Expression of a Chromoplast-Specific Lycopene β-Cyclase Gene (CYC-B) Is Implicated in Carotenoid Accumulation and Coloration in the Loquat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
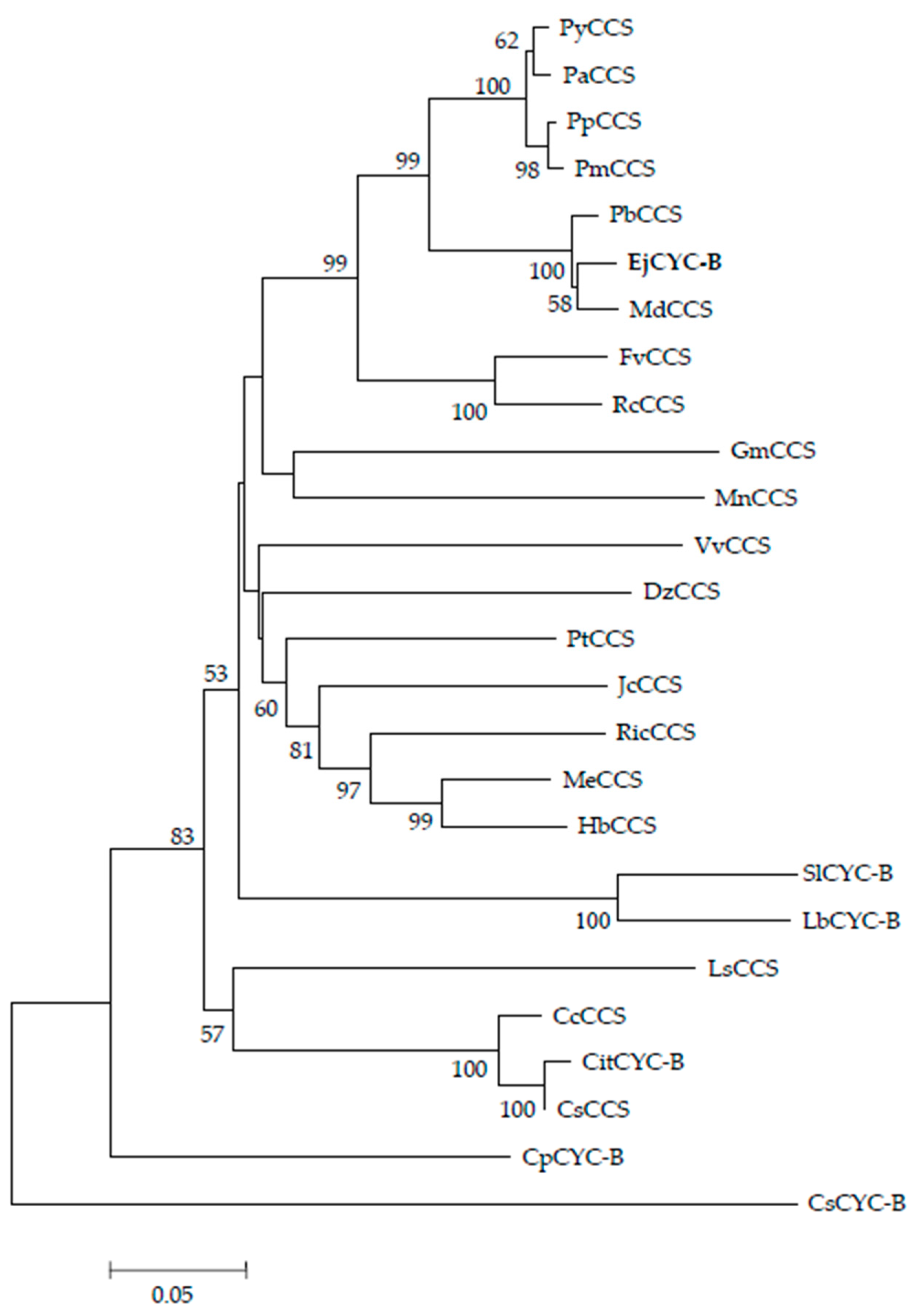
2.2. Identification of Loquat CYC-B Gene Full-Length cDNA and Phylogenetic Analysis
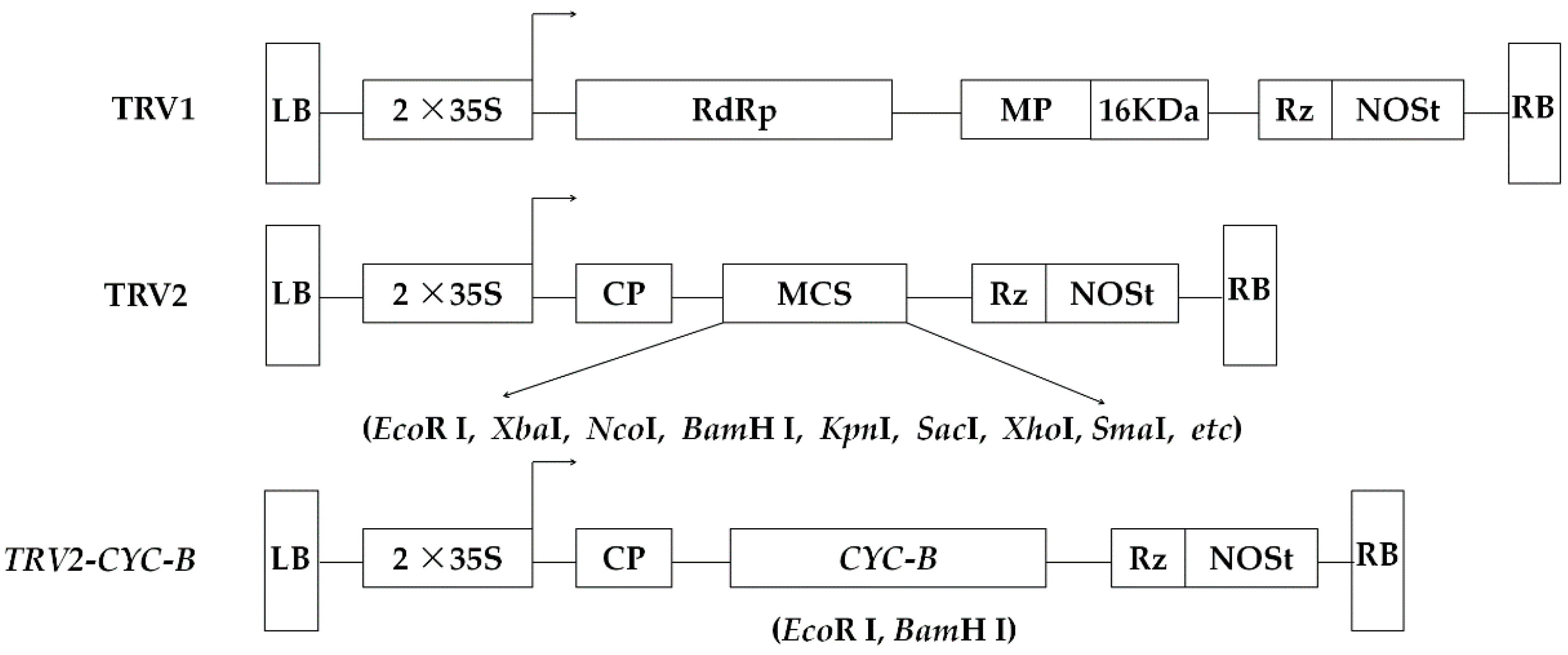
2.3. Plasmid Construction
2.4. Agrobacterium Transformation and Injection
2.5. Quantification of Carotenoid Content
2.6. Transcription Level Analysis
2.7. Statistical Analysis
3. Results
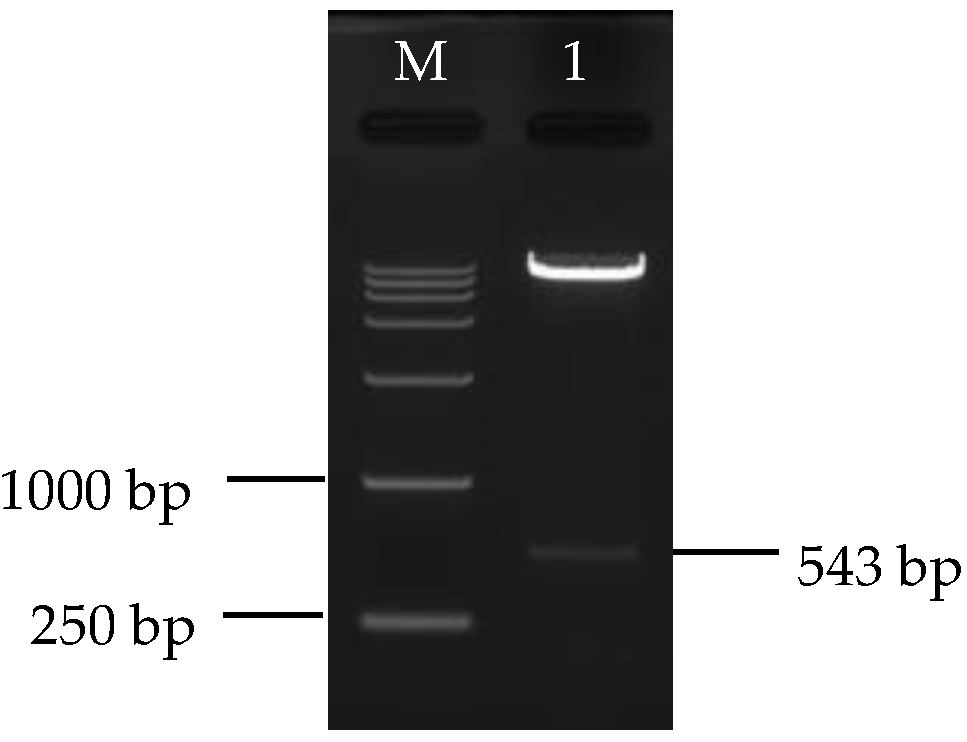
3.1. Isolation of Loquat CYC-B Gene
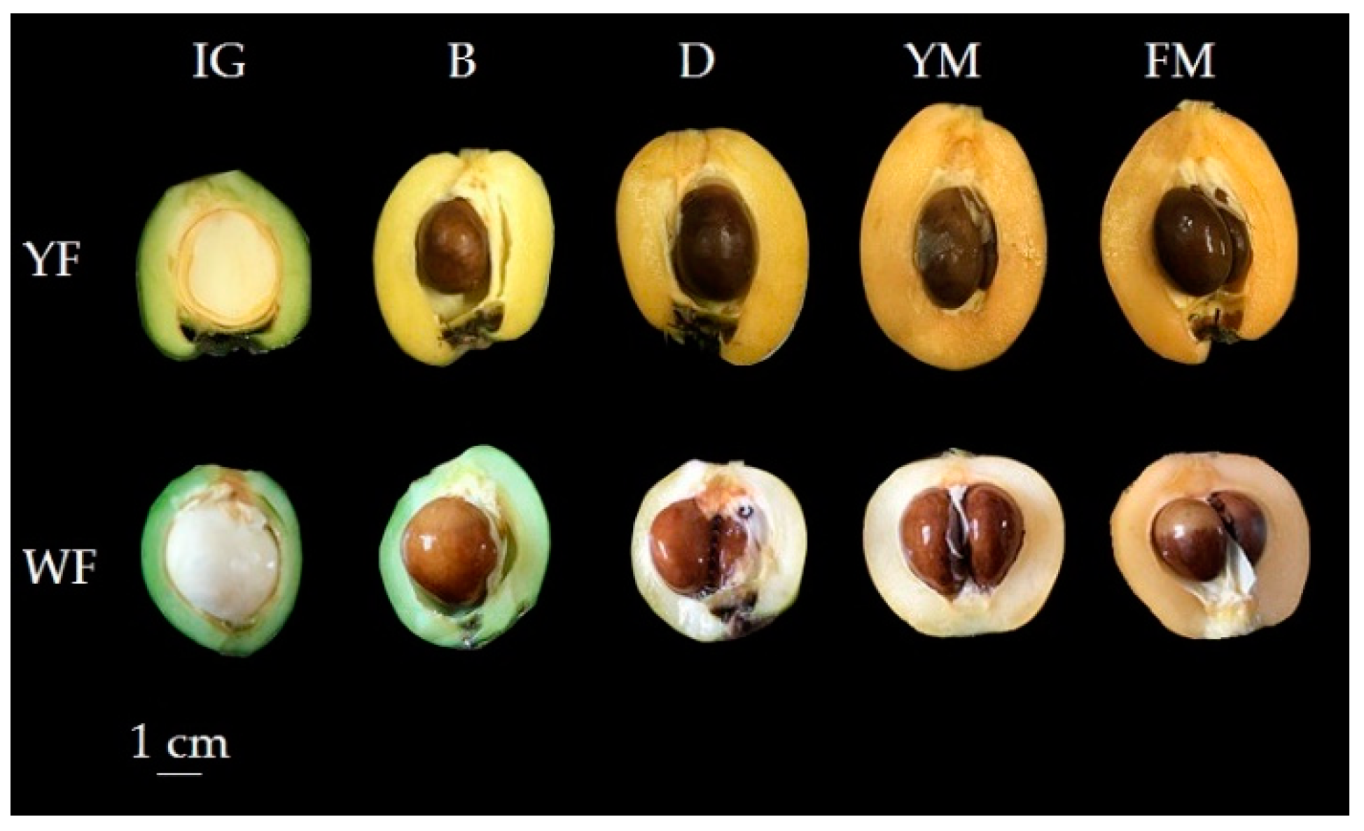
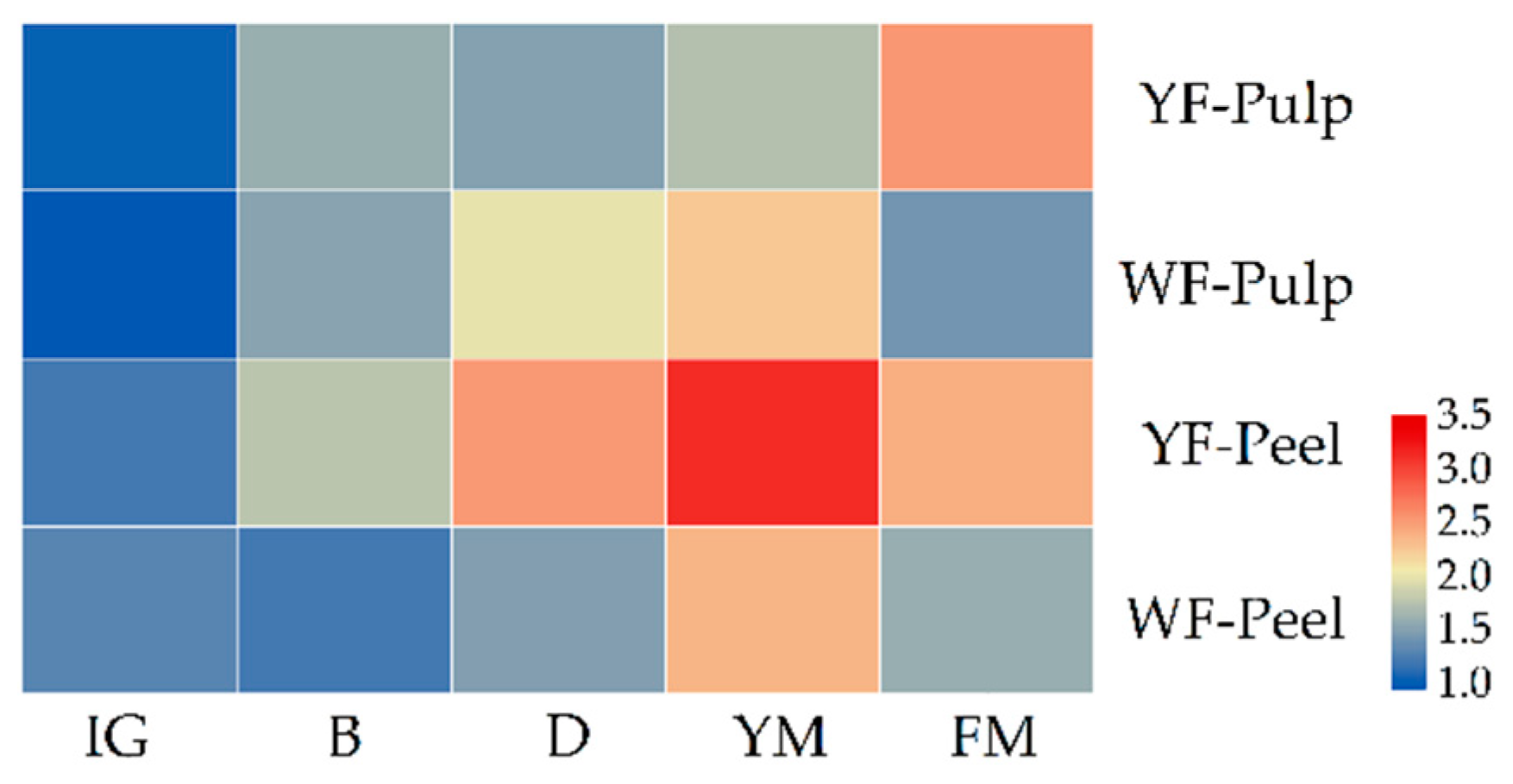
3.2. Differential Accumulation Patterns of Yellow and White-Fleshed Loquats
3.3. Construction and Identification of Silencing Vector
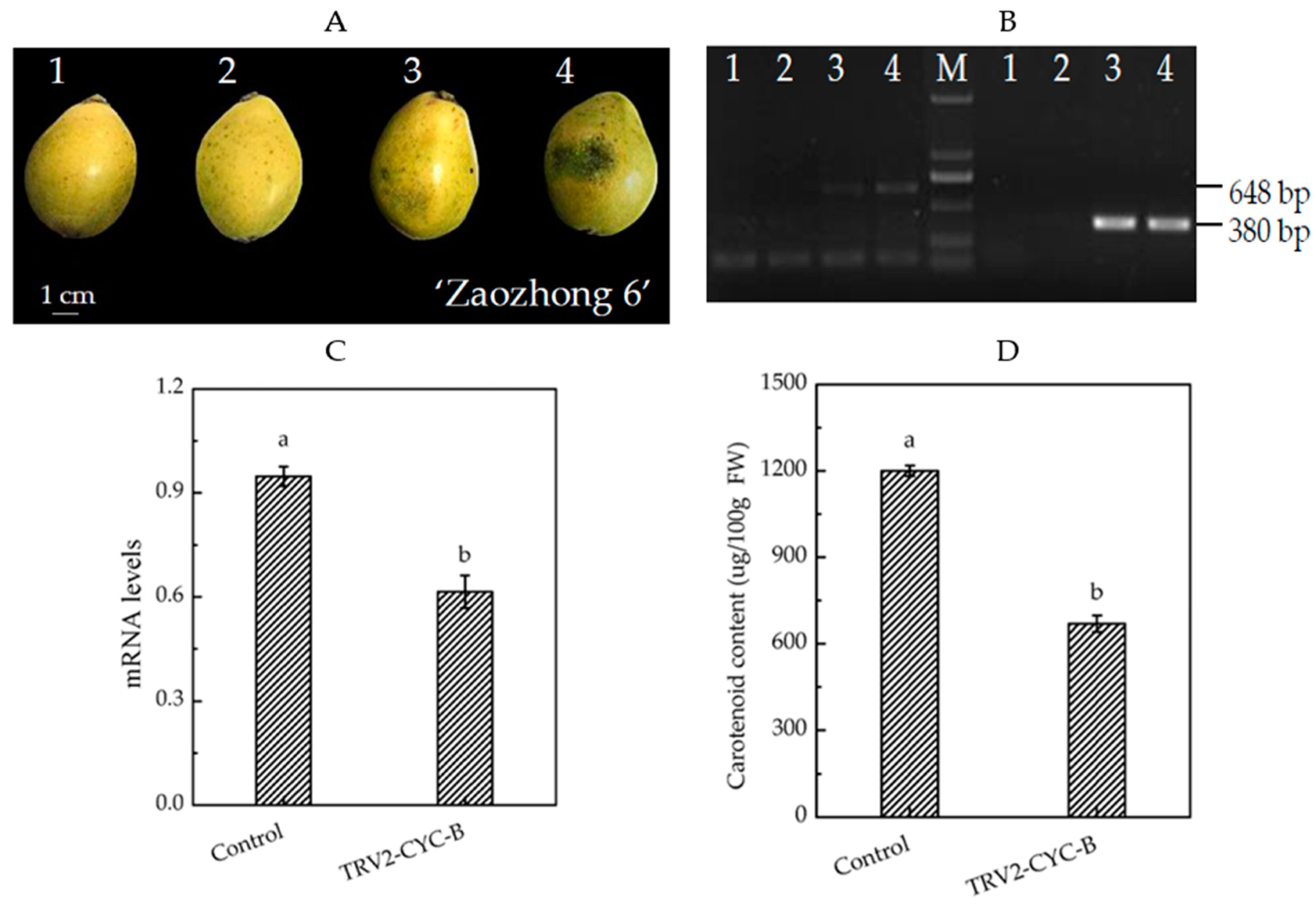
3.4. TRV-Mediated CYC-B Gene Silencing Decreased Carotenoid Accumulation of the Loquat
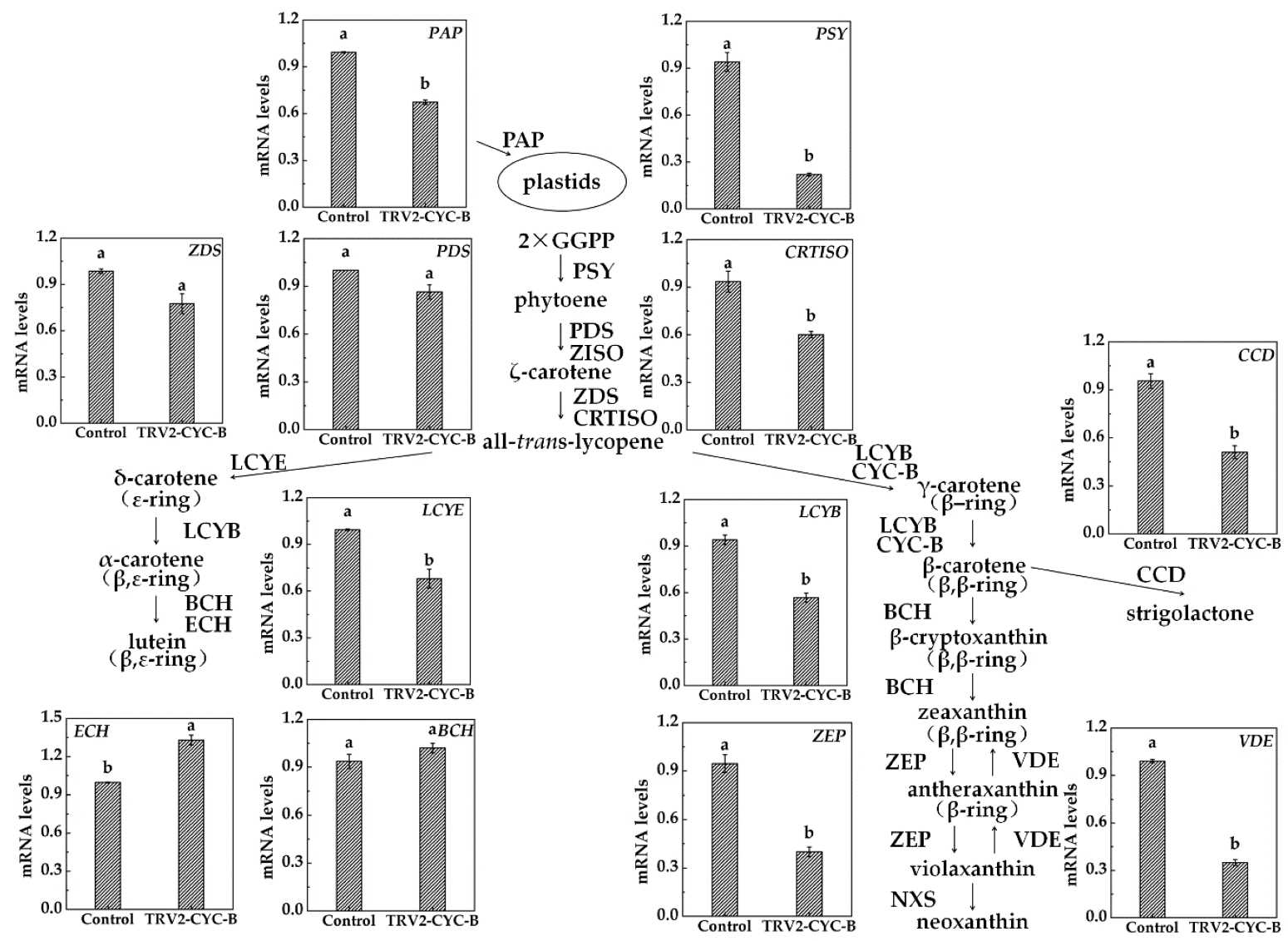
3.5. Expression Analysis of Carotenoid Metabolism Related Genes after TRV-CYC-B Silencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pareek, S.; Benkeblia, N.; Janick, J.; Cao, S.; Yahia, E.M. Postharvest physiology and technology of loquat (Eriobotrya japonica Lindl.) fruit. J. Sci. Food Agric. 2014, 94, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Xu, C.-J.; Sun, C.-D.; Li, X.; Chen, K.-S. Carotenoids in White- and Red-Fleshed Loquat Fruits. J. Agric. Food Chem. 2007, 55, 7822–7830. [Google Scholar] [CrossRef]
- Sadana, J.C. Carotenoids of loquat (Eriobotrya japonica Lindl.). Biochem. J. 1949, 44, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Kong, W.; Peng, G.; Zhou, J.; Azam, M.; Xu, W.; Grierson, D.; Chen, K. Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya Jpn.) fruits. J. Exp. Bot. 2011, 63, 341–354. [Google Scholar] [CrossRef]
- Armstrong, G.A.; Alberti, M.; Hearst, J.E. Conserved enzymes mediate the early reactions of carotenoid biosynthesis in nonphotosynthetic and photosynthetic prokaryotes. Proc. Natl. Acad. Sci. USA 1990, 87, 9975–9979. [Google Scholar] [CrossRef]
- Johnson, J.D. Do carotenoids serve as transmembrane radical channels? Free Radic. Biol. Med. 2009, 47, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, J. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant. Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Howitt, C.A.; Pogson, B.J. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006, 29, 435–445. [Google Scholar] [CrossRef]
- Moise, A.R.; Al-Babili, S.; Wurtzel, E.T. Mechanistic Aspects of Carotenoid Biosynthesis. Chem. Rev. 2013, 114, 164–193. [Google Scholar] [CrossRef]
- Liang, M.-H.; Zhu, J.; Jiang, J.-G. Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit. Rev. Food Sci. Nutr. 2017, 58, 2314–2333. [Google Scholar] [CrossRef]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bramley, P.M. Regulation of carotenoid formation during tomato fruit ripening and development. J. Exp. Bot. 2002, 53, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Koshiba, T. Complex regulation of ABA biosynthesis in plants. Trends. Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid. Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The Strigolactone Story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [PubMed]
- De Saint Germain, A.; Bonhomme, S.; Boyer, F.-D.; Rameau, C. Novel insights into strigolactone distribution and signalling. Curr. Opin. Plant Biol. 2013, 16, 583–589. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in Cardiovascular Health and Disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef]
- Alós, E.; Rodrigo, M.J.; Zacarias, L. Manipulation of Carotenoid Content in Plants to Improve Human Health. In Carotenoids in Nature; Springer: Cham, Switzerland, 2016; pp. 311–343. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Lu, P.; Wang, R.; Zhu, C.; Fu, X.; Wang, S.; Grierson, D.; Xu, C. Microscopic Analyses of Fruit Cell Plastid Development in Loquat (Eriobotrya japonica) during Fruit Ripening. Molecules 2019, 24, 448. [Google Scholar] [CrossRef]
- Colasuonno, P.; Lozito, M.L.; Marcotuli, I.; Nigro, D.; Giancaspro, A.; Mangini, G.; de Vita, P.; Mastrangelo, A.M.; Pecchioni, N.; Houston, K.; et al. The carotenoid biosynthetic and catabolic genes in wheat and their association with yellow pigments. BMC Genom. 2017, 18, 122–139. [Google Scholar] [CrossRef]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to beta -carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Kim, Y.; Han, J.; Nou, I.S. Orange color is associated with CYC-B expression in tomato fleshy fruit. Mol. Breed. 2016, 36, 42–51. [Google Scholar] [CrossRef]
- Blas, A.L.; Ming, R.; Liu, Z.; Veatch, O.J.; Paull, R.E.; Moore, P.H.; Yu, Q. Cloning of the Papaya Chromoplast-Specific Lycopene β-Cyclase, CpCYC-b, Controlling Fruit Flesh Color Reveals Conserved Microsynteny and a Recombination Hot Spot. Plant Physiol. 2010, 152, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Alquézar, B.; Zacarías, L.; Rodrigo, M.J. Molecular and functional characterization of a novel chromoplast-specific lycopene β-cyclase from Citrus and its relation to lycopene accumulation. J. Exp. Bot. 2009, 60, 1783–1797. [Google Scholar] [CrossRef]
- Ahrazem, O.; Rubio-Moraga, A.; Lopez, R.C.; Gomez-Gomez, L. The expression of a chromoplast-specific lycopene beta cyclase gene is involved in the high production of saffron’s apocarotenoid precursors. J. Exp. Bot. 2009, 61, 105–119. [Google Scholar] [CrossRef]
- Mohan, V.; Pandey, A.; Sreelakshmi, Y.; Sharma, R. Neofunctionalization of Chromoplast Specific Lycopene Beta Cyclase Gene (CYC-B) in Tomato Clade. PLoS ONE 2016, 11, 1–22. [Google Scholar] [CrossRef]
- Dalal, M.; Chinnusamy, V.; Bansal, K.C. Isolation and functional characterization of Lycopene beta-cyclase (CYC-B) promoter from Solanum habrochaites. BMC Plant Biol. 2010, 10, 61–75. [Google Scholar] [CrossRef]
- Sasaki, S.; Yamagishi, N.; Yoshikawa, N. Efficient virus-induced gene silencing in apple, pear and Japanese pear using Apple latent spherical virus vectors. Plant Methods 2011, 7, 15–25. [Google Scholar] [CrossRef]
- Bai, S.; Tuan, P.A.; Tatsuki, M.; Yaegaki, H.; Ohmiya, A.; Yamamizo, C.; Moriguchi, T. Knockdown of Carotenoid Cleavage Dioxygenase 4 (CCD4) via Virus-Induced Gene Silencing Confers Yellow Coloration in Peach Fruit: Evaluation of Gene Function Related to Fruit Traits. Plant Mol. Biol. Report. 2015, 34, 257–264. [Google Scholar] [CrossRef]
- Zhai, R.; Wang, Z.; Zhang, S.; Meng, G.; Song, L.; Wang, Z.; Li, P.; Ma, F.; Xu, L. Two MYB transcription factors regulate flavonoid biosynthesis in pear fruit (Pyrus bretschneideri Rehd.). J. Exp. Bot. 2015, 67, 1275–1284. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Tang, Y.-M.; Hu, Y.-Y.; Wang, Y.; Sun, B.; Wang, X.-R.; Tang, H.-R.; Chen, Q. FaTT12-1, a multidrug and toxin extrusion (MATE) member involved in proanthocyanidin transport in strawberry fruits. Sci. Hortic. 2018, 231, 158–165. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Asakura, Y.; Kurosaki, F. Cloning and Expression of Dcga Gene Encoding α Subunit of GTP-Binding Protein in Carrot Seedlings. Biol. Pharm. Bull. 2007, 30, 1800–1804. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Y.; Yan, Q.H.; Ming, F. An effective homologous cloning method for isolating novel miR172s from Phalaenopsis hybrida. Genet. Mol. Biol. 2014, 37, 414–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, A.M.; Li, F.X.; Chen, Y.Q.; Zong, P.; Qu, X.; Gong, D.-P.; Liu, G.S.; Sun, Y.H. Homology-based cloning and expression analysis of Rf genes encoding PPR-containing proteins in tobacco. Genet. Mol. Res. 2014, 13, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Hadjipieri, M.; Georgiadou, E.C.; Marin, A.; Diaz-Mula, H.M.; Goulas, V.; Fotopoulos, V.; Tomás-Barberán, F.A.; Manganaris, G.A. Metabolic and transcriptional elucidation of the carotenoid biosynthesis pathway in peel and flesh tissue of loquat fruit during on-tree development. BMC Plant Biol. 2017, 17, 102–113. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.-K.; Zheng, T.-T.; Wei, W.-L.; Zhu, Y.-M.; Gao, Y.-S.; Yang, X.-H.; Lin, S.-H. Characterization of Carotenoid Accumulation and Carotenogenic Gene Expression during Fruit Development in Yellow and White Loquat Fruit. Hortic. Plant J. 2016, 2, 9–15. [Google Scholar] [CrossRef]
- Leitner-Dagan, Y.; Ovadis, M.; Shklarman, E.; Elad, Y.; David, D.R.; Vainstein, A. Expression and Functional Analyses of the Plastid Lipid-Associated Protein CHRC Suggest Its Role in Chromoplastogenesis and Stress. Plant Physiol. 2006, 142, 233–244. [Google Scholar] [CrossRef]
- Ratcliff, F.; Martin-Hernandez, A.M.; Baulcombe, D.C. Technical Advance: Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 2008, 25, 237–245. [Google Scholar] [CrossRef]
- Fu, D.-Q.; Zhu, B.-Z.; Zhu, H.; Zhang, H.-X.; Xie, Y.-H.; Jiang, W.-B.; Zhao, X.-D.; Luo, K.-B. Enhancement of virus-induced gene silencing in tomato by low temperature and low humidity. Mol. Cells 2006, 21, 153–160. [Google Scholar]
- Senthil-Kumar, M.; Mysore, K.S. Virus-induced gene silencing can persist for more than 2 years and also be transmitted to progeny seedlings in Nicotiana benthamiana and tomato. Plant Biotechnol. J. 2011, 9, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Page, J.E. Optimized cDNA libraries for virus-induced gene silencing (VIGS) using tobacco rattle virus. Plant Methods 2008, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Donson, J.; della-Cioppa, G.; Harvey, D.; Hanley, K.; Grill, L. Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc. Natl. Acad. Sci. USA 1995, 92, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.B.; Eck, J.V.; Conlin, B.; Paolillo, D.; O’Neill, J.; Li, L. Effect of the cauliflower or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J. Exp. Bot. 2008, 59, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Welsch, R.; Yang, Y.; Alvarez, D.; Riediger, M.; Yuan, H.; Fish, T.; Liu, J.; Thannhauser, T.W.; Li, L. ArabidopsisOR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Castillon, A.; Shen, H.; Huq, E. Phytochrome Interacting Factors: Central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007, 12, 514–521. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Rodriguez-Concepcion, M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Chi, Z.-H.; Wang, Y.-Q.; Tang, Y.-M.; Deng, Q.-X.; He, M.-Y.; Wang, R.-K.; He, Y.-Z. Expression of a Chromoplast-Specific Lycopene β-Cyclase Gene (CYC-B) Is Implicated in Carotenoid Accumulation and Coloration in the Loquat. Biomolecules 2019, 9, 874. https://doi.org/10.3390/biom9120874
Hong M, Chi Z-H, Wang Y-Q, Tang Y-M, Deng Q-X, He M-Y, Wang R-K, He Y-Z. Expression of a Chromoplast-Specific Lycopene β-Cyclase Gene (CYC-B) Is Implicated in Carotenoid Accumulation and Coloration in the Loquat. Biomolecules. 2019; 9(12):874. https://doi.org/10.3390/biom9120874
Chicago/Turabian StyleHong, Min, Zhuo-Heng Chi, Yong-Qing Wang, Yue-Ming Tang, Qun-Xian Deng, Ming-Yang He, Ri-Kui Wang, and Yi-Zhong He. 2019. "Expression of a Chromoplast-Specific Lycopene β-Cyclase Gene (CYC-B) Is Implicated in Carotenoid Accumulation and Coloration in the Loquat" Biomolecules 9, no. 12: 874. https://doi.org/10.3390/biom9120874
APA StyleHong, M., Chi, Z.-H., Wang, Y.-Q., Tang, Y.-M., Deng, Q.-X., He, M.-Y., Wang, R.-K., & He, Y.-Z. (2019). Expression of a Chromoplast-Specific Lycopene β-Cyclase Gene (CYC-B) Is Implicated in Carotenoid Accumulation and Coloration in the Loquat. Biomolecules, 9(12), 874. https://doi.org/10.3390/biom9120874




