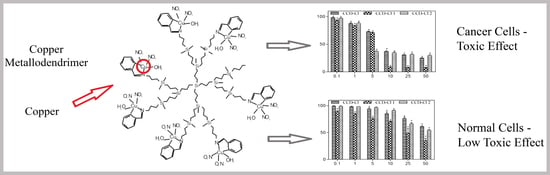
In Vitro Anticancer Properties of Copper Metallodendrimers
Abstract
:1. Introduction
2. Materials and Methods
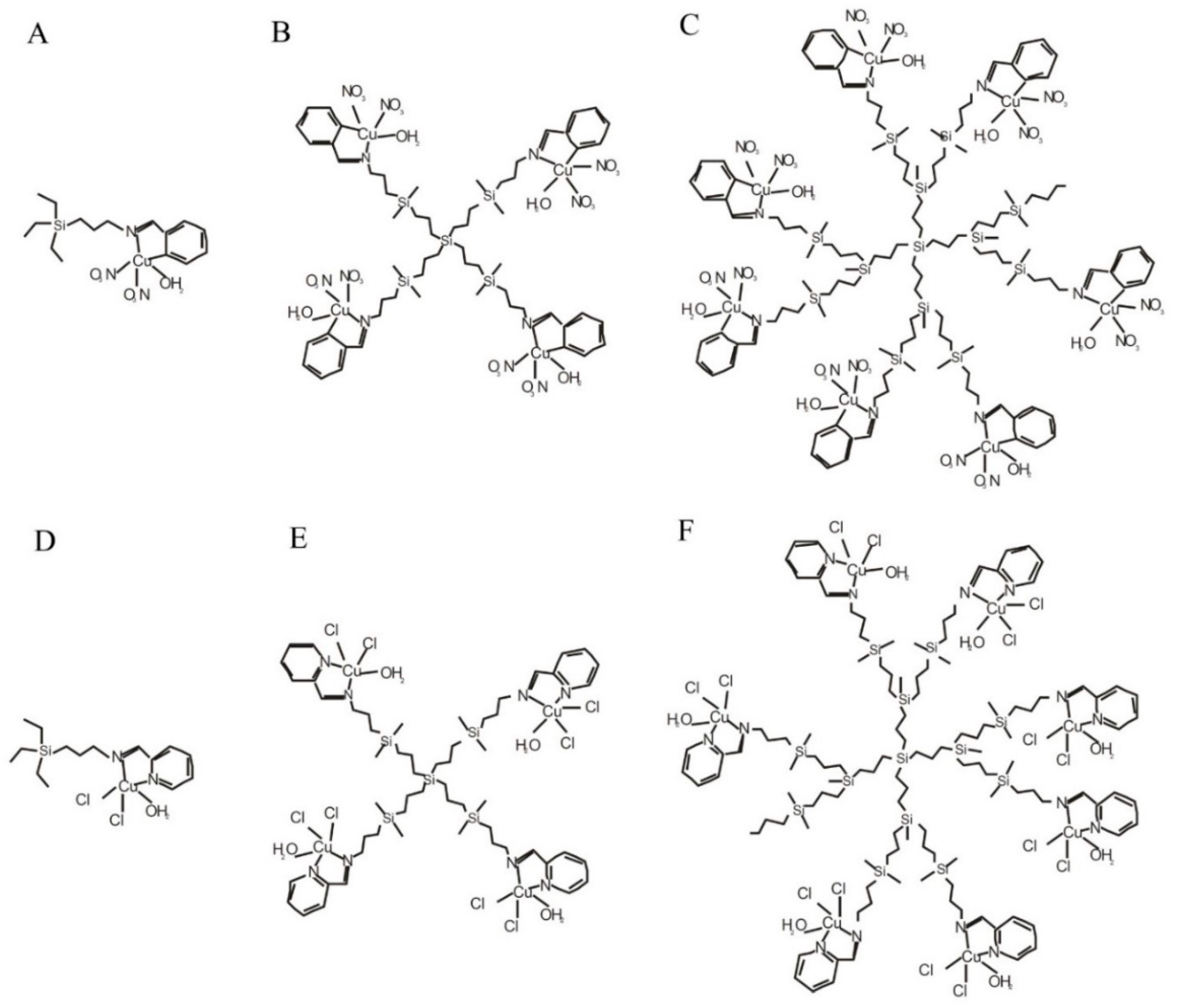
2.1. Dendrimers
2.2. Zeta Potential Technique
2.3. Measurement of the Hydrodynamic Diameter of the Particles
2.4. Transmission Electron Microscopy (TEM)
2.5. Circular Dichroism
2.6. Haemotoxicity
2.7. Erythrocyte Membrane Isolation
2.8. Fluorescence Anisotropy
2.9. Cell Lines
2.10. Cytotoxicity
2.11. Statistical Analysis
3. Results
3.1. Particle Size and Zeta Potential Analysis
3.2. Transmission Electron Microscopy (TEM)
3.3. Circular Dichroism
3.4. Erythrocyte Membrane Fluidity
3.5. Hemotoxicity
3.6. Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanz del Olmo, N.; Maroto-Diaz, M.; Ramirez, R.G.; Ortega, P.; Cangiotti, M.; Ottaviani, M.; de la Mata, F.J. Carbosilane metallodendrimers based on copper (II) complexes: Synthesis, EPR characterization and anticancer activity. J. Inorg. Biochem. 2017, 177, 211–218. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Azuara, L.; Bravo-Gomez, M.E. Copper compounds in cancer chemotherapy. Curr. Med. Chem. 2010, 17, 3606–3615. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Yan, Y.K.; Lee, P.P.F.; Lim, K.H. Copper, gold and silver compounds as potential new anti-tumor metallodrugs. Future Med. Chem. 2010, 10, 1591–1608. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Chen, J.; Yang, Q.; Yang, L.; Xu, D.; Zhang, P.; Wang, X.; Liu, J. Hinokitol copper complex inhibits proteasomal deubiquitination and induces paraptosis-like cell death in human cancer cells. Eur. J. Pharmacol. 2017, 815, 147–155. [Google Scholar] [CrossRef]
- Mignani, S.; Brahmi, N.E.L.; Eloy, L.; Poupon, J.; Nicolas, V.; Steinmetz, A.; Kazzouli, S.E.L.; Bousmina, M.M.; Blanchard-Desce, M.; Caminade, A.M.; et al. Anticancer copper(II) phosphorus dendrimers are potent proapoptotic Bax activators. Eur. J. Med. Chem. 2017, 132, 142–156. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; Wang, Y.; Guo, Z.; Wang, X. Hypotoxic copper complexes with potent anti-metastatic and anti-angiogenic activities against cancer cells. Dalton Trans. 2018, 47, 5049–5054. [Google Scholar] [CrossRef]
- Demkow, U.; Stelmaszczyk-Emmel, A. Cardiotoxicity of cisplatin-based chemotherapy in advanced non-small cell lung cancer patients. Respir. Physiol. Neurobiol. 2013, 187, 64–67. [Google Scholar] [CrossRef]
- Seng, S.; Liu, Z.; Chiu, S.K.; Proverbs-Singh, T.; Sonpavde, G.; Choueiri, T.K.; Tsao, C.K.; Yu, M.; Hahn, N.M.; Oh, W.K.; et al. Risk of venous thromboembolism in patients with cancer treated with Cisplatin: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 4416–4426. [Google Scholar] [CrossRef]
- Boodram, J.N.; Mcgregor, I.J.; Bruno, P.M.; Cressey, P.B.; Hemann, M.T.; Suntharalingam, K. breast cancer stem cell potent copper(ii)-non-steroidal anti-inflammatory drug complexes. Angew. Chem. 2016, 128, 2895–2900. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Maroto-Díaz, M.; Szwed, A.; Ihnatsyeu-Kachan, A.; Loznikova, S.; Shcharbin, D.; Maly, M.; Gomez Ramirez, R.; de la Mata, F.J.; et al. Ruthenium dendrimers as carriers for anticancer siRNA. J. Inorg. Biochem. 2018, 181, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Michlewska, S.; Ionov, M.; Shcharbin, D.; Maroto-Díaz, M.; Gomez Ramirez, R.; de la Mata, F.J.; Bryszewska, M. Ruthenium metallodendrimers with anticancer potential in an acute promyelocytic leukemia cell line (HL60). Eur. Polym. J. 2017, 87, 39–47. [Google Scholar] [CrossRef]
- Fuentes-Paniagua, E.; Serramia, M.J.; Sanchez-Nieves, J.; Alvarez, S.; Munoz-Fernandez, M.A.; Ramirez, R.G.; de la Mata, F.J. Fluorescein labelled cationic carbosilane dendritic systems for biological studies. Eur. Polym. J. 2015, 71, 61–72. [Google Scholar]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L.; Swain, K.A. Health effects related to nanoparticle exposures: Environmental, health and safety considerations for assessing hazards and risks. Pharmacol. Ther. 2008, 120, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, D.B.; Amiji, M.M. Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int. J. Pharm. 2005, 293, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Khalil, M.; Gurbuz, M.; Simone, T.M.; Mousa, S.A. Nanoparticles and cancer therapy: A concise review with emphasis on dendrimers. Int. J. Nanomed. 2009, 4, 1–7. [Google Scholar]
- Malik, P.; Gulati, N.; Kaur Malik, R.; Nagaich, U. Carbon nanotubes, quantum dots and dendrimers as potential nanodevices for nanotechnology drug delivery systems. Int. J. Pharm. Sci. Nanotech. 2013, 6, 2113–2124. [Google Scholar]
- Dehshahri, A.; Sadeghpour, H. Surface decorations of poly(amidoamine) dendrimer by various pendant moieties for improved delivery of nucleic acid materials. Colloids Surf. B Biointerfaces 2015, 132, 85–102. [Google Scholar] [CrossRef]
- Shcharbin, D.; Shcharbina, N.; Milowska, K.; de la Mata, F.J.; Muñoz-Fernandez, M.A.; Mignani, S.; Ramirez, R.G.; Majoral, J.P.; Bryszewska, M. Interference of cationic polymeric nanoparticles with clinical chemistry tests—Clinical relevance. Int. J. Pharm. 2014, 473, 599–606. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Reyna, L.A.; Svenson, S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans. 2007, 35, 61–67. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: A review. Int. J. Biol. Macromol. 2016, 86, 570–586. [Google Scholar] [CrossRef]
- Ionov, M.; Ihnatsyeu-Kachan, A.; Michlewska, S.; Shcharbina, N.; Shcharbin, D.; Majoral, J.P.; Bryszewska, M. Effect of dendrimers on selected enzymes—Evaluation of nano carriers. Int. J. Pharm. 2016, 499, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Ciepluch, K.; Moreno, B.R.; Appelhans, D.; Sánchez-Nieves, J.; Gómez Ramirez, R.; de la Mata, F.J.; Muñoz-Fernández, M.A.; Bryszewska, M. Biophysical characterization of glycodendrimers as nano-carriers for HIV peptides. Curr. Med. Chem. 2013, 20, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Martinho, N.; Florindo, H.; Silva, L.; Brocchini, S.; Zloh, M.; Barata, T. Molecular modeling to study dendrimers for biomedical applications. Moleclues 2014, 19, 20424–20467. [Google Scholar] [CrossRef] [PubMed]
- Pandi, P.; Jain, A.; Kommineni, N.; Ionov, M.; Bryszewska, M.; Khan, W. Dendrimer as a new potential carrier for topical delivery of siRNA: A comparative study of dendriplex vs. lipoplex for delivery of TNF-α siRNA. Int. J. Pharm. 2018, 550, 240–250. [Google Scholar] [PubMed]
- Ionov, M.; Wróbel, D.; Gardikis, K.; Hatziantoniou, S.; Demetzos, C.; Majoral, J.P.; Klajnert-Maculewicz, B.; Bryszewska, M. Effect of phosphorus dendrimers on DMPC lipid membranes. Chem. Phys. Lipids 2012, 165, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Lazniewska, J.; Milowska, K.; Katir, N.; El kadib, A.; Bryszewska, M.; Majoral, J.P.; Gabryelak, T. Viologen-phosphorus dendrimers exhibit minor toxicity against a murine neuroblastoma cell line. Cell. Mol. Biol. Lett. 2013, 18, 459–478. [Google Scholar] [CrossRef]
- Ionov, M.; Klajnert, B.; Gardikis, K.; Hatziantoniou, S.; Palecz, B.; Salakhutdinov, B.; Cladera, J.; Zamaraeva, M.; Demetzos, C.; Bryszewska, M. Effect of amyloid beta peptides Ab1–28 and Ab25–40 on model lipid membranes. J. Therm. Anal. Calorim. 2010, 99, 741–747. [Google Scholar] [CrossRef]
- Ionov, M.; Ciepluch, K.; Garaiova, Z.; Melikishvili, S.; Michlewska, S.; Balcerzak, Ł.; Glińska, S.; Miłowska, K.; Gomez-Ramirez, R.; de la Mata, F.J.; et al. Dendrimers complexed with HIV-1 peptides interact with liposomes and lipid monolayers. Biochim. Biophys. Acta 2015, 1848, 907–915. [Google Scholar] [CrossRef]
- Ciołkowski, M.; Różanek, M.; Szewczyk, M.; Klajnert, B.; Bryszewska, M. The influence of PAMAM-OH dendrimers on the activity of human erythrocytes ATPases. Biochim. Biophys. Acta 2011, 1808, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Domański, D.; Bryszewska, M.; Salamończyk, G. Preliminary evaluation of the behavior of fifth-generation thiophosphate dendrimer in biological systems. Biomacromolecules 2004, 5, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Ciepluch, K.; Katir, N.E.L.; Kadib, A.; Felczak, A.; Zawadzka, K.; Weber, M.; Klajnert, B.; Lisowska, K.; Caminade, A.M.; Bousmina, M.; et al. Biological properties of new viologen-phosphorus dendrimers. Mol. Pharmaceut. 2012, 9, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.M.; Islam, M.T.; Orr, B.G.; Baker, J.R.; Banaszak Holl, M.M. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjugate Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Halets, I.; Shcharbin, D.; Klajnert, B.; Bryszewska, M. Contribution of hydrophobicity, DNA and proteins to the cytotoxicity of cationic PAMAM dendrimers. Int. J. Pharm. 2013, 1, 1–3. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Smith, B.D. High-generation polycationic dendrimers are unusually effective at disrupting anionic vesicles: Membrane bending model. Bioconjugate Chem. 2000, 11, 805–814. [Google Scholar] [CrossRef]
- Hong, S.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.; Balogh, L.; Orr, B.G.; Baker, J.R., Jr.; Banaszak Holl, M.M. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: Hole formation and the relation to transport. Bioconjugate Chem. 2004, 15, 774–782. [Google Scholar] [CrossRef]
- Moreno, S.; Szwed, A.; Brahmi, N.E.; Milowska, K.; Kurowska, J.; Fuentes-Paniagua, E.; Pędziwiatr-Werbicka, E.; Gabryelak, T.; Katir, N.; de la Mata, F.J.; et al. Synthesis, characterization and biological properties of new hybrid carbosilane-viologen-phosphorus dendrimers. RSC Adv. 2015, 5, 25942–25958. [Google Scholar] [CrossRef]
- Miłowska, K.; Rybczyńska, A.; JMosiolek, J.; Durdyn, J.; Szewczyk, E.M.; Katir, N.; Brahmi, Y.; Majoral, J.P.; Bousmina, M.; Bryszewska, M.; et al. Biological activity of mesoporous dendrimer-coated titanium dioxide: Insight on the role of the surface-interface composition and the framework crystallinity. Appl. Mater. Interfaces 2015, 7, 19994–20003. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 57, 2106–2129. [Google Scholar] [CrossRef]
- Shcharbin, D.; Ionov, M.; Abashkin, V.; Loznikova, S.; Dzmitruk, V.; Shcharbina, N.; Matusevich, L.; Miłowska, K.; Gałęcki, K.; Wysocki, S.; et al. Nanoparticle corona for proteins: Mechanisms of interaction between dendrimers and proteins. Colloids Surf. B Biointerfaces 2015, 134, 377–383. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Jiang, J.; Dong, S. pH-dependent protein conformational changes in albumin:gold nanoparticle bioconjugates: A spectroscopic study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.M.; Aubert, G.; Laurent, R.; Caminade, A.M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.P. Original multivalent copper(II)-conjugated phosphorus dendrimers and corresponding mononuclear copper(II) complexes with antitumoral activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef]
- Vogus, D.R.; Evans, M.A.; Pusuluri, A.; Barajas, A.; Zhang, M.; Krishnan, V.; Nowak, M.; Menegatti, S.; Helgeson, M.E.; Squires, T.M.; et al. A hyaluronic acid conjugate engineered to synergistically and sequentially deliver gemcitabine and doxorubicin to treat triple negative breast cancer. J. Control. Release 2017, 267, 191–202. [Google Scholar] [CrossRef]
- Zhao, Z.; Lou, S.; Hu, Y.; Zhu, J.; Zhang, C. A Nano-in-nano polymer–dendrimer nanoparticle-based nanosystem for controlled multidrug delivery. Mol. Pharmaceut. 2017, 14, 2697–2710. [Google Scholar] [CrossRef]
- Lou, S.; Zhao, Z.; Dezort, M.; Lohneis, T.; Zhang, C. Multifunctional nanosystem for targeted and controlled delivery of multiple chemotherapeutic agents for the treatment of drug-resistant breast cancer. ACS Omega 2018, 3, 9210–9219. [Google Scholar] [CrossRef]






| CCD-NO-0 | CCD-NO-1 | CCD-NO-2 | CCD-Cl-0 | CCD-Cl-1 | CCD-Cl-2 | |
|---|---|---|---|---|---|---|
| Generation | 0 | 1 | 2 | 0 | 1 | 2 |
| Surface groups number | 1 | 4 | 8 | 1 | 4 | 8 |
| Molecular weight [g/Mol] | 468.04 | 1840.10 | 3992.90 | 414.93 | 1627.68 | 3696.01 |
| Solubility | MeOH/DMF/DMSO | DMF/DMSO/CHCl3/CH2Cl2 | ||||
| Dendrimer | Zeta Potential, [mV] | Zeta Size, [nm] |
|---|---|---|
| CCD-NO-0 | 14.79 ± 1.92 | 135.28 ± 9.27 |
| CCD-NO-1 | 25.90 ± 2.32 | 51.59 ± 6.74 |
| CCD-NO-2 | 39.23 ± 3.78 | 63.12 ± 5.28 |
| CCD-Cl-0 | 10.45 ± 1.25 | 152.13 ± 7.52 |
| CCD-Cl-1 | 19.68 ± 1.78 | 59.53 ± 8.92 |
| CCD-Cl-2 | 37.48 ± 3.09 | 74.27 ± 7.26 |
| Dendrimer | PDI |
|---|---|
| CCD-NO-0 | 0.537 ± 0.146 |
| CCD-NO-1 | 0.370 ± 0.087 |
| CCD-NO-2 | 0.229 ± 0.022 |
| CCD-Cl-0 | 0.542 ± 0.122 |
| CCD-Cl-1 | 0.423 ± 0.068 |
| CCD-Cl-2 | 0.356 ± 0.062 |
| Dendrimer | PBMC | 1301 | HL60 |
|---|---|---|---|
| CCD-NO-0 | 62.64 ± 0.2 | 27.84 ± 1.2 | 29.05 ± 0.6 |
| CCD-NO-1 | 56.55 ± 0.4 | 5.03 ± 0.2 | 24.32 ± 1.7 |
| CCD-NO-2 | 35.02 ± 0.2 | 4.85 ± 2.4 | 4.01 ± 0.4 |
| CCD-Cl-0 | 62.23 ± 0.3 | 12.44 ± 0.7 | 5.94 ± 0.4 |
| CCD-Cl-1 | 31.57 ± 7.3 | 4.58 ± 2.4 | 5.57 ± 0.2 |
| CCD-Cl-2 | 50.95 ± 1.1 | 7.12 ± 0.3 | 2.37 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hołota, M.; Magiera, J.; Michlewska, S.; Kubczak, M.; Sanz del Olmo, N.; García-Gallego, S.; Ortega, P.; de la Mata, F.J.; Ionov, M.; Bryszewska, M. In Vitro Anticancer Properties of Copper Metallodendrimers. Biomolecules 2019, 9, 155. https://doi.org/10.3390/biom9040155
Hołota M, Magiera J, Michlewska S, Kubczak M, Sanz del Olmo N, García-Gallego S, Ortega P, de la Mata FJ, Ionov M, Bryszewska M. In Vitro Anticancer Properties of Copper Metallodendrimers. Biomolecules. 2019; 9(4):155. https://doi.org/10.3390/biom9040155
Chicago/Turabian StyleHołota, Marcin, Jakub Magiera, Sylwia Michlewska, Małgorzata Kubczak, Natalia Sanz del Olmo, Sandra García-Gallego, Paula Ortega, Francisco Javier de la Mata, Maksim Ionov, and Maria Bryszewska. 2019. "In Vitro Anticancer Properties of Copper Metallodendrimers" Biomolecules 9, no. 4: 155. https://doi.org/10.3390/biom9040155
APA StyleHołota, M., Magiera, J., Michlewska, S., Kubczak, M., Sanz del Olmo, N., García-Gallego, S., Ortega, P., de la Mata, F. J., Ionov, M., & Bryszewska, M. (2019). In Vitro Anticancer Properties of Copper Metallodendrimers. Biomolecules, 9(4), 155. https://doi.org/10.3390/biom9040155