Rational Design, Synthesis and Preliminary Evaluation of Novel Fusarinine C-Based Chelators for Radiolabeling with Zirconium-89
Abstract
:1. Introduction
2. Materials and Methods
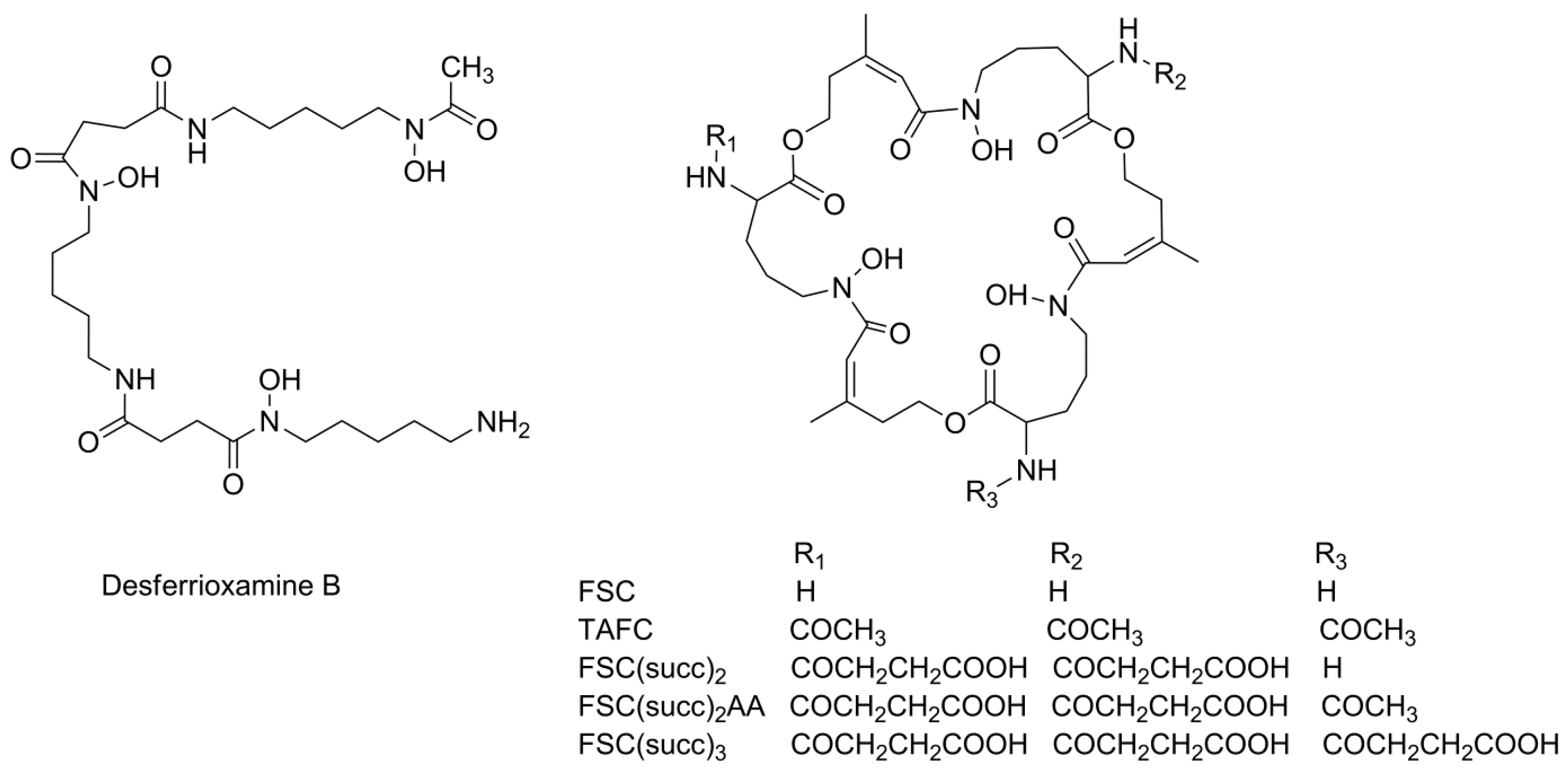
2.1. Fusarinine C
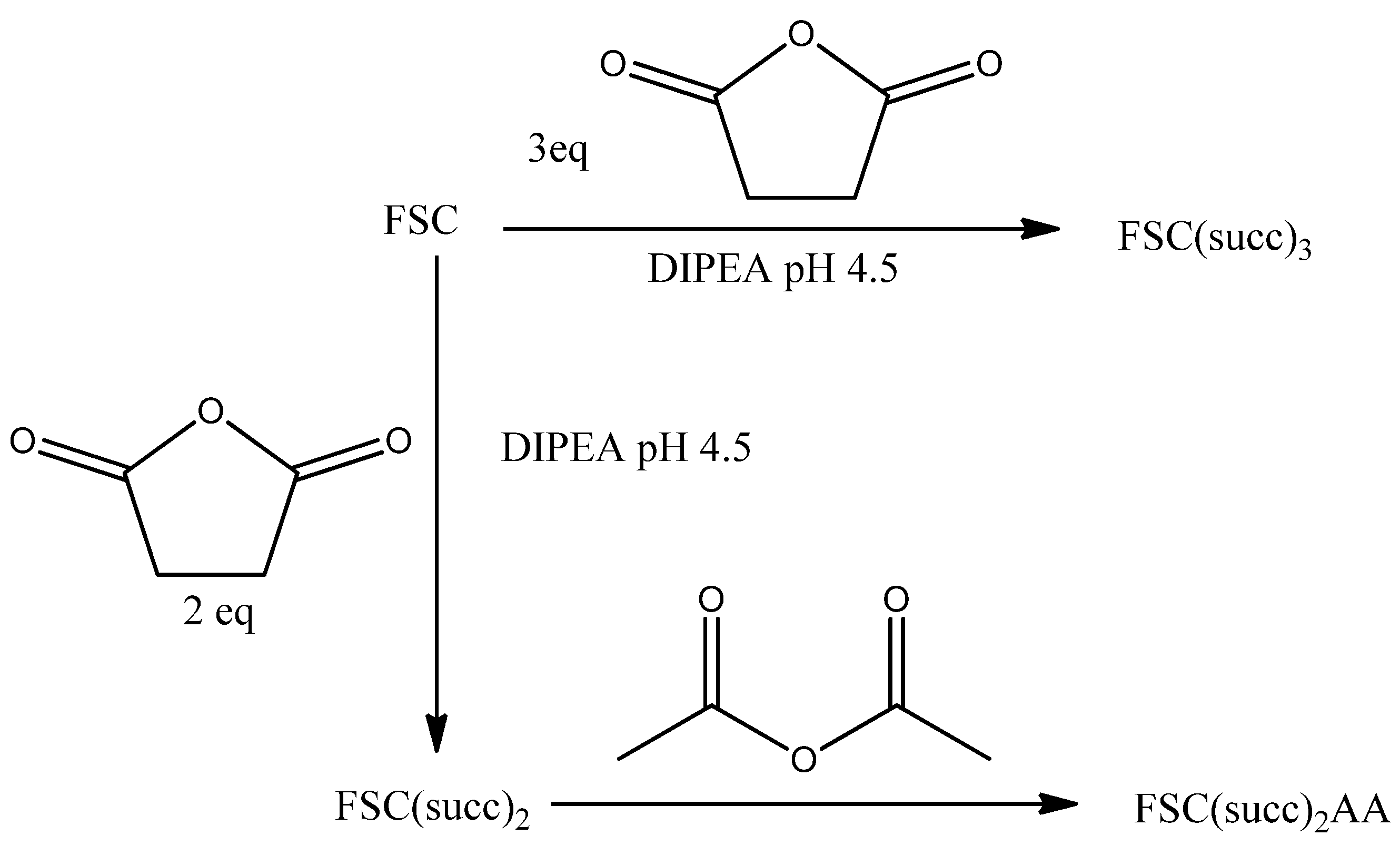
2.2. Fusarinine C (succ)3
2.3. Fusarinine C(succ)2
2.4. Fusarinine C(succ)2AA
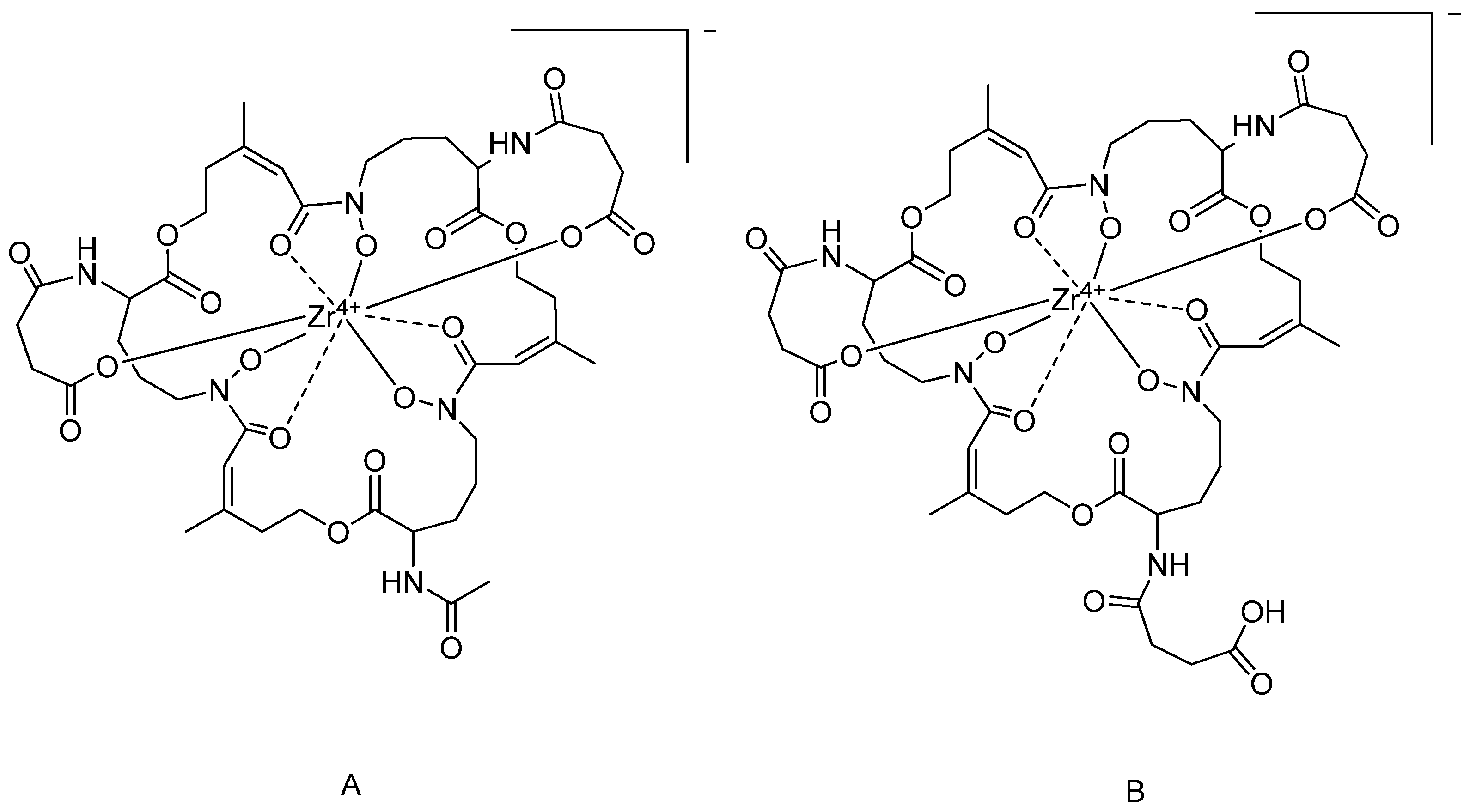
2.5. Zr-Fusarinine C(succ)2AA and Zr-Fusarinine C(succ)3.
2.6. 89Zr-Labeling
2.7. In Vitro Characterization
2.7.1. Distribution Coefficient
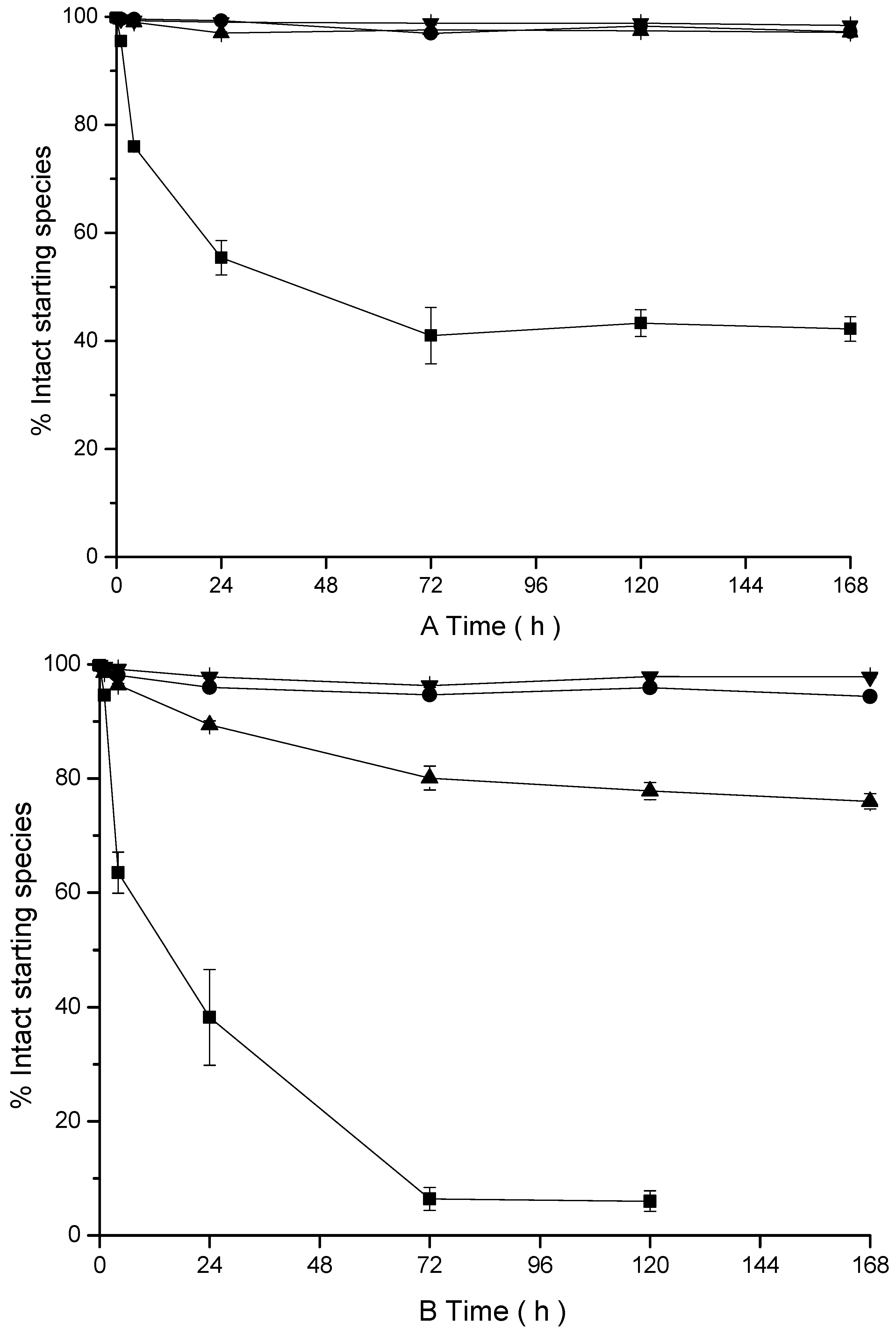
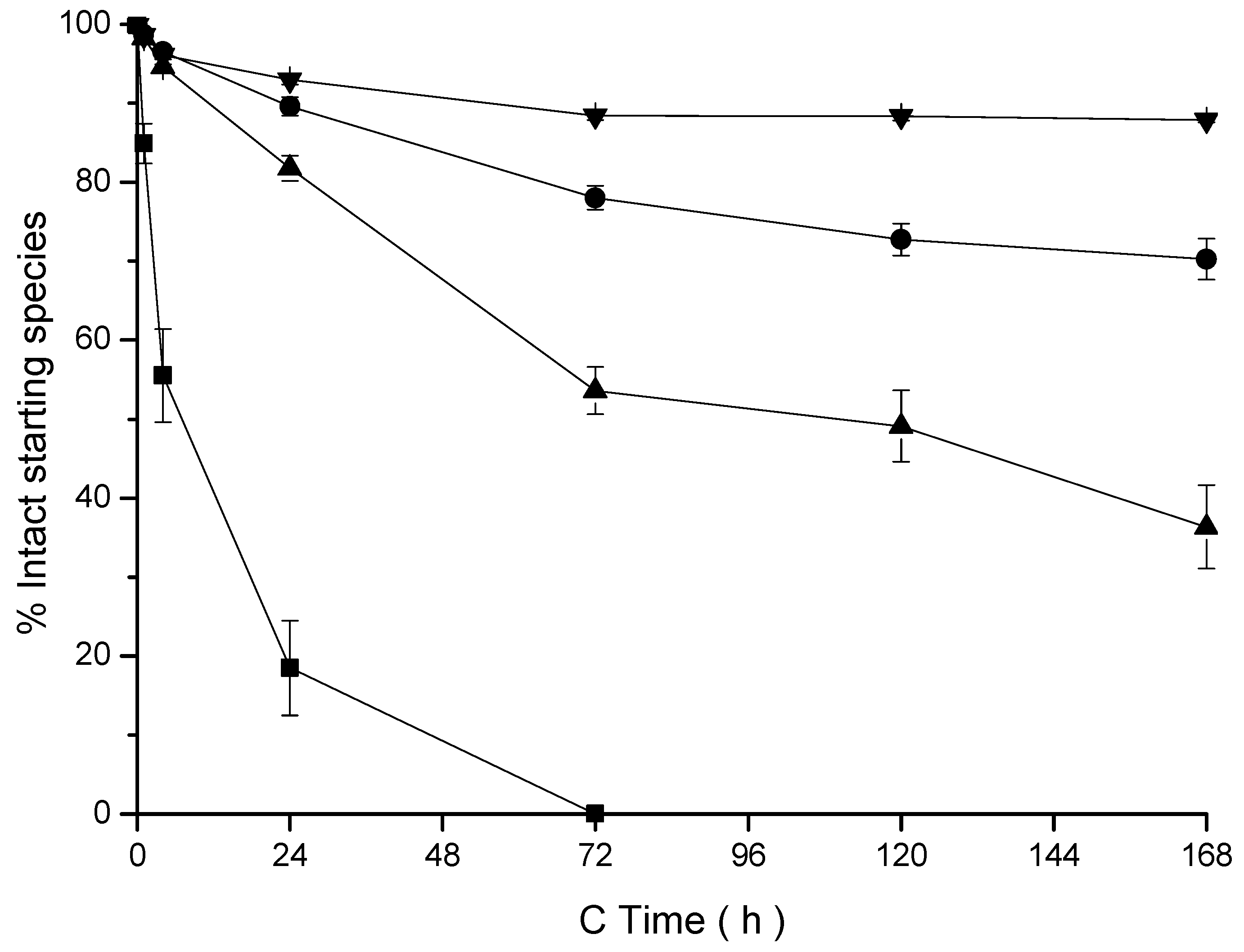
2.7.2. Stability Assay
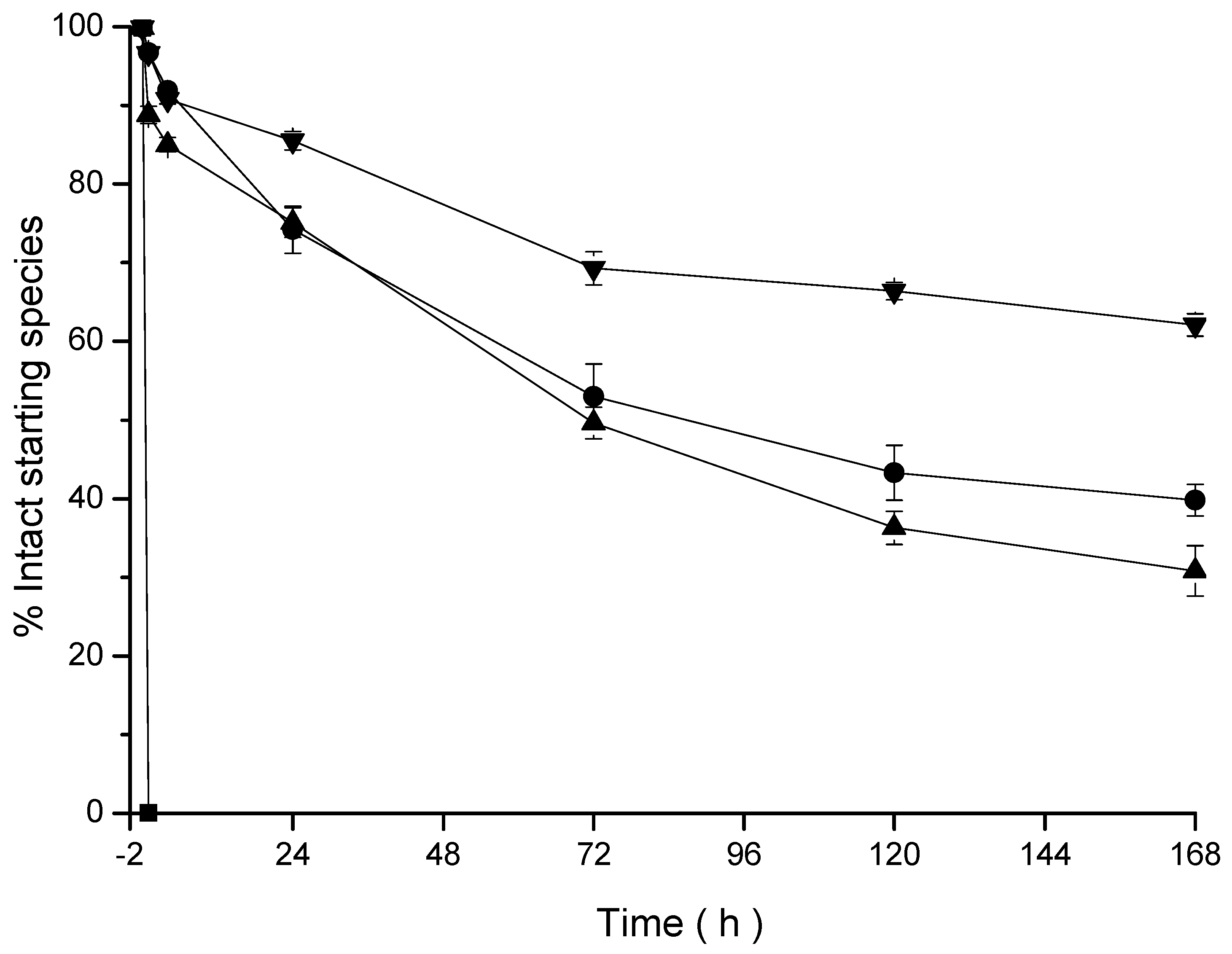
2.7.3. Transchelation Study
2.7.4. Protein Binding Assay
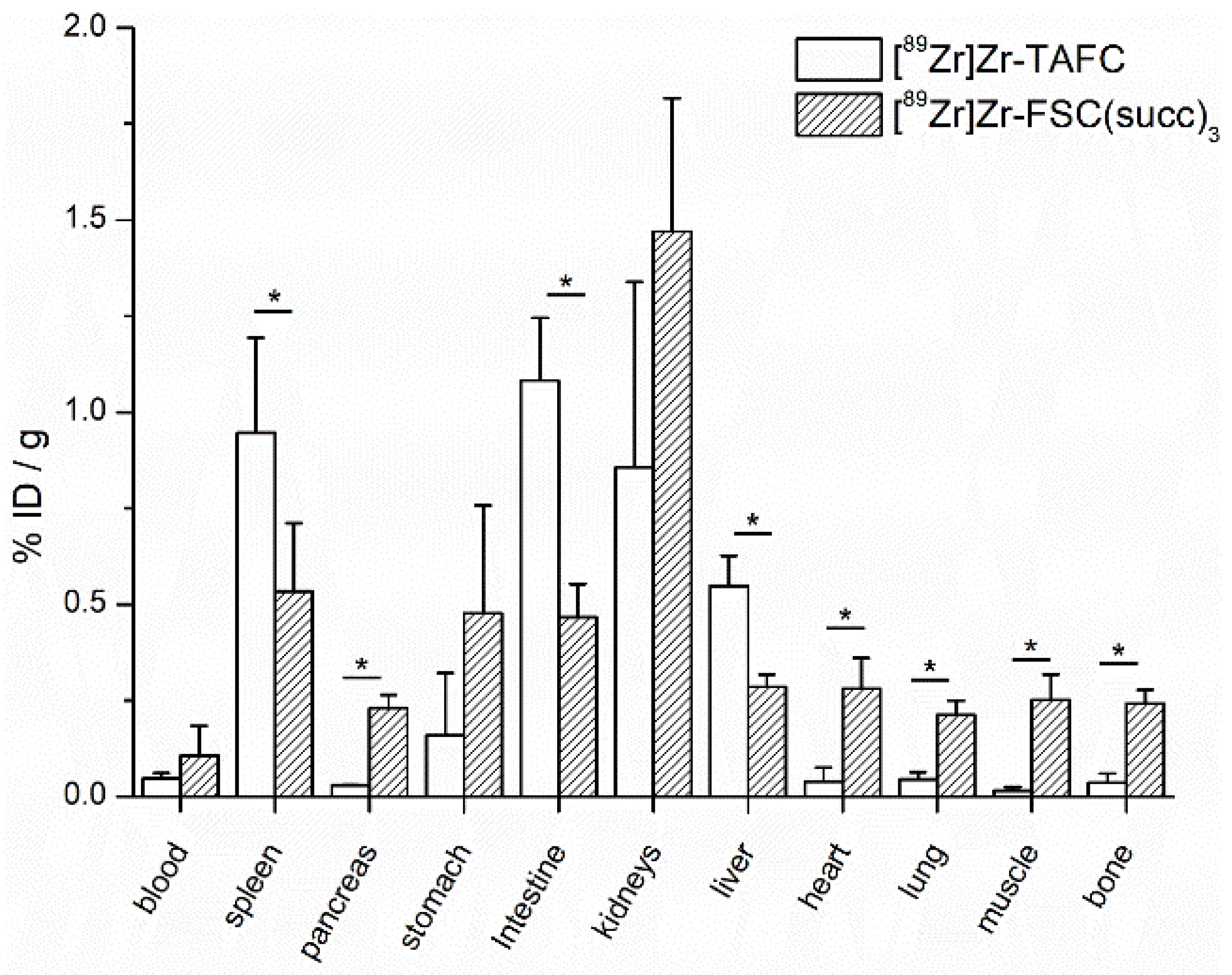
2.7.5. Biodistribution Study
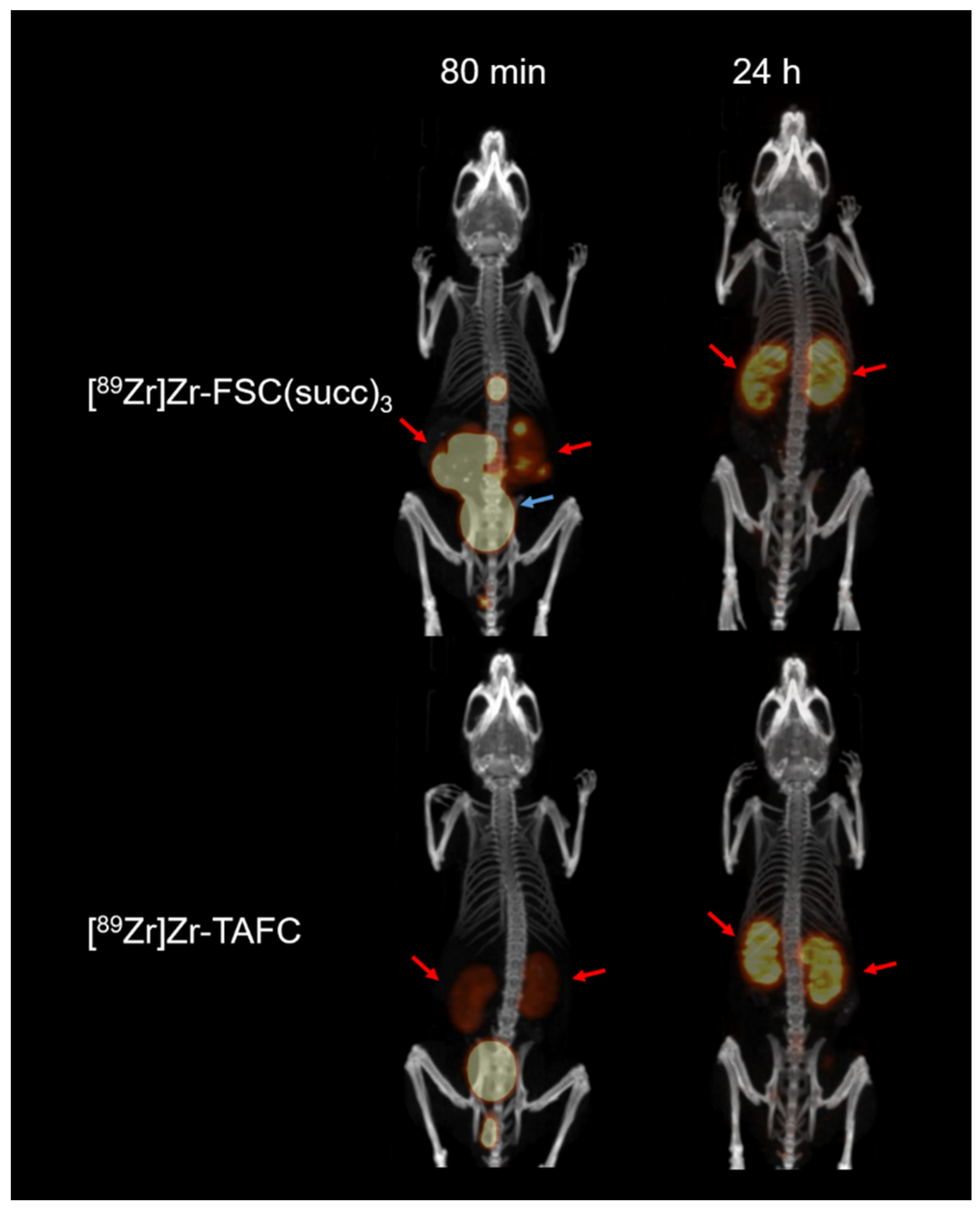
2.7.6. Micro-Positron Emission Tomography/Computer Tomography Imaging
3. Results
3.1. Synthesis
3.2. 89Zr-Labeling
3.3. Ex Vitro Characterization
3.4. Biodistribution Study and Micro-Positron Emission Tomography/Computer Tomography (microPET/CT) Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Dongen, G.A.; Visser, G.W.; Lub-de Hooge, M.N.; De Vries, E.G.; Perk, L.R. Immuno-PET: A navigator in monoclonal antibody development and applications. Oncologist 2007, 12, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Seibold, U.; Schirrmacher, R.; Wangler, B.; Wangler, C. 89Zr, a radiometal nuclide with high potential for molecular imaging with PET: Chemistry, applications and remaining challenges. Molecules 2013, 18, 6469–6490. [Google Scholar] [CrossRef] [PubMed]
- Stillebroer, A.B.; Franssen, G.M.; Mulders, P.F.; Oyen, W.J.; van Dongen, G.A.; Laverman, P.; Oosterwijk, E.; Boerman, O.C. ImmunoPET imaging of renal cell carcinoma with 124I-and 89Zr-labeled anti-CAIX monoclonal antibody cG250 in mice. Cancer Biother. Radiopharm. 2013, 28, 510–515. [Google Scholar] [CrossRef] [PubMed]
- van de Watering, F.C.; Rijpkema, M.; Perk, L.; Brinkmann, U.; Oyen, W.J.; Boerman, O.C. Zirconium-89 labeled antibodies: A new tool for molecular imaging in cancer patients. BioMed Res. Int. 2014, 2014, 203601. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.J.; DeSilva, R.; Jain, S.; Lears, K.; Rogers, B.; Lapi, S. 89Zr-radiolabeled trastuzumab imaging in orthotopic and metastatic breast tumors. Pharmaceuticals 2012, 5, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Dubois, L.; Perk, L.; Vermaelen, P.; van Dongen, G.A.; Wouters, B.G.; Lambin, P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med. 2009, 50, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Mohindra, P.; Weissmann, G.I.; Divilov, V.; Hilderbrand, S.A.; Weissleder, R.; Lewis, J.S. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand diels–Alder click chemistry. Bioconjug. Chem. 2011, 22, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- van Rij, C.M.; Sharkey, R.M.; Goldenberg, D.M.; Frielink, C.; Molkenboer, J.D.; Franssen, G.M.; van Weerden, W.M.; Oyen, W.J.G.; Boerman, O.C. Imaging of prostate cancer with immuno-PET and immuno-SPECT using a radiolabeled anti-EGP-1 monoclonal antibody. J. Nucl. Med. 2011, 52, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Heskamp, S.; van Laarhoven, H.W.; Molkenboer-Kuenen, J.D.; Franssen, G.M.; Versleijen-Jonkers, Y.M.; Oyen, W.J.; van der Graaf, W.T.; Boerman, O.C. ImmunoSPECT and immunoPET of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J. Nucl. Med. 2010, 51, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Guérard, F.; Lee, Y.-S.; Tripier, R.; Szajek, L.P.; Deschamps, J.R.; Brechbiel, M.W. Investigation of Zr(IV) and 89Zr(IV) complexation with hydroxamates: Progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem. Commun. 2013, 49, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Bauman, A.; Mari, C.; Fischer, C.A.; Blacque, O.; Häussinger, D.; Gasser, G.; Mindt, T.L. An octadentate bifunctional chelating agent for the development of stable zirconium-89 based molecular imaging probes. Chem. Commun. 2014, 50, 11523–11525. [Google Scholar] [CrossRef] [PubMed]
- Guérard, F.; Lee, Y.S.; Brechbiel, M.W. Rational Design, Synthesis, and Evaluation of Tetrahydroxamic Acid Chelators for Stable Complexation of Zirconium (IV). Chem. A Eur. J. 2014, 20, 5584–5591. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Pailloux, S.; Tatum, D.; Magda, D.; Wadas, T.J. Di-macrocyclic terephthalamide ligands as chelators for the PET radionuclide zirconium-89. Chem. Commun. 2015, 51, 2301–2303. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.T.; Meszaros, L.K.; Paterson, B.M.; Berry, D.J.; Cooper, M.S.; Ma, Y.; Hider, R.C.; Blower, P.J. Tripodal tris(hydroxypyridinone) ligands for immunoconjugate PET imaging with 89Zr 4+: Comparison with desferrioxamine-B. Dalton Trans. 2015, 44, 4884–4900. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Ponnala, S.; Zeglis, B.M.; Pohl, G.; Dannenberg, J.J.; Lewis, J.S.; Francesconi, L.C. An alternative chelator for 89Zr radiopharmaceuticals: Radiolabeling and evaluation of 3,4,3-(LI-1, 2-HOPO). J. Med. Chem. 2014, 57, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Seibold, U.; Wangler, B.; Wangler, C. Rational Design, Development, and Stability Assessment of a Macrocyclic Four-Hydroxamate-Bearing Bifunctional Chelating Agent for 89Zr. ChemMedChem 2017, 12, 1555–1571. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.; Zhang, Z.; Dias, G.M.; Zhang, C.; Colpo, N.; Benard, F.; Lin, K.S. Design, synthesis and evaluation of novel bifunctional tetrahydroxamate chelators for PET imaging of 89Zr-labeled antibodies. Bioorg. Med. Chem. Lett. 2017, 27, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Holland, J.P.; Kenton, N.; Rotile, N.; Caravan, P. Macrocycle-Based Hydroxamate Ligands for Complexation and Immunoconjugation of 89Zirconium for Positron Emission Tomography (PET) Imaging. Chempluschem 2016, 81, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Buchwalder, C.; Rodriguez-Rodriguez, C.; Schaffer, P.; Karagiozov, S.K.; Saatchi, K.; Hafeli, U.O. A new tetrapodal 3-hydroxy-4-pyridinone ligand for complexation of 89Zirconium for positron emission tomography (PET) imaging. Dalton Trans. 2017, 46, 9654–9663. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Wilson, J.J.; Boros, E. Multifunctional Desferrichrome Analogues as Versatile 89Zr(IV) Chelators for ImmunoPET Probe Development. Mol. Pharm. 2017, 14, 2831–2842. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Bhatt, N.; Yuan, H.; Day, C.S.; Ehrmann, B.M.; Wright, M.; Bierbach, U.; Wadas, T.J. Zirconium tetraazamacrocycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem. Sci. 2017, 8, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Summer, D.; Rangger, C.; Franssen, G.; Laverman, P.; Haas, H.; Petrik, M.; Haubner, R.; Decristoforo, C. Novel bifunctional cyclic chelator for 89Zr labeling–radiolabeling and targeting properties of RGD conjugates. Mol. Pharm. 2015, 12, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Rangger, C.; Klingler, M.; Laverman, P.; Franssen, G.M.; Lechner, B.E.; Orasch, T.; Haas, H.; von Guggenberg, E.; Decristoforo, C. Exploiting the Concept of Multivalency with 68Ga- and 89Zr-Labelled Fusarinine C-Minigastrin Bioconjugates for Targeting CCK2R Expression. Contrast Media Mol. Imaging 2018, 2018, 3171794. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Garousi, J.; Oroujeni, M.; Mitran, B.; Andersson, K.G.; Vorobyeva, A.; Lofblom, J.; Orlova, A.; Tolmachev, V.; Decristoforo, C. Cyclic versus Noncyclic Chelating Scaffold for 89Zr-Labeled ZEGFR:2377 Affibody Bioconjugates Targeting Epidermal Growth Factor Receptor Overexpression. Mol. Pharm. 2018, 15, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Zhai, C.; Novy, Z.; Urbanek, L.; Haas, H.; Decristoforo, C. In vitro and in vivo comparison of selected Ga-68 and Zr-89 labelled siderophores. Mol. Imaging Biol. 2016, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
| 89Zr-Complex | Log D | Incubation Time (days) | Protein Binding (%) | Stability in Human Serum (%) |
|---|---|---|---|---|
| [89Zr]Zr-DFO | −3.0 ± 0.1 | 7 | 8.7 ± 1.0 | 99.3 |
| [89Zr]Zr-TAFC | −2.0 ± 0.0 | 7 | 6.8 ± 0.5 | 99.5 |
| [89Zr]Zr-FSC(succ)2AA | −3.3 ± 0.1 | 7 | 5.9 ± 1.6 | 99.4 |
| [89Zr]Zr-FSC(succ)3 | −3.5 ± 0.4 | 7 | 8.3 ± 3.5 | 99.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, C.; He, S.; Ye, Y.; Rangger, C.; Kaeopookum, P.; Summer, D.; Haas, H.; Kremser, L.; Lindner, H.; Foster, J.; et al. Rational Design, Synthesis and Preliminary Evaluation of Novel Fusarinine C-Based Chelators for Radiolabeling with Zirconium-89. Biomolecules 2019, 9, 91. https://doi.org/10.3390/biom9030091
Zhai C, He S, Ye Y, Rangger C, Kaeopookum P, Summer D, Haas H, Kremser L, Lindner H, Foster J, et al. Rational Design, Synthesis and Preliminary Evaluation of Novel Fusarinine C-Based Chelators for Radiolabeling with Zirconium-89. Biomolecules. 2019; 9(3):91. https://doi.org/10.3390/biom9030091
Chicago/Turabian StyleZhai, Chuangyan, Shanzhen He, Yunjie Ye, Christine Rangger, Piriya Kaeopookum, Dominik Summer, Hubertus Haas, Leopold Kremser, Herbert Lindner, Julie Foster, and et al. 2019. "Rational Design, Synthesis and Preliminary Evaluation of Novel Fusarinine C-Based Chelators for Radiolabeling with Zirconium-89" Biomolecules 9, no. 3: 91. https://doi.org/10.3390/biom9030091
APA StyleZhai, C., He, S., Ye, Y., Rangger, C., Kaeopookum, P., Summer, D., Haas, H., Kremser, L., Lindner, H., Foster, J., Sosabowski, J., & Decristoforo, C. (2019). Rational Design, Synthesis and Preliminary Evaluation of Novel Fusarinine C-Based Chelators for Radiolabeling with Zirconium-89. Biomolecules, 9(3), 91. https://doi.org/10.3390/biom9030091






