence of Fe (III)–TPTZ, and changing it to dark blue after interaction with antioxidants illustrates the presence of Fe (II)–TPTZ in the reagent. To this end, FRAP stock solution was prepared freshly by mixing TPTZ (10 mM), HCl (40 mM), and FeCl3 (20 mM) in an acetate buffer (300 mM, pH 3.6). Next, 900 µL of FRAP reagent was allowed to react with 30 µL of plant extract for 30 min in a test tube. The tube was vortexed and placed in bain-marie to reach 37 °C. The standard calibration curve was obtained by using different concentrations of FeSO4 as the standard for calculation of the FRAP values for both the quercetin and herbal mixture. Afterwards, absorbance at 595 nm wavelength was monitored against control solution [28]. Finally, the result was expressed in µM Fe (II)/g dry mass and compared with that of quercetin as the standard antioxidant.
2.2.4. Total Phenolic Content
Total amount of the phenolic components was determined by Folin-Ciocalteau method. One mL of methanolic extract (5 mg/mL in acetone-water solution) was mixed with 200 µL of Folin-Ciocalteau reagent and 1 mL of 2% Na2CO3, and the new solution was incubated for 30 min at room temperature. Then the absorbance was determined at 750 nm using Thermo Fisher spectrophotometer. Various concentrations of gallic acid were utilized as the standard, and control samples did not contain any extract. All evaluations were performed in triplicate.
2.2.5. Total Flavonoid Content
Total flavonoids were determined using the AlCl3 method. Eighty percent methanol was used as the test solution. With this end in view, 133 mg crystalline AlCl3 and 400 mg crystalline NaCOOCH3 were dissolved in 100 mL of 80% methanol and used as an AlCl3 reagent. To estimate the flavonoid content of extract, 2 mL of extract solution, 400 µL of water, and 1 mL of AlCl3 reagent were mixed and the absorbance was set at 430 nm using Thermo Fisher spectrophotometer. The blank solution containing no AlCl3 reagent and different concentrations of quercetin were used as the standard. The number of flavonoids was estimated based on the calibration curve of quercetin. All measurements were performed in triplicate.
2.2.6. Essential Oil Extraction
The herbal mixture powder (120 g) was exposed to hydrodistillation using a Clevenger type apparatus for about 4 h. Then, the obtained dark yellow oil was dried over anhydrous sodium sulfate, measured, and stored in a dark glass at 4 °C for further analyses. The essential oil (0.5% w/w) was assessed on the dry weight basis.
2.2.7. Gas Chromatography–Mass Spectrometry and Gas Chromatography–Flame Ionization Detector Analyses
Gas chromatography–mass spectrometry (GC–MS) and gas chromatography with flame ionization detector (GC–FID) analyses were performed on a Shimadzu GC-MSQP-5050A and GC-17A equipped with a DB-1 fused silica column (60 m × 0.25 mm i.d., 0.25 µm film thickness), with an oven temperature of 50 °C rising to 260 °C at a rate of 3 °C/min. The total running time for a sample was about 82 min. Helium was used as the carrier gas at a flow rate of 1.3 mL/min. The essential oil was diluted 1:100 in n-hexane and 1 µL was injected into the column. Split ratio, ionization energy, scan time, and acquisition mass range were 1:33, 70 eV, 1 s, and 30–600 amu, respectively.
2.2.8. Identification of Components
Identification of the essential oil components was based on the comparison of the standard alkanes (C8–C20) from Sigma-Aldrich (St. Louis, MO, USA) with the retention times and mass spectral data, and computer matching with the NIST 21, NIST 107, and WILEY 229 library by comparing the fragmentation patterns of the mass spectra with those reported in the library [29].
2.3. Clinical Trial Study
This single-blind, parallel randomized clinical trial was authorized by the Ethics Committee of Tabriz University of Medical Sciences (code: TBZMED.REC.1394.576) on 26 November 2015, and was registered in the Iranian Registry of Clinical Trials (IRCT201509295563N7) on 9 January 2016. A total of 60 women with PCOS aged 18–35 years old with primary and secondary infertility, a body mass index (BMI) between 26.5 and 28.5 kg/m2, and willing to be pregnant were included in the study. The participants were from the Infertility Clinic, Alzahra Hospital, Tabriz, Iran, and contributed to the study for approximately nine months (from 16 January 2016 to 25 October 2016). The diagnosis of PCOS was based on Rotterdam criteria (2003), satisfying at least two out of three of the following criteria: oligomenorrhea /or amenorrhea, clinical /or biochemical sign of hyperandrogenism, and presence of PCOS by ultrasonography [30]. The criteria for inclusion were PCOS women diagnosed with primary or secondary infertility, aged between 18 and 35 years, and having a BMI < 30. The exclusion criteria included the patients with diabetes mellitus, the use of medications such as those helping ovulation or insulin sensitizers, thyroid disorders, cholesterol-lowering drugs, smoking, current treatment of infertility, hypertension, cardiovascular diseases, Cushing syndrome, and allergy to spearmint, ginger, cinnamon, and C. sinensis.
The sample size based on:
95% confidence interval, 80% power, , and α = 0.05 was calculated 20 individuals per group. Final sample was estimated 25 women, considering 25% probable drop-out for each group.
All participants were informed about the purpose of the study, and an informed consent was obtained from each of them. They were also asked to keep their daily intake of food during three months of study. Compliance of the subjects was followed up via phone consultation every week. The subjects were randomly allocated into the three groups: group 1 (n = 20) received routine dose of CC pills (50–150 mg) for three menstrual cycles from the fifth day of menstruation for five days; group 2 (n = 20) consumed 700 mg herbal mixture capsule daily; and group 3 used up 700 mg herbal mixture capsule along with CC for three months.
2.3.1. Randomization
The participants were divided into three groups by random allocation software (RAS/ version 1.0.0, M Saghaei, Isfahan, Iran) [31] through randomized blocks of three and six with an allocation ratio of 1:1:1 by a person who was not involved in the study. For allocation concealment, according to sequence generation opaque and sealed envelopes numbered from 1 to 75; each contain a letter designating the allocation. The first envelope was dedicated to first participant and this process was followed to the end of the research. Only the statistician was blind to the study.
2.3.2. Collection of Serum Samples
Ten mL of blood samples was collected twice from antecubital veins in the morning after an overnight fasting, on the second day of the women’s menstrual cycle; first, as the pre-intervention, and second, as the post intervention (three months later). The blood samples were centrifuged for 10 min at 4000 rpm to separate the serum. The extracted serum was divided into four aliquots and kept frozen at −70 °C until assay.
2.3.3. Measurement of Biomarkers of Oxidative Stress
Serum MDA levels were measured by thiobarbituric acid (TBA) test with ±0.01 µMol/L sensitivity. Concentration of plasma MDA was determined with a spectrophotometric detector at 532 nm. A 1,1′,3,3′-tetramethoxypropane was used to construct the calibration curve as the standard [32] (reference value: 0.54–1.32 pg/mL). In addition, serum SOD levels were measured by colorimetric method using an ELISA kit (RANDOX, Antrim, North Ireland UK) according to the manufacturers’ instruction. Sensitivity of the assay was ± 0.01 IU/mL (Cat. No. (SD) = 124; reference value: 164–240 IU/mL). Moreover, serum GPx levels were measured by UV method using the ELISA kit (RANDOX). Sensitivity of this assay was ± 1.15 IU/mL (Cat. No. RS 2318; reference value: 4171–10881 IU/mL). Furthermore, serum CAT levels were measured by immunoturbimetric assay using an ELISA commercial kit according to the manufacturers’ protocol (CUSABIO Kit, WUHAN HUAMEI BIOTECH Co., Ltd. Wuhan, China). Sensitivity of the assays was ± 3.9 pg/mL (Cat. No. CSB-E13635h; reference value: 19.8–66.4 pg/mL).
2.3.4. Measurement of Glycemic Biomarkers
Insulin concentration was determined through a fully automated chemiluminescence assay (LIAISON C-Peptide, Byk-Sangtec; reference value: 3.21–16.32 µIU/mL) [33]. Blood glucose level (FBS) was measured by enzymatic methods using commercial kits (Pars Azemun, Isfahan, Iran) and the auto-analyzer system (Selectra E, Vitalab, Netherlands; reference value: 70–115 mg/dL). Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated according to formula. It is noteworthy to mention that the 75th percentiles of HOMA-IR in the whole population, in normal-weight, and in obese people are 3.027, 1.68, and 3.42, respectively.
2.3.5. Ultrasonography
Volume of ovary, numbers as well as size of follicles were probed by vaginal ultrasound (5 MHz Ulramark 4 Plus; Advanced Technology Laboratories, Bothell, WA, USA).
2.3.6. Checklist of Side Effects
The participants were also asked to complete a checklist encompassing the side effects of medications during the intervention.
2.4. Statistical Analysis
Normality of all quantitative variables for each of the groups was confirmed using the Kolmogorov-Smirnov test. Descriptive statistics, including the frequency and percentage, and measures of central tendency and dispersion, including the mean and standard deviation (SD), were also used to describe the study variable. Moreover, the results were analyzed using one-way analysis of variance (ANOVA) with a post-hoc Tukey test for baseline quantitative variables. The paired t-test was also applied for comparison of quantitative data before and after the trial within the 3 groups, and analysis of covariance (ANCOVA) was used for between-group analysis after intervention adjusted for baseline values. p-Value ≤ 0.05 was considered statistically significant. The data were analyzed by SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Preparation and Analysis of the Herbal Mixture
In this study, the total methanolic extract was obtained from the herbal mixture powder which was 12.04 g. Then, the levels of DPPH, FRAP, TPC, and TFC were measured from this methanolic extract.
Herbal mixture showed potent antioxidant activity (RC50: 0.018 ± 0.0007 mg/mL) in comparison to quercetin (RC50: 0.004 ± 0.0001 mg/mL) as the positive control; this activity could be attributed to the presence of phenolic structures in the extract. The methanolic extract of herbal mixture had the ability to decrease TPRZ-Fe (III) to TPTZ-Fe (II), and FRAP values for methanolic extract of herbal mixture was 720 ± 35 µmol Fe (II)/g, which was lower than that of quercetin (2880 ± 41 µmol Fe (II)/g) as the standard antioxidant. In addition, the assessment of TPC and TFC contents proved the presence of 24.062 ± 0.2 mg gallic acid/100 mg and 8.93 ± 0.09 mg quercetin/100 mg in herbal mixture (Table 1).

Table 1.
DPPH, FRAP, TPC, and TFC of methanolic extract of herbal mixture.
The amount of extracted essential oil was 0.5% w/w, which was assessed on the dry weight basis. The GC–MS and GC–FID analysis of the herbal powder essential oil indicated that the phytochemicals of the essential oil, including sesquiterpenes and derivatives (55.49%), aldehydes (19.38%), and monoterpenes (15.45%) were the main groups of oil components. Moreover, it was shown that zingiberene (13.58%), α-curcumene (10.77%), β-sesquiphellandrene (9.86%), α-farnesene (5.30%), and β-bisabolene (5.93%), isolated from ginger with sesquiterpenes hydrocarbons, were the most abundant components in essential oil of herbal mixture powder. In the same way, cinnamic aldehyde (13.06%) was separated from cinnamon with the organic compounds of aldehyde, and pulegone (5.61%) was extracted from spearmint with monoterpene structure. No substance was extracted that could be related to C. sinensis, which might be due to its lowest dosage (Table 2, Figure 1).

Table 2.
Volatile compounds identified in the essential oil of herbal mixture.
Figure 1.
Gas chomatography–mass chromatogram (GC–MS) of the essential oil of herbal mixture. TIC*1.00: total ion current (1.0 std).
Based on our results, the antioxidant activity increased proportionally to the levels of polyphenol component, and a tremendous effect was seen on the treatment of PCOS regarding antioxidant activity. Therefore, in standardization of herbal mixture, different proportions of each herbal powder were mixed, and phenolic and flavonoid contents were measured. Moreover, the highest levels of phenolic and flavonoid contents were obtained in the weight ratios of 5 (250 mg) spearmint): 4 (200 mg) ginger: 3 (150 mg) cinnamon: 2 (100 mg) C. sinensis in comparison with other ratios.
3.2. Clinical Trial Data
In the present study, the initial sample consisted of 90 women with PCOS, from whom 11 were excluded because they did not meet the Rotterdam criteria, and 4 women’s husbands suffered from azoospermia. Then, 75 participants were randomly allocated into 3 groups including group 1: CC (n = 25); group 2: herbal mixture (n = 25); and group 3: CC with herbal mixture (n = 25). Twenty-two women out of 25 in group 1, 23 out of 25 in group 2, and 24 out of 25 in group 3 received allocated interventions. However, 3, 2, and 1 of them in groups 1, 2, and 3, respectively, did not feel comfortable enough to participate. During the follow-up stage, 2 participants in group 1 were lost to be followed up with because of consuming other medication along with treatment, 3 of them in group 2 discontinued intervention on account of deciding to get treated with intrauterine insemination (IUI) or in vitro fertilization (IVF), and 2 of them in group 3 did not take an initial blood test, while 2 of them wanted to be treated with IUI or IVF. Finally, 60 participants (n = 20 in each group) finalized the intervention and their blood samples were analyzed at the end of three months (Figure 2).
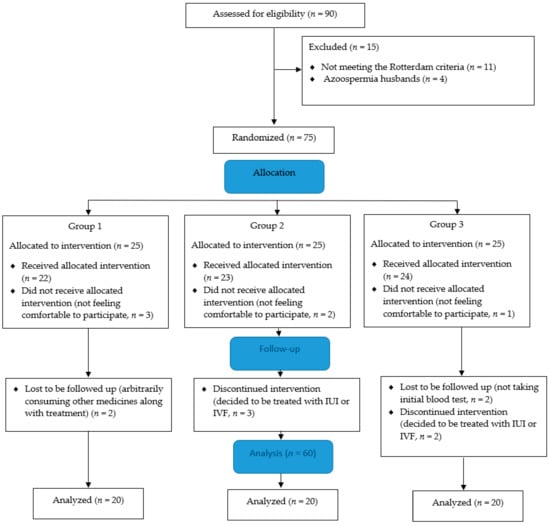
Figure 2.
Polycystic ovary syndrome (PCOS) patients’ flow diagram. Group 1: Clomiphene citrate (CC), group 2: Herbal mixture, and group 3: CC along with herbal mixture. IUI: intrauterine insemination, IVF: in vitro fertilization.
Before intervention, there were no significant differences between groups in terms of socio-demographic characteristics. The mean (SD) age was 25.7(4.1) years; the mean (SD) marital age was 20.2 (3.5) years, and the mean (SD) infertility history was 3.9 (3.0) years. Likewise, the mean (SD) weight was 73.8 (11.4) kg, and the mean (SD) BMI was 27.5 (3.8) kg/m2. Furthermore, the numbers of women with primary and secondary infertility were 53 (83.3%) and 7 (16.7%), respectively. None of the participants were smokers (Table 3).

Table 3.
General characteristics of the women with PCOS at baseline and after intervention.
Before intervention, there were no significant differences between groups regarding the serum levels of CAT (p = 0.725), GPx (p = 0.751), SOD (p = 0.793), and MDA (p = 0.835). After intervention, however, with adjusting baseline values, significant differences were observed between groups in terms of CAT (p = 0.001), GPx (p < 0.001), and SOD (p < 0.001) levels except for MDA (p = 0.420). In a binary comparison, the CAT levels in group 2 (aMD = 9.0; CI 95% = 1.1–16.9, p = 0.021) and group 3 (aMD = 12.2; CI 95% = 4.5–19.9, p < 0.001), the GPx levels in group 2 (aMD = 986.1; CI 95% = 141.1–1831.1, p = 0.017) and group 3 (aMD = 1781.2; CI 95% = 960.7–2601.8, p < 0.001), and the SOD levels in group 2 (aMD = 55.1; CI 95% = 26.0–84.2, p < 0.001) and group 3 (aMD = 55.9; CI 95% = 27.5–84.2, p < 0.001) significantly increased in comparison with group 1. However, the binary comparison displayed no significant decrease in the MDA levels in group 2 (aMD = −0.1; CI 95% = −0.4 to 0.1, p = 0.502) and group 3 (aMD = −0.1; CI 95% = −0.4 to 0.1, p = 0.725). In addition, the binary comparison indicated no significant difference in the SOD, GPx, CAT, and MDA levels between groups 2 and 3 (p > 0.05).
Yet, in within-group analysis, a significant increase was observed after intervention compared to baseline considering the serum levels of CAT in group 2 (mean difference (MD) = 8.6; CI 95% = 1.9–15.2, p = 0.014) and group 3 (MD = 12.8; CI 95% = 6.5–19.1, p < 0.001), GPx in group 2 (MD = 1063.3; CI 95% = 509.7–1616.9, p < 0.001) and group 3 (MD = 1682.3; CI 95% = 794.3–2570.3, p < 0.001), and SOD in group 2 (MD = 49.2; CI 95% = 29.1–69.2, p < 0.001) and group 3 (MD = 54.1; CI 95% = 27.4–80.8, p < 0.001). Meanwhile, serum levels of MDA in group 2 (MD = –0.4; CI 95% = −0.6 to −0.2, p < 0.001) and group 3 (MD = −0.3; CI 95% = −0.5 to −0.1, p = 0.004) significantly decreased. Moreover, no significant difference was observed in group 1 in terms of CAT, SOD, and MDA levels (p > 0.05) except for GPx level (MD= −52.3; CI 95% = −100.8 to –3.7, p = 0.036), which reduced remarkably (Table 4).

Table 4.
Comparison of mean ± SD serum levels of CAT, GPx, SOD, and MDA in participants receiving CC, herbal mixture, and CC along with herbal mixture.
Before intervention, there were no significant differences between groups in terms of serum levels of insulin (p = 0.135) and HOMA-IR (p = 0.188) except for FBS (p = 0.003). After intervention, however, with adjusting baseline values, significant differences were observed in terms of FBS (p = 0.003), insulin (p = 0.029), and HOMA-IR (p = 0.017) levels. In the binary comparison, a significant decrease was seen in serum levels of FBS (aMD = −8.7; CI 95%= −14.7 to 2.7, p = 0.002) in group 3, insulin (aMD = −5.6; CI 95% = −10.8 to −0.4, p = 0.029) in group 2, and HOMA-IR (aMD = −1.3; CI 95% = −2.4 to −0.2, p = 0.013) in group 2 in comparison with group 1. Moreover, the binary comparison indicated no significant difference in the FBS level (p = 0.212) in group 2, the insulin level (p = 0.842) in group 3, and the HOMA-IR level (p = 0.403) in group 3 compared to group 1. Furthermore, based on our results from the binary comparison between groups 2 and 3 (p > 0.05), there was no significant difference in the FBS, insulin, and HOMA-IR levels.
Additionally, in within-group analysis, no significant decrease was detected after intervention compared to baseline regarding the serum levels of FBS (MD = −6.4; CI 95%= −10.5 to −2.3, p = 0.004) in group 3, insulin (MD = −4.3; CI 95% = −7.7 to −2.2, p < 0.001) in group 2, and HOMA-IR (MD = −1.0; CI 95% = −1.4 to −0.4, p = 0.002) in group 2; yet, in the within-group comparison, there were no significant differences in the FBS levels in groups 1 and 2, the insulin levels in groups 1 and 3, and the HOMA-IR levels in groups 1 and 3 (p > 0.05, Table 5).

Table 5.
Comparison of mean ± SD serum levels of FBS, insulin, and HOMA-IR in participants receiving CC, herbal mixture, CC along with herbal mixture.
Ultrasonography for PCOS women on the second day of menstrual cycle after three-month treatment demonstrated no significant variations in the number, size of basal antral follicle count (AFC), and volume of ovary in all three groups that were 10–12, 2–12 mm, and 10 cm3, respectively. In addition, sonography in the mid-cycle of the third month showed that in group 1, the antral follicles of 14 out of 20 patients reached the ovulation stage and 4 of them became pregnant (20%). While in group 2, 11 PCOS women out of 20 showed dominant follicles, 2 of which experienced the pregnancy (10%). In group 3, 17 out of 20 PCOS participants had dominant follicles (18–20 mm), which resulted in 5 pregnancies (25%; one of them was pregnant with twins).
Our results also showed that after intervention, in groups 1, 2, and 3, 35%, 22.2%, and 35% had oligomenorrhea versus 65% (p = 0.014), 55.6% (p = 0.014), and 100% (p < 0.001) at the baseline. Furthermore, after intervention, 25% of women in group 1 had amenorrhea versus 45% at the baseline (p = 0.46), and 25% in group 3 had amenorrhea versus 65% at the baseline (p = 0.005). However, after intervention, in group 2, 16.7% had amenorrhea versus 33.3% at the baseline; though the difference between the groups was not significant (p = 0.083). Meanwhile, no side effects were reported in all three study groups during the 12 weeks of intervention.
4. Discussion
According to our results presented in Table 1, herbal mixture showed potent antioxidant activity, as well as total phenol and flavonoid content. Based on previous studies, the antioxidant activity of extracts could mainly be attributed to the polyphenolic compounds [34]. The FRAP assay is widely used to determine the antioxidant compounds in dietary polyphenols [35]. In fact, polyphenols determine the antioxidant activity, and this positive relationship renders a trend in many medicinal plants [36]. It has been proven that cinnamon contains a great variety of flavonoids and polyphenols with free-radical-scavenging properties and antioxidant activities [37]. Ginger with a wide range of antioxidants and polyphenol compounds such as β-carotene, ascorbic acid, terpenoids, alkaloids, and polyphenols like flavonoids, flavone glycosides, and rutin is regarded the greatest source of phytochemical antioxidants [38]. Phenolic phytochemicals of spearmint significantly enhance the antioxidant defense [39]. Peel of C. sinensis is also very rich in phenolic compounds, including flavonoids and phenolic acids, which can be consumed as natural antioxidants [40].
The GC–MS and GC–FID analyses in Table 2 showed the presence of zingiberene (13.58%), α-curcumene (10.77%), β-sesquiphellandrene (9.86%), α-farnesene (5.30%), and β-bisabolene (5.93%) with sesquiterpene hydrocarbons in ginger; cinnamic aldehyde (13.06%) with the organic compounds of aldehyde in cinnamon, and finally, pulegone (5.61%) with monoterpenes extracted from spearmint. A review of the literature indicated that sesquiterpene hydrocarbons and monoterpenes could be effective in various biological activities such as antioxidant effects [41,42]. Zhan et al. extracted α-zingiberene (22.29%), β-sesquiphellandrene (8.58%), α-farnesene (3.93%), β-bisabolene (3.87%), α-curcumene (2.63%) with sesquiterpene hydrocarbons from ginger volatile oil, which had antioxidant potency [43]. In addition, it was shown in another study that the antioxidant effects of essential oils of cinnamon barks and ginger rhizomes could be ascribed to the presence of antioxidant constituents such as cinnamaldehyde, sesquiphellandrene, and zingiberene [44]. Using the GC–MS analysis, Telci et al. determined the main composition of spearmint as oxygenated monoterpenes including pulegone (26.71–29.56%) and piperitone (22.17–28.16%) as the major terpenoid group [45]. Significant difference in the percentage of oil compositions in comparison to that of our study might depend on variation in environmental factors as well as difference in ecologies.
Regarding Table 4, our results clearly showed that after a 12-week intervention, levels of CAT, GPx, and SOD significantly increased in groups 2 and 3 compared to group 1. Although a significant decrease in case of MDA was seen in groups 2 and 3, there was no significant difference in comparison with group 1. Moreover, according to Table 5, levels of FBS in group 3, and insulin and HOMA-IR in group 2 significantly decreased compared to group 1.
Many studies have indicated low serum antioxidant levels and insulin resistance in PCOS women [46,47,48]. In fact, hyperglycemia can enhance OS through numerous pathways. However, through a major non-enzymatic mechanism, it induces the intracellular ROS, generates electrochemical proton gradient produced by mitochondrial electron transport chain (mETC), and results in enhanced derivation of superoxide [49]. In addition, reactive species by impairing glucose uptake in muscle and fat [50], and also by reducing insulin secretion from pancreatic β cells play a crucial role in insulin resistance [7]. On the other hand, an imbalance between oxidant-antioxidant is responsible for ovarian disturbance, insulin resistance, and chronic inflammation that is associated with pancreatic β cell dysfunction in women with PCOS [10]. Although ROS and RNS at low concentrations play physiological roles in cellular signaling pathways, in excess levels they may damage cellular functions [6]. Reactive oxygen species react with lipids, causing them to boost peroxidation products such as MDA [47].
Cinnamon also reduces insulin resistance by enhancing phosphatidylinositol 3-kinase activity in the insulin signaling pathway [51]. In several studies, the authors proved that cinnamon contained proanthocyanidins and phenolic compounds with antioxidant activities that not only neutralized free radicals, but also reduced blood glucose and insulin [52,53]. Moselhy et al. indicated a strong hepatoprotective effect of cinnamon ethanolic extract against carbon tetrachloride (CCl4)-induced oxidative stress by increasing the SOD and CAT levels and decreasing the MDA level, the possible mechanism of which could be attributed to the free radical scavenging activity of polyphenol compounds [54]. In a study examining the effect of ginger and onion (Allium cepa) on the levels of sexual hormones and OS, the reduction of MDA serum level was significant in the within-ginger group, but was not significant in comparison to the control group; this result was in line with ours [55]. A use of the combination of ginger and cinnamon in diabetic male rats in the study of Khaki et al. particularly illustrated an increase in the SOD, CAT, and GPx levels, while the MDA level more significantly decreased compared to other groups using only one herb [17]. In an animal study, Al-Amin et al. demonstrated that ginger could decrease the FBS level by the mechanism of serotonin receptors which activate pancreatic β cells to release insulin [56]. Additionally, Bayani et al. showed that phenolic phytochemicals of spearmint possessed hypoglycemic, and antioxidant attributes [57]. Deep et al. also revealed that methanolic extract of spearmint was a more potent antioxidant than ascorbic acid, which is recognized as the standard antioxidant. Furthermore, the levels of SOD, GPx, and CAT were duplicated, while MDA level fell by half [58]. In other research, Selmi et al. approved that hesperidin as one of the main flavonoids of C. sinensis reversed lipoperoxidation and hydrogen peroxide production, reduced MDA level, and increased antioxidant status (CAT, GPX, SOD levels) drastically [59].
Volatile oil from C. sinensis peel contains naringin and naringenin as two kinds of flavonoids which have anti-diabetic and antioxidant properties [60]. Polymethoxylated flavones (PMFs) of C. sinensis have hypolipidemic effects, resulting in a significant reduction of insulin tolerance and glucose levels [61]. Furthermore, according to other research, a mixture of onion, ginger, basil, cinnamon, orange peel, yellow and red watermelon seeds, and carrot seed could significantly affect the CAT level and reduce the OS [62]. All of this research is consistent with our study.
In this study, regular menstrual cycle, ovulation, and pregnancy occurred in all three groups. The main cause of this syndrome is the reduction in the sensitivity of pre-antral follicles to follicle stimulating hormone (FSH), and enhancement of follicular activity to luteinizing hormone (LH) leading to the inhibition of follicle maturation [63]. Similarly, excessive secretion of LH and gonadotropin-releasing hormone (GnRH) stimulate ovarian theca cells to produce androgens [64]. As a consequence, hyperandrogenism not only arrests antral follicle growth, but also stimulates apoptosis of its granulose cells which convert androgen to estradiol employing aromatase enzyme [65].
The study of Khodaeifar et al. certified that cinnamon could improve ovulatory menstrual cycle by enhancing progesterone levels in the luteal phase of PCOS women [66]. Similarly, Ataabadi et al. demonstrated that spearmint, through reducing body weight and level of testosterone and having antioxidant properties, matured follicles, and induced ovulation, led to higher number of Graafian follicles and corpus lutea and lower number of ovarian cysts and atretic follicles [24]. Ginger not only stimulated blood circulation for the treatment of inflammation and menstrual irregularities, but also enhanced AFC and ovarian reserve function [67]. Clomiphene citrate stimulated ovulation and increased fertility rate via stimulating the secretion of gonadotropin-releasing hormone as well as anti-estrogenic effect [68].
The results of the present study pointed out the fact that herbal mixture could increase the serum levels of CAT, GPx, SOD, and reduce the MDA level. Likewise, CC along with herbal mixture could exert such effects. This result may contribute to the point that although CC could not alter serum level of the antioxidant, it did not interfere in the activity of herbal supplement. Although the FBS level did not reduce in the groups administered with CC and herbal mixture solely, their combination could decrease the FBS; this result might be ascribed to the synergistic effects of their combination. Further studies are needed to prove these findings.
Limitations of the Study
One of the major strengths of this study was not only the analysis of essential pharmacophores, TPC, TFC, DPPH, and FRAP in a herbal mixture, but also the evaluation of its efficacy on serum antioxidant levels and glycemic biomarkers in infertile PCOS women. Furthermore, in previous studies, the effects of all aforementioned herbs were investigated separately, while we evaluated the effects of CC and herbal mixture as a new combination, which might have synergistic effects. Nonetheless, the relatively small sample size, short-term follow-up, single-blind design, and using a fixed dose of herbal mixture in the clinical study were the limitations of this study.
We recommend that further research should be undertaken regarding the effects of herbal mixtures on hormonal factors of PCOS women such as sexual hormones in both proliferative and secretory phases, and lipid profile in a longer follow-up.
5. Conclusions
In summary, the results indicated that phenolics and phyto-antioxidant constituents of the herbal mixture including zingiberene, α-curcumene, β-sesquiphellandrene, α-farnesene, β-bisabolene, cinnamic aldehyde, and pulegone could effectively improve antioxidant potential, and the levels of FBS, HOMA-IR, and insulin in the serum of PCOS patients. It can be concluded that consumption of a herbal mixture as a supplement alongside CC can improve the antioxidant activity, glycemic status, and pregnancy rate in PCOS patients. Hence, it could be considered as a beneficial supplementary medicament for patients suffering from PCOS.
Author Contributions
Conceptualization, N.A. and E.O.S.M.; Methodology, A.K. and E.O.S.M.; Statistical analysis, A.F.-K.; Managed patient recruitment and sample collection, N.A.; Laboratory analysis, N.A. and A.K.; Resources, A.K. and M.H.; Writing—Original Draft Preparation, N.A.; Writing—Review & Editing, A.K., E.O.S.M., A.F.-K. and M.H; Supervision, E.O.S.M.; Project Administration E.O.S.M; Funding Acquisition, E.O.S.M.; All authors read and approved the final manuscript.
Funding
This research was funded by “Tabriz University of Medical Sciences, Tabriz, Iran (grant number: 5.4.9505)” and “Iran National Science Foundation (INSF) and Deputy of Research and Technology of Ministry of Health and Medical Education (grant number: 96010160)”.
Acknowledgments
We would like to thank the Pharmaceutical Aras Part Medicinal Herbs International Company for the preparing herbal mixture capsules, Women’s Reproductive Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran for their infertility clinic, and Iran National Science Foundation (INSF) and Deputy of Research and Technology of Ministry of Health and Medical Education for the financial support. Our further appreciation goes to all of the investigators, coordinators, and patients who assisted or took part in this study.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Szczuko, M.; Skowronek, M.; Zapalowska-Chwyc, M.; Starczewski, A. Quantitative assessment of nutrition in patients with polycystic ovary syndrome (PCOS). Roczniki Panstwowego Zakladu Higieny 2016, 67, 419–426. [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Luque-Ramirez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Updat. 2013, 19, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, D.A. Polycystic ovary syndrome. N. Engl. J. Med. 2005, 352, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Allamaneni, S.S. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod. Biomed. Online 2004, 9, 338–347. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Arai, H.; Yamamoto, H.; Okumura, H.; Taketani, Y. Control of oxidative stress and metabolic homeostasis by the suppression of postprandial hyperglycemia. J. Med. Investig. 2005, 52, 259–265. [Google Scholar] [CrossRef]
- Gao, D.; Nong, S.; Huang, X.; Lu, Y.; Zhao, H.; Lin, Y.; Man, Y.; Wang, S.; Yang, J.; Li, J. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J. Biol. Chem. 2010, 285, 29965–29973. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef]
- Zuo, T.; Zhu, M.; Xu, W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxidative Med. Cell. Longev. 2016, 2016, 8589318. [Google Scholar] [CrossRef]
- Mitwally, M.F.; Casper, R.F. Aromatase inhibitors in ovulation induction. Semin. Reprod. Med. 2004, 22, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Gerli, S.; Gholami, H.; Manna, C.; Di Frega, A.S.; Vitiello, C.; Unfer, V. Use of ethinyl estradiol to reverse the antiestrogenic effects of clomiphene citrate in patients undergoing intrauterine insemination: A comparative, randomized study. Fertil. Steril. 2000, 73, 85–89. [Google Scholar] [CrossRef]
- Wang, J.G.; Anderson, R.A.; Graham, G.M., 3rd; Chu, M.C.; Sauer, M.V.; Guarnaccia, M.M.; Lobo, R.A. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: A pilot study. Fertil. Steril. 2007, 88, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Maurya, S.; DeLampasona, M.P.; Catalan, C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Biazar, G.; Nabi, B.N.; Sedighinejad, A.; Moghadam, A.D.; Farzi, F.; Atrkarroushan, Z.; Mirblook, F.; Mirmansouri, L. Herbal Products Use During Pregnancy in North of Iran. Int. J. Womens Health Reprod. Sci. 2019, 7, 134–137. [Google Scholar] [CrossRef]
- Khaki, A.; Bayatmakoo, R.; Nouri, M.; Khaki, A.A. Remedial Effect of Cinnamon zeylanicum on serum anti-oxidants levels in male diabetic Rat. Life Sci. J. 2013, 10, 103–107. [Google Scholar]
- Khaki, A.; Khaki, A.A.; Hajhosseini, L.; Golzar, F.S.; Ainehchi, N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Chen, Z.T.; Chu, H.L.; Chyau, C.C.; Chu, C.C.; Duh, P.D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012, 135, 2119–2127. [Google Scholar] [CrossRef]
- Khaki, A.; Fathiazad, F.; Nouri, M.; Khaki, A.F.; Ghanbari, Z.; Ghanbari, M.; Ouladsahebmadarek, E.; Javadi, L.; Farzadi, L. Anti-oxidative effects of citro flavonoids on spermatogenesis in rat. Afr. J. Pharm. Pharmacol. 2011, 5, 721–725. [Google Scholar] [CrossRef]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Makhdoomi Arzati, M.; Mohammadzadeh Honarvar, N.; Saedisomeolia, A.; Anvari, S.; Effatpanah, M.; Makhdoomi Arzati, R.; Yekaninejad, M.S.; Hashemi, R.; Djalali, M. The Effects of Ginger on Fasting Blood Sugar, Hemoglobin A1c, and Lipid Profiles in Patients with Type 2 Diabetes. Int. J. Endocrinol. Metab. 2017, 15, e57927. [Google Scholar] [CrossRef]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Preventive and Protective Properties of Zingiber officinale (Ginger) in Diabetes Mellitus, Diabetic Complications, and Associated Lipid and Other Metabolic Disorders: A Brief Review. Evid. Based Complement. Altern. Med. 2012, 2012, 516870. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ataabadi, M.; Alaee, S.; Bagheri, M.J.; Bahmanpoor, S. Role of Essential Oil of Mentha Spicata (Spearmint) in Addressing Reverse Hormonal and Folliculogenesis Disturbances in a Polycystic Ovarian Syndrome in a Rat Model. Adv. Pharm. Bull. 2017, 7, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and Volatile Composition of a Dry Spearmint (Mentha spicata L.) Extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef]
- Al-Fartosi, K.G.; Radi, H.; Al-Rekabi, E.A. Lipid Profile of Diabetic Male Rats Treated with Phenolic Compounds of Leaves Extracts from Mentha longifolia and Mentha spicata. Int. J. Pharm. Biol. Med. Sci. 2014, 3, 26–31. [Google Scholar]
- Heshmati Afshar, F.; Delazar, A.; Nazemiyeh, H.; Esnaashari, S.; Bamdad Moghadam, S. Comparison of the Total Phenol, Flavonoid Contents and Antioxidant Activity of Methanolic Extracts of Artemisia spicigera and A. splendens Growing in Iran. Pharm. Sci. 2012, 18, 165–170. [Google Scholar]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Asnaashari, S.; Afshar, F.H.; Ebrahimi, A.; Moghadam, S.B.; Delazar, A. Chemical composition and radical scavenging activity of essential oil and methanolic extract of Eremostachys azerbaijanica Rech.f. from Iran. Res. Pharm. Sci. 2016, 11, 113–119. [Google Scholar]
- Azziz, R. Diagnosis of polycystic ovarian syndrome: The Rotterdam criteria are premature. J. Clin. Endocrinol. Metab. 2006, 91, 781–785. [Google Scholar] [CrossRef]
- Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 2004, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.F.; Jacob, R.F.; Jeffers, B.; Ghadanfar, M.M.; Preston, G.M.; Buch, J.; Mason, R.P. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: A longitudinal analysis of the PREVENT study. J. Am. Coll. Cardiol. 2004, 44, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Pfutzner, A.; Lobig, M.; Fortunato, A.; Forst, T. Evaluation of a new fully automated one-step C-peptide chemiluminescence assay (LIAISON C-Peptid). Clin. Lab. 2003, 49, 227–232. [Google Scholar] [PubMed]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006, 95, 200–204. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef] [PubMed]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Al-Rekabi, E.A. Anti-oxidant and hepatoprotective activity of phenolic compounds of leaves extracts from mentha longifolia and mentha spicata in diabetic male rats. World J. Pharm. Res. 2015, 4, 346–354. [Google Scholar]
- Omoba, O.S.; Obafaye, R.O.; Salawu, S.O.; Boligon, A.A.; Athayde, M.L. HPLC-DAD Phenolic Characterization and Antioxidant Activities of Ripe and Unripe Sweet Orange Peels. Antioxidants 2015, 4, 498–512. [Google Scholar] [CrossRef]
- Wu, Q.F.; Wang, W.; Dai, X.Y.; Wang, Z.Y.; Shen, Z.H.; Ying, H.Z.; Yu, C.H. Chemical compositions and anti-influenza activities of essential oils from Mosla dianthera. J. Ethnopharmacol. 2012, 139, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhao, X.; Yin, L.; Zhang, Y.; Wang, B.; Wu, X.; Zhang, X.; Fu, X.; Sun, W. The essential oil of Artemisia capillaris protects against CCl4-induced liver injury in vivo. Rev. Bras. Farmacogn. 2016, 26, 369–374. [Google Scholar] [CrossRef]
- Zhan, K.; Wang, C.; Xu, K.; Yin, H. Analysis of volatile and non-volatile compositions in ginger oleoresin by gas chromatography-mass spectrometry. Se Pu 2008, 26, 692–696. [Google Scholar] [PubMed]
- El-Baroty, G.S.; Abd El Baky, H.H.; Farag, R.S.; Saleh, M.A. Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr. J. Biomed. Res. 2010, 4, 167–174. [Google Scholar]
- Telci, I.; Demirtas, I.; Bayram, E.; Arabaci, O.; Kacar, O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.). Ind. Crops Prod. 2010, 32, 588–592. [Google Scholar] [CrossRef]
- Al-kataan, M.A.; Ibrahim, M.A.; Al-Jammas, M.H.H.; Shareef, Y.S.; Sulaiman, M.A. Serum antioxidant vitamins changes in women with Polycystic Ovarian Syndrome. J. Bahrain Med. Soc. 2010, 22, 68–71. [Google Scholar]
- Deepika, M.L.; Nalini, S.; Maruthi, G.; Ramchander, V.; Ranjith, K.; Latha, K.P.; Rani, V.U.; Jahan, P. Analysis of oxidative stress status through MN test and serum MDA levels in PCOS women. Pak. J. Biol. Sci. 2014, 17, 574–577. [Google Scholar]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Rudich, A.; Tirosh, A.; Potashnik, R.; Hemi, R.; Kanety, H.; Bashan, N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 1998, 47, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Nagasaki, M.; Ren, M.; Bajotto, G.; Oshida, Y.; Sato, Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res. Clin. Pract. 2003, 62, 139–148. [Google Scholar] [CrossRef]
- Anderson, R.A.; Zhan, Z.; Luo, R.; Guo, X.; Guo, Q.; Zhou, J.; Kong, J.; Davis, P.A.; Stoecker, B.J. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J. Tradit. Complement. Med. 2016, 6, 332–336. [Google Scholar] [CrossRef]
- Muchuweti, M.; Kativu, E.; Mupure, C.H.; Chidewe, C.; Ndhlala, A.R.; Benhura, M.A.N. Phenolic composition and antioxidant properties of some spices. Am. J. Food Technol. 2007, 2, 414–420. [Google Scholar] [CrossRef]
- Moselhy, S.S.; Ali, H.K. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol. Res. 2009, 42, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Khaki, A.; Farnam, A.; Badie, A.D.; Nikniaz, H. Treatment Effects of Onion (Allium cepa) and Ginger (Zingiber officinale) on Sexual Behavior of Rat after Inducing an Antiepileptic Drug (lamotrigine). Balk. Med. J. 2012, 29, 236–242. [Google Scholar] [CrossRef]
- Al-Amin, Z.M.; Thomson, M.; Al-Qattan, K.K.; Peltonen-Shalaby, R.; Ali, M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006, 96, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Bayani, M.; Ahmadi-Hamedani, M.; Jebelli Javan, A. Study of Hypoglycemic, Hypocholesterolemic and Antioxidant Activities of Iranian Mentha Spicata Leaves Aqueous Extract in Diabetic Rats. Iran. J. Pharm. Res. 2017, 16, 75–82. [Google Scholar] [PubMed]
- Deep, A.; Rana, P.; Gosal, S.S.; Soni, G. Antioxidant potential of tissue cultured Mentha spicata. BioMedRx 2013, 1, 90–96. [Google Scholar]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat. Lipids Health Dis. 2017, 16, 152. [Google Scholar] [CrossRef]
- Constantin, R.P.; Constantin, R.P.; Bracht, A.; Yamamoto, N.S.; Ishii-Iwamoto, E.L.; Constantin, J. Molecular mechanisms of citrus flavanones on hepatic gluconeogenesis. Fitoterapia 2014, 92, 148–162. [Google Scholar] [CrossRef]
- Li, R.W.; Theriault, A.G.; Au, K.; Douglas, T.D.; Casaschi, A.; Kurowska, E.M.; Mukherjee, R. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sci. 2006, 79, 365–373. [Google Scholar] [CrossRef]
- Alizadeh, H.; Khaki, A.; Farzadi, L.; Nouri, M.; Ahmadi-Asrbadr, Y.; Seyed-Ghiasi, G.; Shahnazi, V. The therapeutic effects of a medicinal plant mixture in capsule form on catalase levels in the semen of men with oligospermia. Crescent J. Med. Biol. Sci. 2015, 2, 6–9. [Google Scholar]
- Dupont, J.; Scaramuzzi, R.J. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem. J. 2016, 473, 1483–1501. [Google Scholar] [CrossRef] [PubMed]
- Gilling-Smith, C.; Story, H.; Rogers, V.; Franks, S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin. Endocrinol. 1997, 47, 93–99. [Google Scholar] [CrossRef]
- Azhary, J.M.K.; Harada, M.; Takahashi, N.; Nose, E.; Kunitomi, C.; Koike, H.; Hirata, T.; Hirota, Y.; Koga, K.; Wada-Hiraike, O.; et al. Endoplasmic Reticulum Stress Activated by Androgen Enhances Apoptosis of Granulosa Cells via Induction of Death Receptor 5 in PCOS. Endocrinology 2019, 160, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Khodaeifar, F.; Fazljou, S.M.B.; Khaki, A.; Torbati, M.; Madarek, E.O.S.; Khaki, A.A.; Shokoohi, M.; Dalili, A.H. Investigating the Role of Hydroalcoholic Extract of Apium graveolens and Cinnamon zeylanicum on Metabolically Change and Ovarian Oxidative Injury in a Rat Model of Polycystic Ovary Syndrome. Int. J. Womens Health Reprod. Sci. 2019, 7, 92–98. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, Y.; Wu, X.; Wang, Y.; Fan, Q.; Wu, S. Effects of ginger-separated moxibustion at Baliao points combined with Bushen Huoxue formula on patients with decreased ovarian reserve function. Zhongguo Zhen Jiu 2017, 37, 1057–1060. [Google Scholar] [CrossRef]
- Brown, J.; Farquhar, C. Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome. Cochrane Database Syst. Rev. 2016, 12, Cd002249. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).