Conserved Glycines Control Disorder and Function in the Cold-Regulated Protein, COR15A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sequence Selection and Alignment
2.2. Plasmids and Cell Lines
2.3. Protein Production and Purification
2.3.1. Protein Production and Purification for Nuclear Magnetic Resonance Experiments
2.3.2. Protein Production and Purification for Fluorescence Spectroscopy and Circular Dichroism Experiments
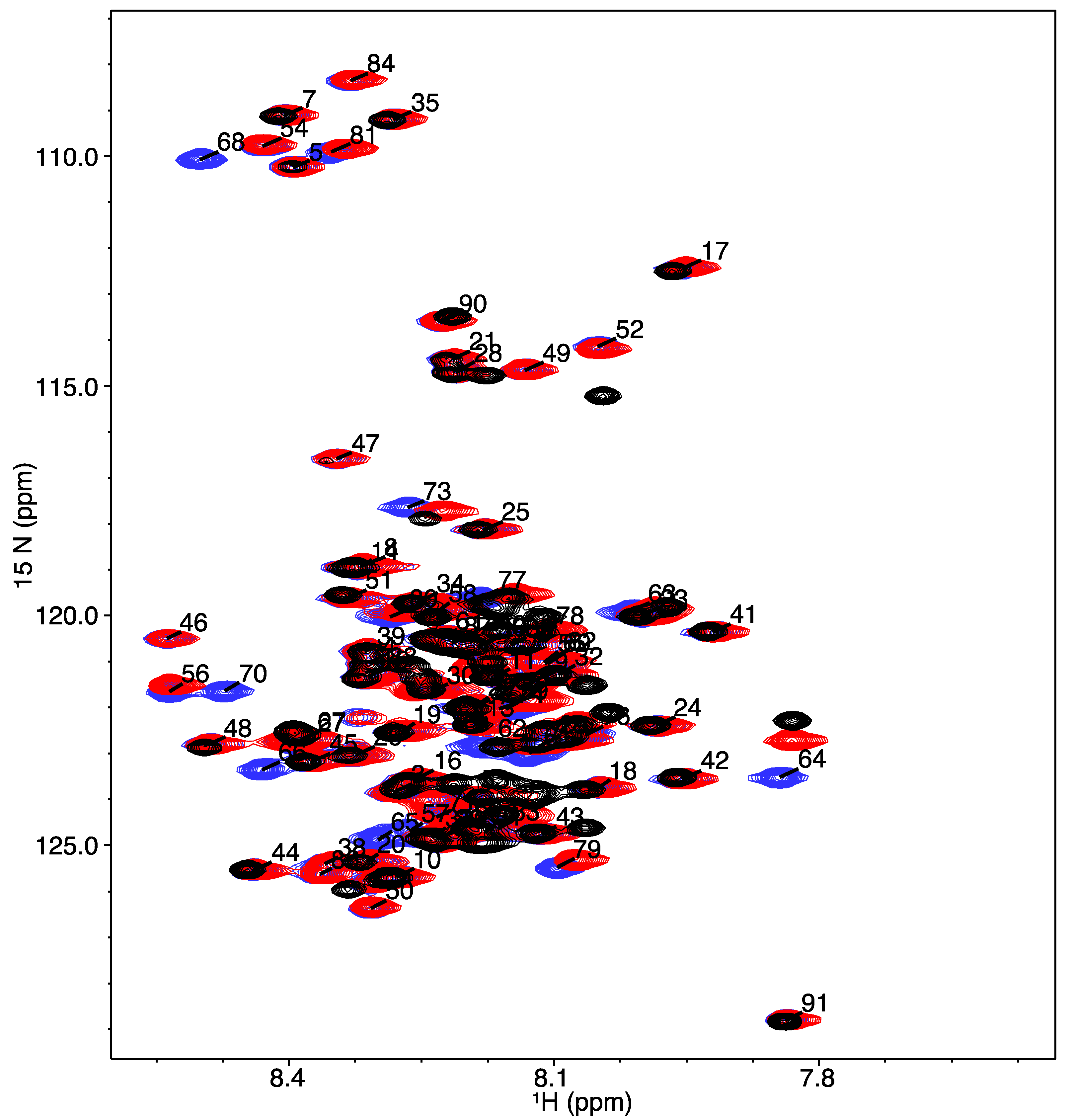
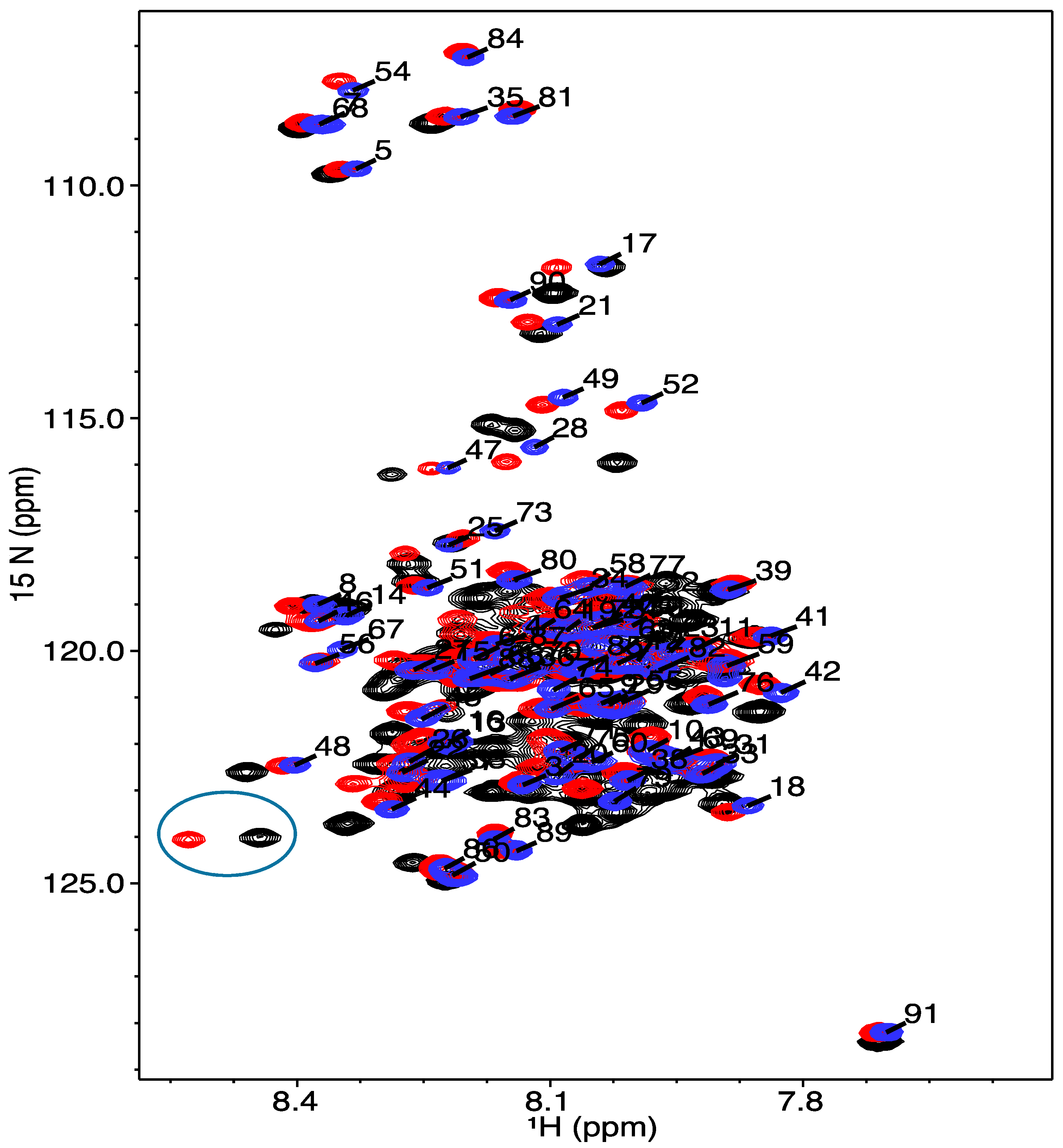
2.4. Nuclear Magnetic Resonance Spectroscopy
2.5. Circular Dichroism Spectroscopy
2.6. Carboxy Fluorescein (CF) Leakage Assay
3. Results and Discussion
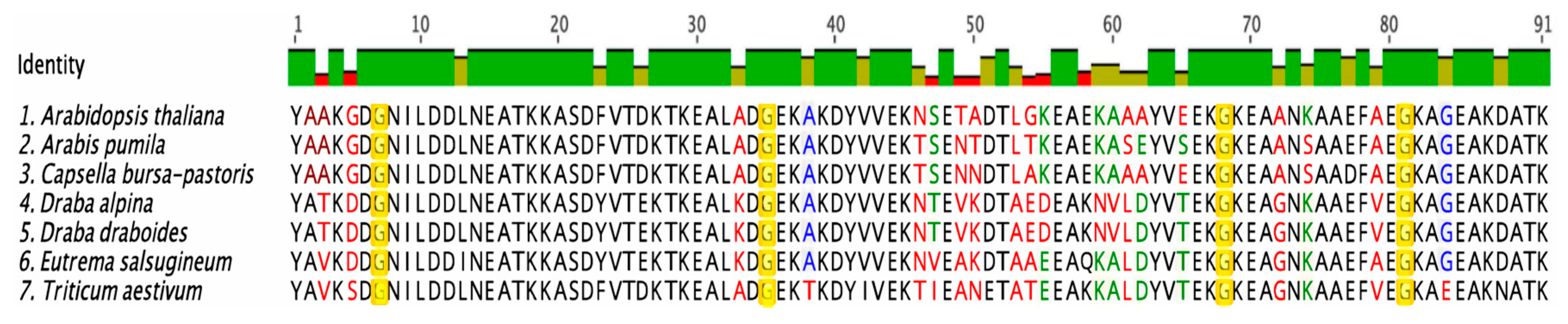
3.1. Sequence Alignments of COR15A
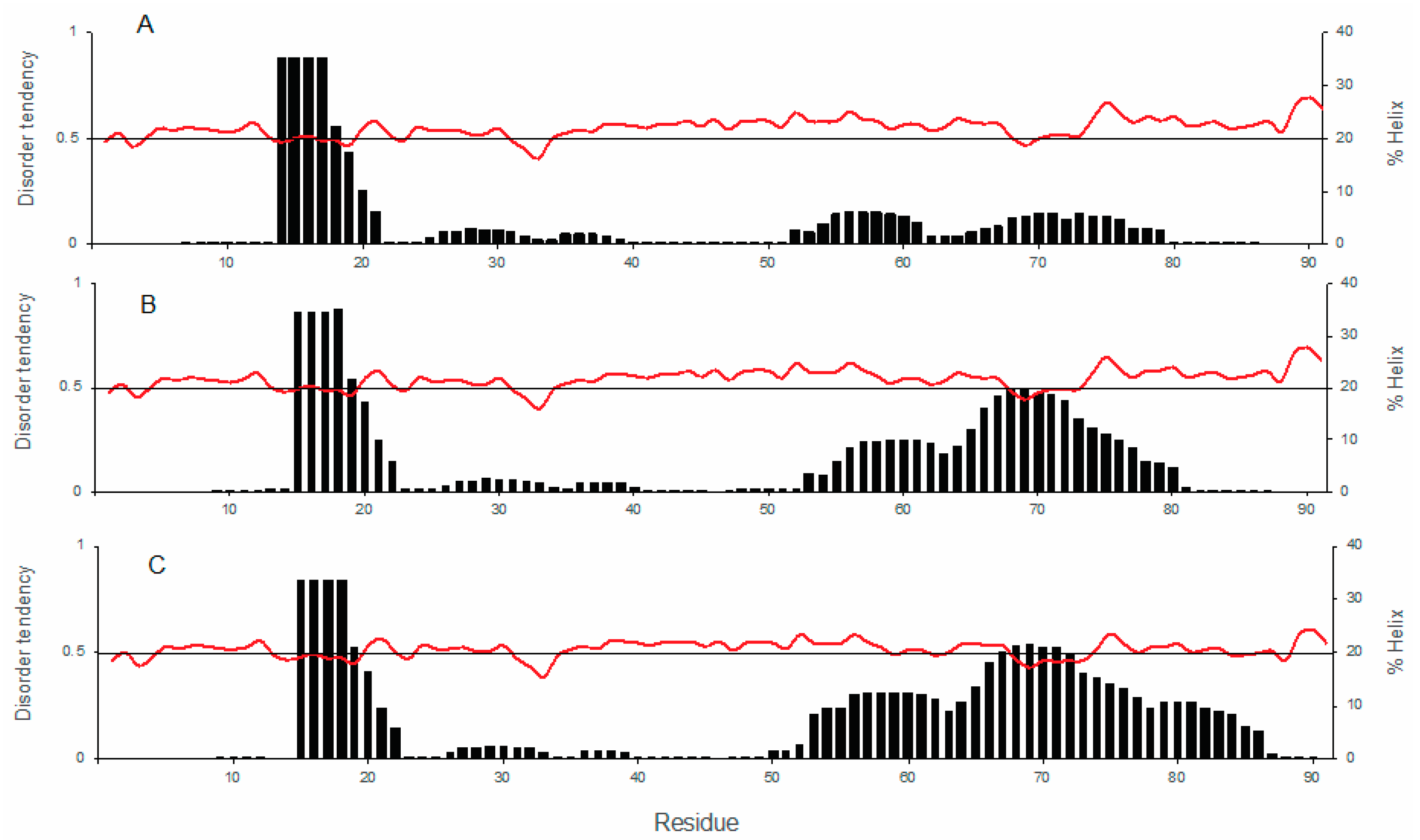
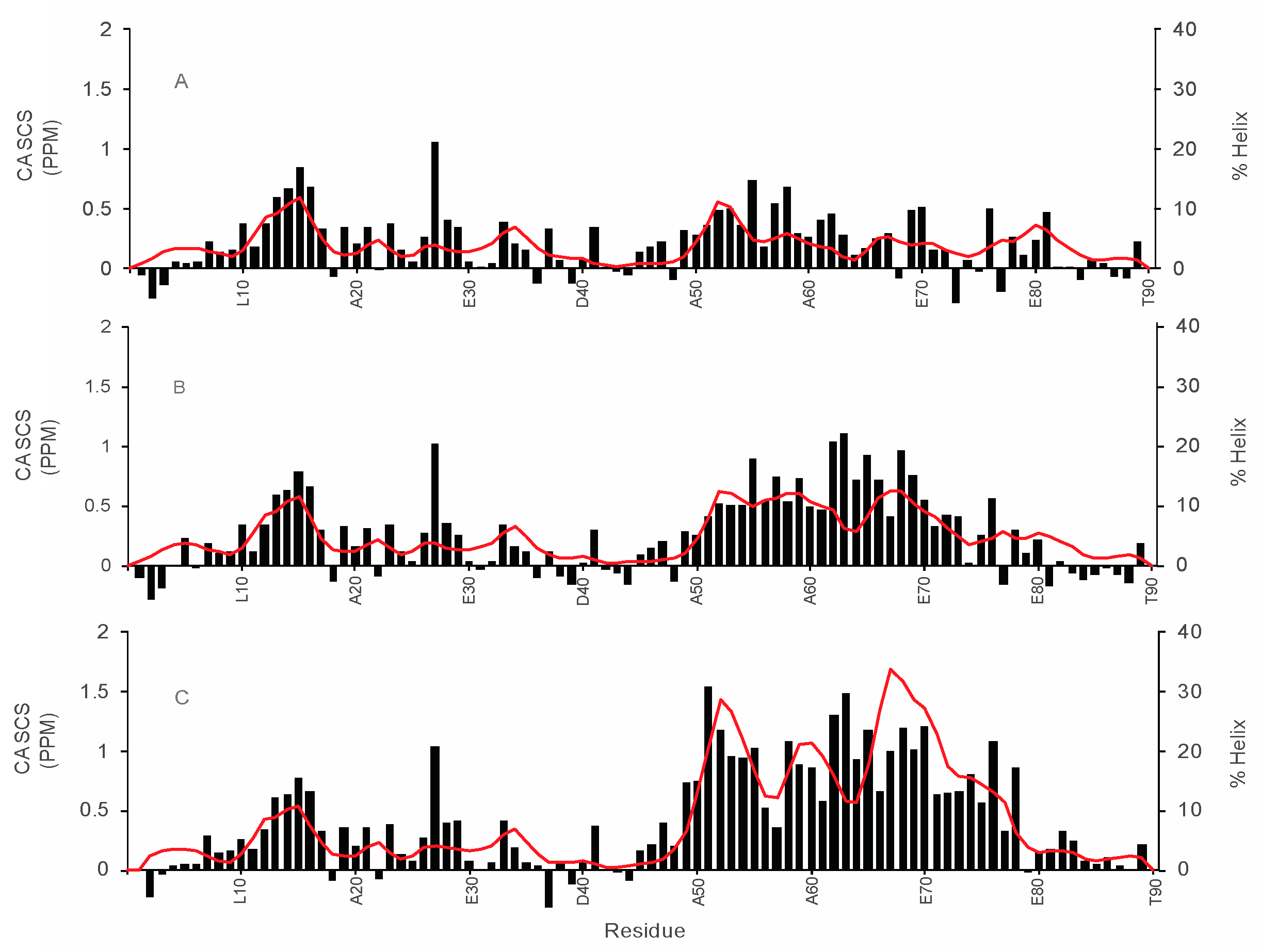
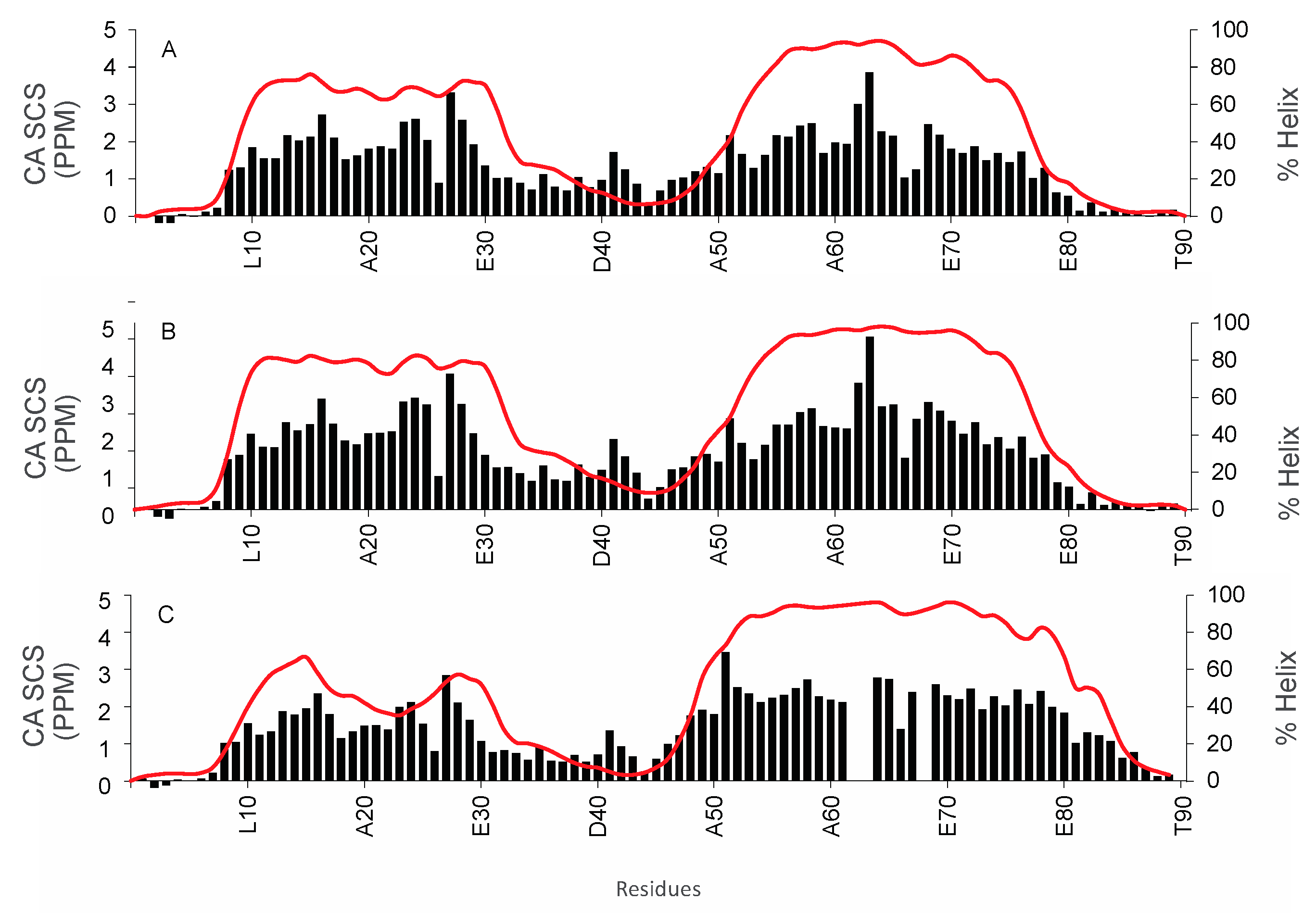
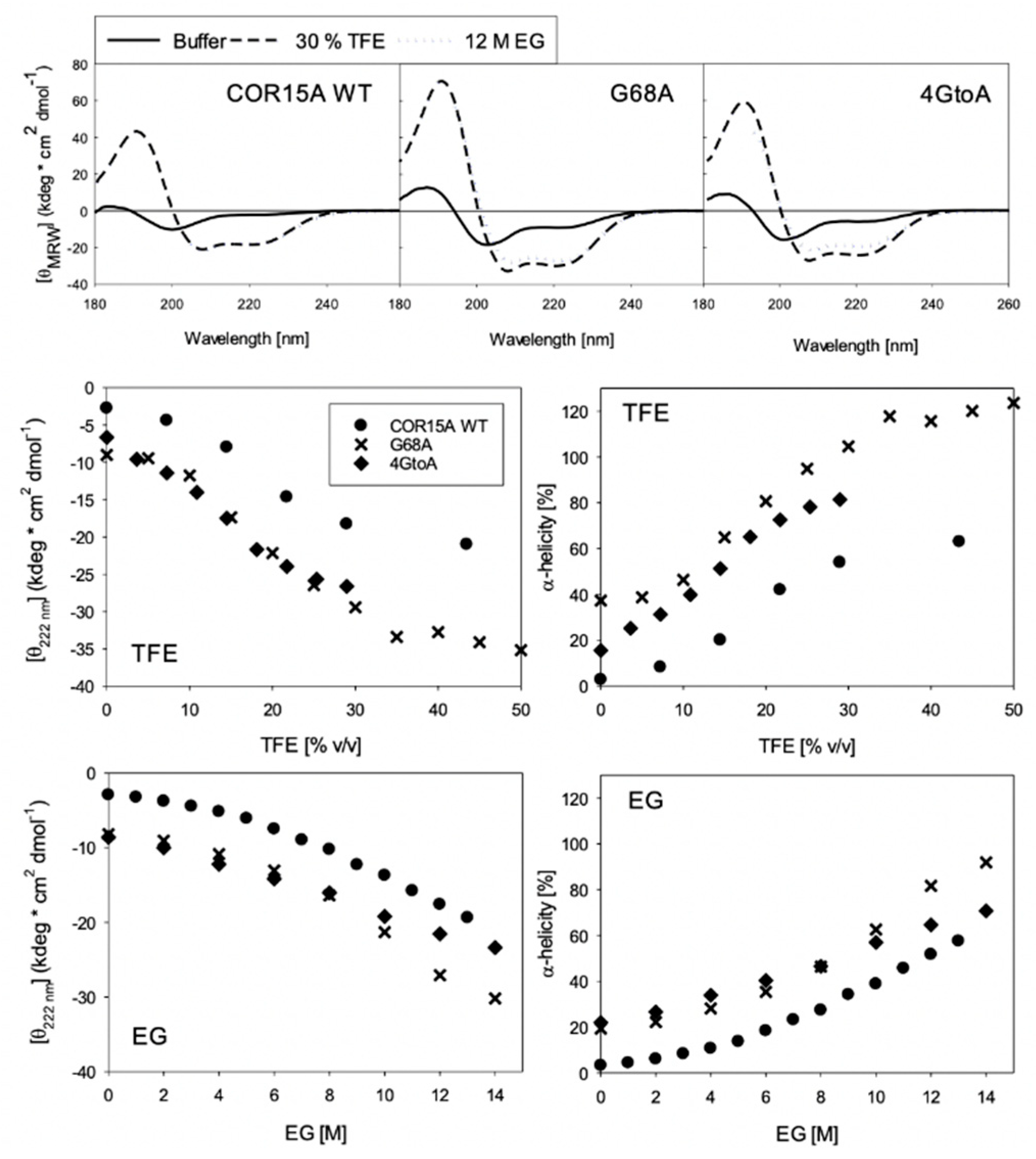
3.2. Structural Characterization of COR15A
3.3. Functional Characterization of COR15A
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uversky, V.N. Intrinsically disordered proteins and their environment: Effects of strong denaturants, temperature, pH, counter ions, membranes, binding partners, osmolytes, and macromolecular crowding. Protein J. 2009, 28, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Dunker, A.K. Understanding protein non-folding. Biochim. Biophys. Acta 2010, 1804, 1231–1264. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Obradovic, Z.; Romero, P.; Garner, E.C.; Brown, C.J. Intrinsic protein disorder in complete genomes. Genome Inform. 2000, 11, 161–171. [Google Scholar] [PubMed]
- Brown, C.J.; Johnson, A.K.; Dunker, A.K.; Daughdrill, G.W. Evolution and disorder. Curr. Opin. Struct. Biol. 2011, 21, 441–446. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Covarrubias, A.A. Late Embryogenesis abundant (LEA) proteins in legumes. Front. Plant Sci. 2013, 4, 190. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 2008, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Hincha, D.K.; Thalhammer, A. LEA proteins: IDPs with versatile functions in cellular dehydration tolerance. Biochem. Soc. Trans. 2012, 40, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Bies-Etheve, N.; Gaubier-Comella, P.; Debures, A.; Lasserre, E.; Jobet, E.; Raynal, M.; Cooke, R.; Delseny, M. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Mol. Biol. 2008, 67, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Retamal, C.; Bremer, A.; Alzate-Morales, J.; Caballero, J.; Hincha, D.K.; Gonzalez, W.; Thalhammer, A. Molecular dynamics simulations and CD spectroscopy reveal hydration-induced unfolding of the intrinsically disordered LEA proteins COR15A and COR15B from Arabidopsis thaliana. Phys. Chem. Chem. Phys. 2016, 18, 25806–25816. [Google Scholar] [CrossRef] [PubMed]
- Thalhammer, A.; Hundertmark, M.; Popova, A.V.; Seckler, R.; Hincha, D.K. Interaction of two intrinsically disordered plant stress proteins (COR15A and COR15B) with lipid membranes in the dry state. Biochim. Biophys. Acta 2010, 1798, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.; Kent, B.; Hauss, T.; Thalhammer, A.; Yepuri, N.R.; Darwish, T.A.; Garvey, C.J.; Bryant, G.; Hincha, D.K. Intrinsically disordered stress protein COR15A resides at the membrane surface during dehydration. Biophys. J. 2017, 113, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Artus, N.N.; Uemura, M.; Steponkus, P.L.; Gilmour, S.J.; Lin, C.; Thomashow, M.F. Constitutive expression of the cold-regulated arabidopsis thaliana COR15A gene affects both chloroplast and protoplast freezing tolerance. Proc. Natl. Acad. Sci. USA 1996, 93, 13404–13409. [Google Scholar] [CrossRef] [PubMed]
- Thalhammer, A.; Bryant, G.; Sulpice, R.; Hincha, D.K. Disordered cold regulated 15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol. 2014, 166, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Thomashow, M.F. A cold-regulated arabidopsis gene encodes a polypeptide having potent cryoprotective activity. Biochem. Biophys. Res. Commun. 1992, 183, 1103–1108. [Google Scholar] [CrossRef]
- Nakayama, K.; Okawa, K.; Kakizaki, T.; Honma, T.; Itoh, H.; Inaba, T. Arabidopsis COR15am is a chloroplast stromal protein that has cryoprotective activity and forms oligomers. Plant Physiol. 2007, 144, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Okawa, K.; Kakizaki, T.; Inaba, T. Evaluation of the protective activities of a late embryogenesis abundant (LEA) related protein, COR15am, during various stresses in vitro. Biosci. Biotechnol. Biochem. 2008, 72, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.V.; Hundertmark, M.; Seckler, R.; Hincha, D.K. Structural transitions in the intrinsically disordered plant dehydration stress protein LEA7 upon drying are modulated by the presence of membranes. Biochim. Biophys. Acta 2011, 1808, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Steponkus, P.L.; Uemura, M.; Joseph, R.A.; Gilmour, S.J.; Thomashow, M.F. Mode of action of the COR15A gene on the freezing tolerance of arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1998, 95, 14570–14575. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Retamal, C.; Bremer, A.; Ingolfsson, H.I.; Alzate-Morales, J.; Caballero, J.; Thalhammer, A.; Gonzalez, W.; Hincha, D.K. Folding and lipid composition determine membrane interaction of the disordered protein COR15A. Biophys. J. 2018, 115, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Buck, M. Trifluoroethanol and colleagues: Cosolvents come of age. Recent studies with peptides and proteins. Q. Rev. Biophys. 1998, 31, 297–355. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, E.; Viguera, A.R.; Serrano, L. Elucidating the folding problem of alpha-helices: Local motifs, long-range electrostatics, ionic-strength dependence and prediction of NMR parameters. J. Mol. Biol. 1998, 284, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Munoz, V.; Serrano, L. Development of the multiple sequence approximation within the AGADIR model of alpha-helix formation: Comparison with Zimm-Bragg and Lifson-Roig formalisms. Biopolymers 1997, 41, 495–509. [Google Scholar] [CrossRef]
- Camilloni, C.; De Simone, A.; Vranken, W.F.; Vendruscolo, M. Determination of secondary structure populations in disordered states of proteins using nuclear magnetic resonance chemical shifts. Biochemistry 2012, 51, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Tamiola, K.; Acar, B.; Mulder, F.A. Sequence-specific random coil chemical shifts of intrinsically disordered proteins. J. Am. Chem. Soc. 2010, 132, 18000–18003. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Yang, J.T.; Martinez, H.M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry 1972, 11, 4120–4131. [Google Scholar] [CrossRef] [PubMed]
- Hincha, D.K. Release of two peripheral proteins from chloroplast thylakoid membranes in the presence of a hofmeister series of chaotropic anions. Arch. Biochem. Biophys. 1998, 358, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.E.; Hincha, D.K.; Crowe, L.M.; Crowe, J.H. Interactions of arbutin with dry and hydrated bilayers. Biochim. Biophys. Acta 1998, 1370, 87–97. [Google Scholar] [CrossRef]
- Provencher, S.W. Contin: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Eftink, M.R. Fluorescence techniques for studying protein structure. Methods Biochem. Anal. 1991, 35, 127–205. [Google Scholar] [PubMed]
- Hincha, D.K. Effects of calcium-induced aggregation on the physical stability of liposomes containing plant glycolipids. Biochim. Biophys. Acta 2003, 1611, 180–186. [Google Scholar] [CrossRef]
- Hincha, D.K.; Zuther, E.; Hellwege, E.M.; Heyer, A.G. Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology 2002, 12, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Coordinators, N.R. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Llano, J.; Campos, L.A.; Sancho, J. Alpha-helix stabilization by alanine relative to glycine: Roles of polar and apolar solvent exposures and of backbone entropy. Proteins 2006, 64, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Dosztanyi, Z. Prediction of protein disorder based on iupred. Protein Sci. 2018, 27, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Dosztanyi, Z.; Csizmok, V.; Tompa, P.; Simon, I. Iupred: Web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 2005, 21, 3433–3434. [Google Scholar] [CrossRef] [PubMed]
- Vise, P.D.; Baral, B.; Latos, A.J.; Daughdrill, G.W. NMR chemical shift and relaxation measurements provide evidence for the coupled folding and binding of the p53 transactivation domain. Nucl. Acids Res. 2005, 33, 2061–2077. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.; Wolff, M.; Thalhammer, A.; Hincha, D.K. Folding of intrinsically disordered plant LEA proteins is driven by glycerol-induced crowding and the presence of membranes. FEBS J. 2017, 284, 919–936. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, N.; Woody, R. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Bakaltcheva, I.; Schmitt, J.M.; Hincha, D.K. Time- and temperature-dependent solute loading of isolated thylakoids during freezing. Cryobiology 1992, 29, 607–615. [Google Scholar] [CrossRef]
- Contreras-Martos, S.; Nguyen, H.H.; Nguyen, P.N.; Hristozova, N.; Macossay-Castillo, M.; Kovacs, D.; Bekesi, A.; Oemig, J.S.; Maes, D.; Pauwels, K.; et al. Quantification of intrinsically disordered proteins: A problem not fully appreciated. Front. Mol. Biosci. 2018, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Colmenero-Flores, J.M.; Moreno, L.P.; Smith, C.E.; Covarrubias, A.A. Pvlea-18, a member of a new late-embryogenesis-abundant protein family that accumulates during water stress and in the growing regions of well-irrigated bean seedlings. Plant Physiol. 1999, 120, 93–104. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowemimo, O.T.; Knox-Brown, P.; Borcherds, W.; Rindfleisch, T.; Thalhammer, A.; Daughdrill, G.W. Conserved Glycines Control Disorder and Function in the Cold-Regulated Protein, COR15A. Biomolecules 2019, 9, 84. https://doi.org/10.3390/biom9030084
Sowemimo OT, Knox-Brown P, Borcherds W, Rindfleisch T, Thalhammer A, Daughdrill GW. Conserved Glycines Control Disorder and Function in the Cold-Regulated Protein, COR15A. Biomolecules. 2019; 9(3):84. https://doi.org/10.3390/biom9030084
Chicago/Turabian StyleSowemimo, Oluwakemi T., Patrick Knox-Brown, Wade Borcherds, Tobias Rindfleisch, Anja Thalhammer, and Gary W. Daughdrill. 2019. "Conserved Glycines Control Disorder and Function in the Cold-Regulated Protein, COR15A" Biomolecules 9, no. 3: 84. https://doi.org/10.3390/biom9030084
APA StyleSowemimo, O. T., Knox-Brown, P., Borcherds, W., Rindfleisch, T., Thalhammer, A., & Daughdrill, G. W. (2019). Conserved Glycines Control Disorder and Function in the Cold-Regulated Protein, COR15A. Biomolecules, 9(3), 84. https://doi.org/10.3390/biom9030084






