Development of a Competition-Binding Assay to Determine Binding Affinity of Molecules to Neuromelanin via Fluorescence Spectroscopy
Abstract
1. Introduction
1.1. Neuromelanin and Parkinson’s Disease
1.2. Role of Nicotine in Protection from PD-Inducing Agents
1.3. Hypothesis of Role for NM in Binding Dopamine
1.4. Summary
2. Materials and Methods
2.1. Synthesis of Model Neuromelanin
2.2. Selection of Materials
2.3. Nicotine Fluorescence Binding Assay
2.4. Nicotine Fluorescence Assays Performed under Reducing Conditions
2.5. Cell Culture and Immunohistochemistry
3. Results and Discussion
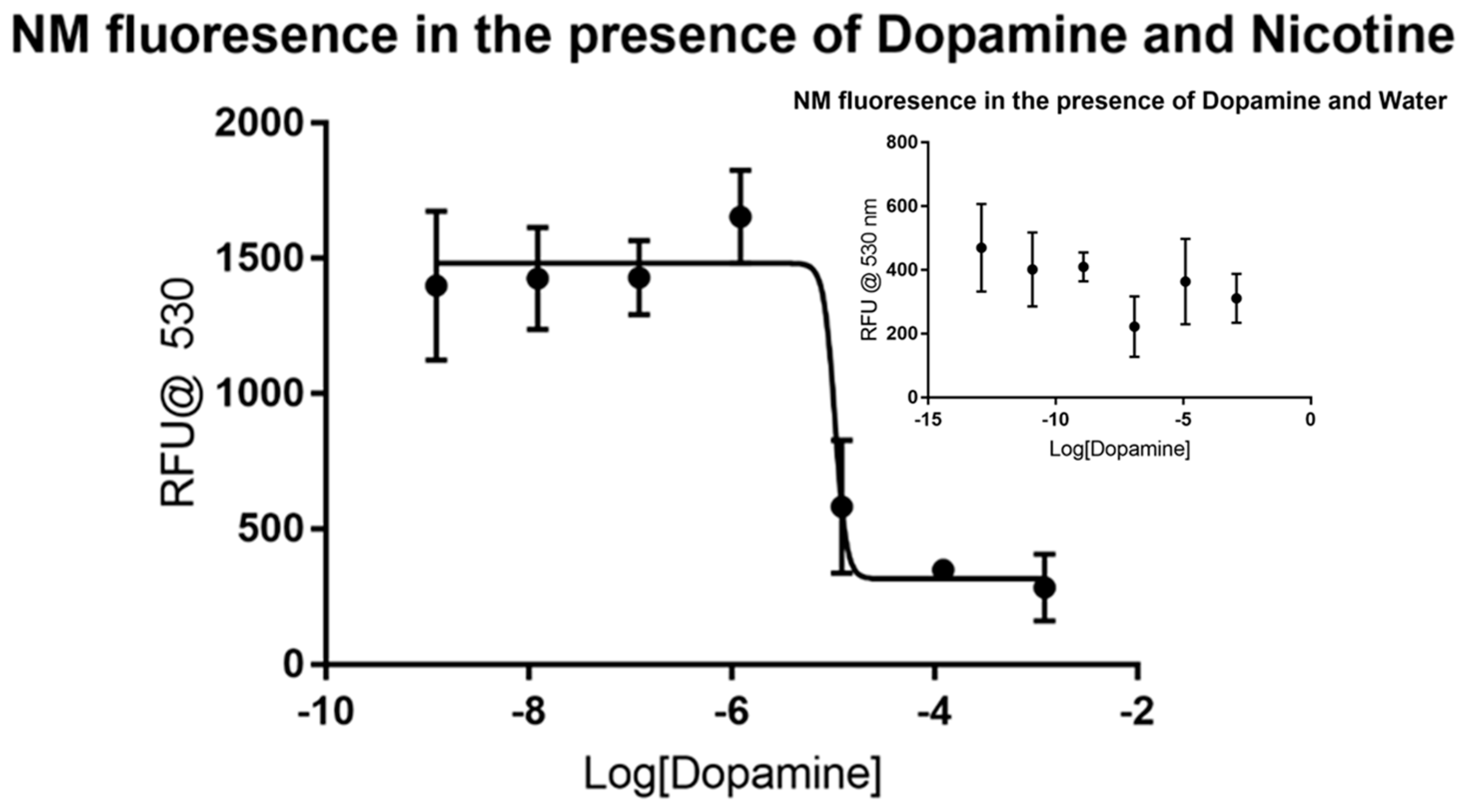
3.1. General Utility of Nicotine as a Signal Molecule for Measurement of Competition-Binding to Synthetic Neuromelanin
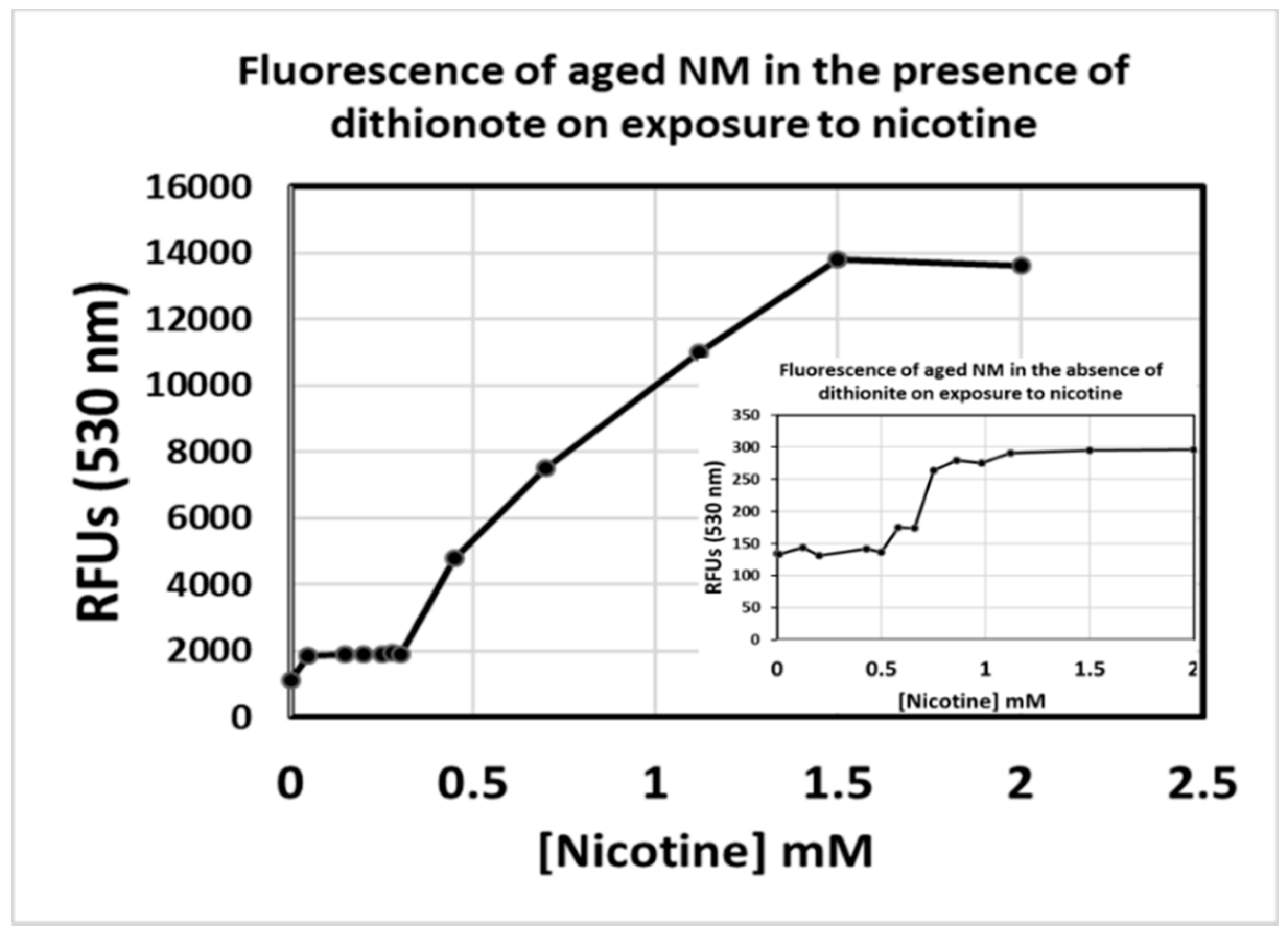
3.2. Redox State of Iron-Bound to Synthetic NM
3.3. Polymerization and Incorporation of Dopamine, 6-Hydroxydopamine, or Combinations of Both into Neuromelanin Granules in Cultured Neurons
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Kd | Dissociation constant |
| NM | Neuromelanin |
| NM-Fe3+ | iron-treated neuromelanin |
| PBS | phosphate buffered saline |
| RFU | relative fluorescence units |
| PD | Parkinson’s disease |
| UV/Vis or UV/visible spectroscopy | ultraviolet/visible spectroscopy |
References
- Davie, C.A. A Review of Parkinson’s Disease. Br. Med. Bull. 2008, 86, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.L.; Nelson, O.; Yazdani, U.; Pasbakhsh, P.; German, D.C. Inverse Relationship between the Contents of Neuromelanin Pigment and the Vesicular Monoamine Transporter-2: Human Midbrain Dopamine Neurons. J. Comp. Neurol. 2004, 47, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zoccarato, F.; Toscano, P.; Alexandre, A. Dopamine-Derived Dopaminochrome Promotes H2O2 Release at Mitochondrial Complex I. J. Biol. Chem. 2005, 280, 15587–15594. [Google Scholar] [CrossRef] [PubMed]
- Lohr, K.M.; Miller, G.W. VMAT2 and Parkinson’s Disease: Harnessing the Dopamine Vesicle. Expert Rev. Neurother. 2014, 14, 1115–1117. [Google Scholar] [CrossRef]
- Sulzer, D.; Zecca, L.; Larsen, K.E.; Bogulavsky, J.; Karatekin, E.; Krantz, D.; Behr, G.; Turro, N.; Edwards, R.H.; Greene, L.A.; et al. Neuromelanin Biosynthesis Is Driven by Excess Cytosolic Catecholamines Not Accumulated by Synaptic Vesicles. Proc. Natl. Acad. Sci. USA 2002, 97, 11869–11874. [Google Scholar] [CrossRef] [PubMed]
- Donatien, P.D.; Orlow, S.J. Interaction of Melanosomal Proteins with Melanin. Eur. J. Biochem. 1995, 232, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Fedorow, H.; Pickford, R.; Hook, J.M.; Double, K.L.; Halliday, G.M.; Gerlach, M.; Riederer, P.; Garner, B. Dolichol Is the Major Lipid Component of Human Substantia Nigra Neuromelanin. J. Neurochem. 2005, 92, 990–995. [Google Scholar] [CrossRef]
- Lees, A.J. Unresolved Issues Relating to the Shaking Palsy on the Celebration of James Parkinson’s 250th Birthday. Mov. Disord. 2007, 22, S327–S334. [Google Scholar] [CrossRef]
- Zecca, L.; Youdim, M.B.H.; Gerlach, M.; Double, K.L.; Becker, G.; Berg, D.; Riederer, P. Brain Iron Pathways and Their Relevance to Parkinson’s Disease. J. Neurochem. 2003, 79, 225–236. [Google Scholar] [CrossRef]
- Zecca, L.; Stroppolo, A.; Gatti, A.; Tampellini, D.; Toscani, M.; Gallorini, M.; Giaveri, G.; Arosio, P.; Santambrogio, P.; Fariello, R.G.; et al. The Role of Iron and Copper Molecules in the Neuronal Vulnerability of Locus Coeruleus and Substantia Nigra during Aging. Proc. Natl. Acad. Sci. USA 2004, 101, 9843–9848. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, H.; Wang, Y.; Wang, J.; Hui, F.; Sha, Q.; Wang, E.; Lu, S. Studies of Interaction between Iron (III) and Intermediates of Synthetic Neuromelanin by Means of Cyclic Voltammetry of and Dopamine. J. Electroanal. Chem. 2006, 594, 59–64. [Google Scholar] [CrossRef]
- LARSSON, B.S. Interaction Between Chemicals and Melanin. Pigment Cell Res. 1993, 6, 127–133. [Google Scholar] [CrossRef]
- Zecca, L.; Zucca, F.; Albertini, A.; Rizzio, E.; Fariello, R.G. A Proposed Dual Role of Neuromelanin in the Pathogenesis of Parkinson ’ s Disease. Neurology 2006, 67, S8–S11. [Google Scholar] [CrossRef]
- Moscatelli, A.; Ward, W.C.; Bellei, C.; Turro, N.J.; Zucca, F.A.; Wakamatsu, K.; Zecca, L.; Gallorini, M.; Eisner, M.; Casella, L.; et al. New Melanic Pigments in the Human Brain That Accumulate in Aging and Block Environmental Toxic Metals. Proc. Natl. Acad. Sci. USA 2008, 105, 17567–17572. [Google Scholar] [CrossRef]
- Javitch, J.A.; D’Amato, R.J.; Strittmatter, S.M.; Snyder, S.H. Parkinsonism-Inducing Neurotoxin, N-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine: Uptake of the Metabolite N-Methyl-4-Phenylpyridine by Dopamine Neurons Explains Selective Toxicity. Proc. Natl. Acad. Sci. USA 1985, 82, 2173–2177. [Google Scholar] [CrossRef]
- D’Amato, R.J.; Lipman, Z.P.; Snyder, S.H. Selectivity of the Parkinsonian Neurotoxin MPTP: Toxic Metabolite MPP+ Binds to Neuromelanin. Science 1986, 231, 987–989. [Google Scholar] [CrossRef]
- Karlsson, O.; Lindquist, N.G. Melanin Affinity and Its Possible Role in Neurodegeneration. J. Neural Transm. 2013, 120, 1623–1630. [Google Scholar] [CrossRef]
- Double, K.L.; Ben-Shachar, D.; Youdim, M.B.; Zecca, L.; Riederer, P.; Gerlach, M. Influence of Neuromelanin on Oxidative Pathways within the Human Substantia Nigra. Neurotoxicol. Teratol. 2002, 24, 621–628. [Google Scholar] [CrossRef]
- Ryan, R.E.; Ross, S.A.; Drago, J.; Loiacono, R.E. Dose-Related Neuroprotective Effects of Chronic Nicotine in 6-Hydroxydopamine Treated Rats, and Loss of Neuroprotection in Α4 Nicotinic Receptor Subunit Knockout Mice. Br. J. Pharmacol. 2001, 132, 1650–1656. [Google Scholar] [CrossRef]
- Chen, X.; Lan, X.; Roche, I.; Liu, R.; Geiger, J.D. Caffeine Protects against MPTP-Induced Blood-Brain Barrier Dysfunction in Mouse Striatum. J. Neurochem. 2008, 107, 1147–1157. [Google Scholar] [CrossRef]
- Chen, X.; Ghribi, O.; Geiger, J.D. Caffeine Protects against Disruptions of the Blood-Brain Barrier in Animal Models of Alzheimer’s and Parkinson’s Diseases. J. Alzheimer’s Dis. 2010, 20. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Cuevas, C.; Villa, M.; Caviedes, P.; Segura-Aguilar, J.; Tizabi, Y. Protective Effects of Nicotine Against Aminochrome-Induced Toxicity in Substantia Nigra Derived Cells: Implications for Parkinson’s Disease. Neurotox. Res. 2012, 22, 177–180. [Google Scholar] [CrossRef][Green Version]
- Haining, R.L.; Jones, T.M.; Hernandez, A. Saturation Binding of Nicotine to Synthetic Neuromelanin Demonstrated by Fluorescence Spectroscopy. Neurochem. Res. 2016, 41. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L.M.; Burcar, B.T.; Stevens, W.; Moriarty, E.M.; McGown, L.B. Guanine-Centric Self-Assembly of Nucleotides in Water: An Important Consideration in Prebiotic Chemistry. Astrobiology 2014, 14, 876–886. [Google Scholar] [CrossRef]
- Haining, R.L.; Achat-Mendes, C. Neuromelanin, One of the Most Overlooked Molecules in Modern Medicine, Is Not a Spectator. Neural Regen. Res. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Vanna, R.; Cupaioli, F.A.; Bellei, C.; De Palma, A.; Di Silvestre, D.; Mauri, P.; Grassi, S.; Prinetti, A.; Casella, L.; et al. Neuromelanin Organelles Are Specialized Autolysosomes That Accumulate Undegraded Proteins and Lipids in Aging Human Brain and Are Likely Involved in Parkinson’s Disease. npj Park. Dis. 2018, 4, 17. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Neumann, S.; Gao, X. Nicotine from Cigarette Smoking and Diet and Parkinson Disease: A Review. Transl. Neurodegener. 2017, 6, 18. [Google Scholar] [CrossRef]
- Zucca, F.A.; Seguura-Aguilar, J.; Ferrari, E.; Munoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of Iron, Dopamine and Neuromelanin Pathways in Brain Aging and Parkinson’s Disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef]
- Alvarado-Swaisgood, A.E.; Gallas, J.M.; Cheng, J.; Moss, S.C.; Eisner, M.; Zajac, G.W. The Fundamental Unit of Synthetic Melanin: A Verification by Tunneling Microscopy of X-Ray Scattering Results. Biochim. Biophys. Acta Gen. Subj. 2003, 1199, 271–278. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Fujikawa, K.; Zucca, F.A.; Zecca, L.; Ito, S. The Structure of Neuromelanin as Studied by Chemical Degradative Methods. J. Neurochem. 2003, 86, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Kim, Y.J.; Chun, S.-E.; Khetan, A.; Bettinger, C.J.; Viswanathan, V.; Whitacre, J.F. Evidence of Porphyrin-Like Structures in Natural Melanin Pigments Using Electrochemical Fingerprinting. Adv. Mater. 2016, 28, 3173–3180. [Google Scholar] [CrossRef]
- Chen, C.-T.; Martin-Martinez, F.J.; Jung, G.S.; Buehler, M.J. Polydopamine and Eumelanin Molecular Structures Investigated with Ab Initio Calculations. Chem. Sci. 2017, 8, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Punitha, V.; Kannan, P.; Saravanabhavan, S.; Thanikaivelan, P.; Rao, J.R.; Nair, B.U. Chemical Degradation of Melanin in Enzyme Based Dehairing and Fiber Opening of Buff Calfskins. Clean Technol. Environ. Policy 2009, 11, 299–306. [Google Scholar] [CrossRef]
- Madhurakkat Perikamana, S.K.; Lee, J.; Lee, Y.B.; Shin, Y.M.; Lee, E.J.; Mikos, A.G.; Shin, H. Materials from Mussel-Inspired Chemistry for Cell and Tissue Engineering Applications. Biomacromolecules 2015, 16, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Sharma, J.; Kaushik, N.; Kaushik, N.; Pardeshi, S.; Lee, S. Biomedical and Clinical Importance of Mussel-Inspired Polymers and Materials. Mar. Drugs 2015, 13, 6792–6817. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Wang, S.; Yang, Y.; Qin, B.; Wang, K.; Xie, T.; Wei, Y.; Ji, Y. Polydopamine Coated Shape Memory Polymer: Enabling Light Triggered Shape Recovery, Light Controlled Shape Reprogramming and Surface Functionalization. Chem. Sci. 2016, 7, 4741–4747. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fink, J.; Pathak, H.; Smith, J.; Achat-Mendes, C.; Haining, R.L. Development of a Competition-Binding Assay to Determine Binding Affinity of Molecules to Neuromelanin via Fluorescence Spectroscopy. Biomolecules 2019, 9, 175. https://doi.org/10.3390/biom9050175
Fink J, Pathak H, Smith J, Achat-Mendes C, Haining RL. Development of a Competition-Binding Assay to Determine Binding Affinity of Molecules to Neuromelanin via Fluorescence Spectroscopy. Biomolecules. 2019; 9(5):175. https://doi.org/10.3390/biom9050175
Chicago/Turabian StyleFink, Jackson, Heather Pathak, John Smith, Cindy Achat-Mendes, and Robert L. Haining. 2019. "Development of a Competition-Binding Assay to Determine Binding Affinity of Molecules to Neuromelanin via Fluorescence Spectroscopy" Biomolecules 9, no. 5: 175. https://doi.org/10.3390/biom9050175
APA StyleFink, J., Pathak, H., Smith, J., Achat-Mendes, C., & Haining, R. L. (2019). Development of a Competition-Binding Assay to Determine Binding Affinity of Molecules to Neuromelanin via Fluorescence Spectroscopy. Biomolecules, 9(5), 175. https://doi.org/10.3390/biom9050175




