Critical Factors in Human Antizymes that Determine the Differential Binding, Inhibition, and Degradation of Human Ornithine Decarboxylase
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Recombinant ODC and AZ
2.2. Site-Directed Mutagenesis
2.3. Assay of ODC Activity in the Presence of AZ
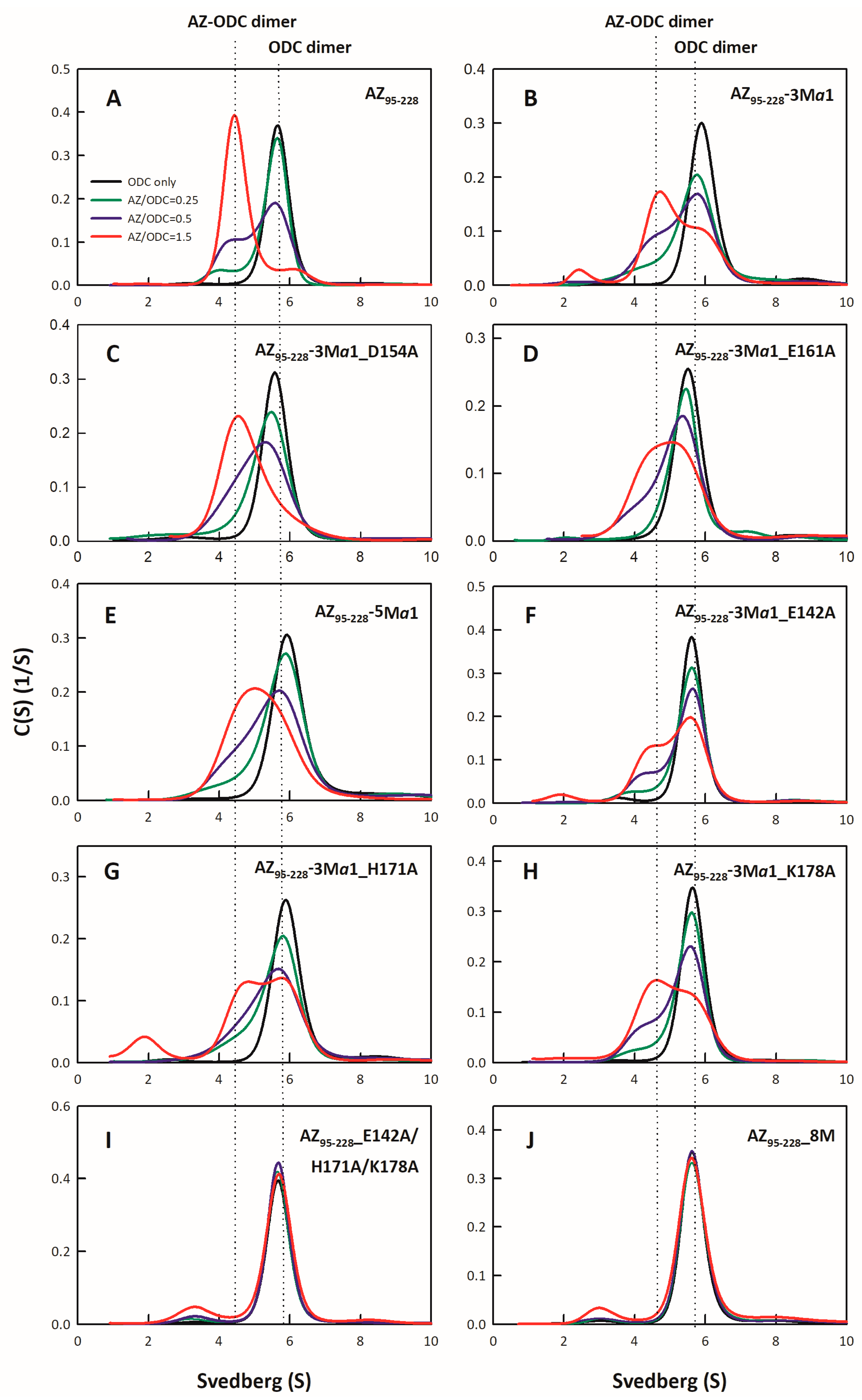
2.4. Analysis of Size Distributions of the AZ-ODC Heterodimer by Analytical Ultracentrifugation
2.5. In Vitro Degradation Assay
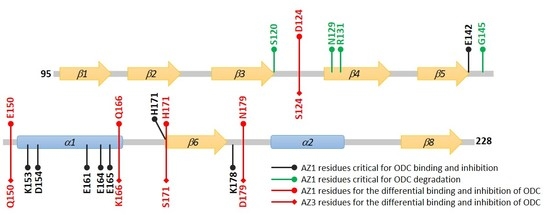
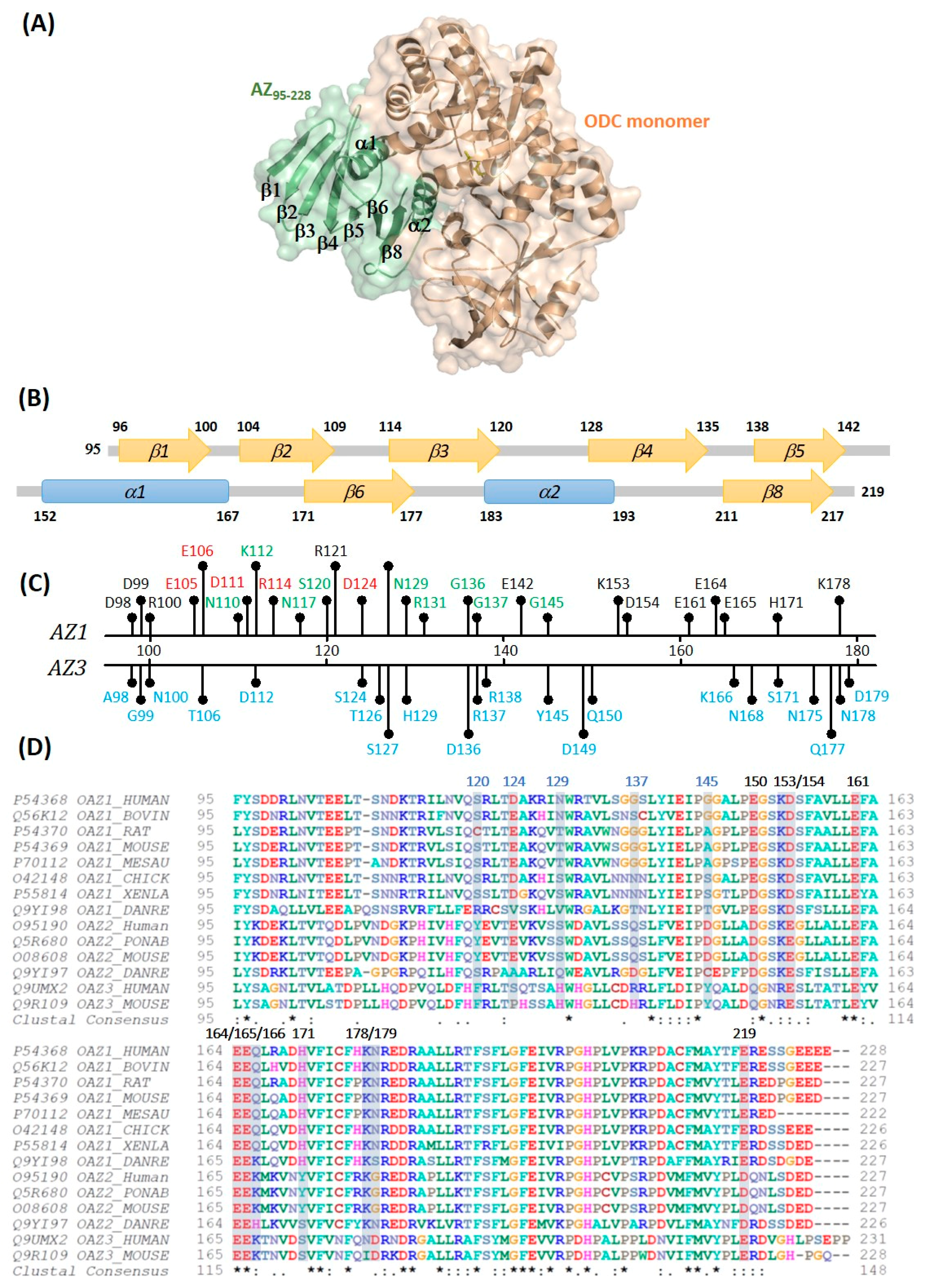
3. Results and Discussion
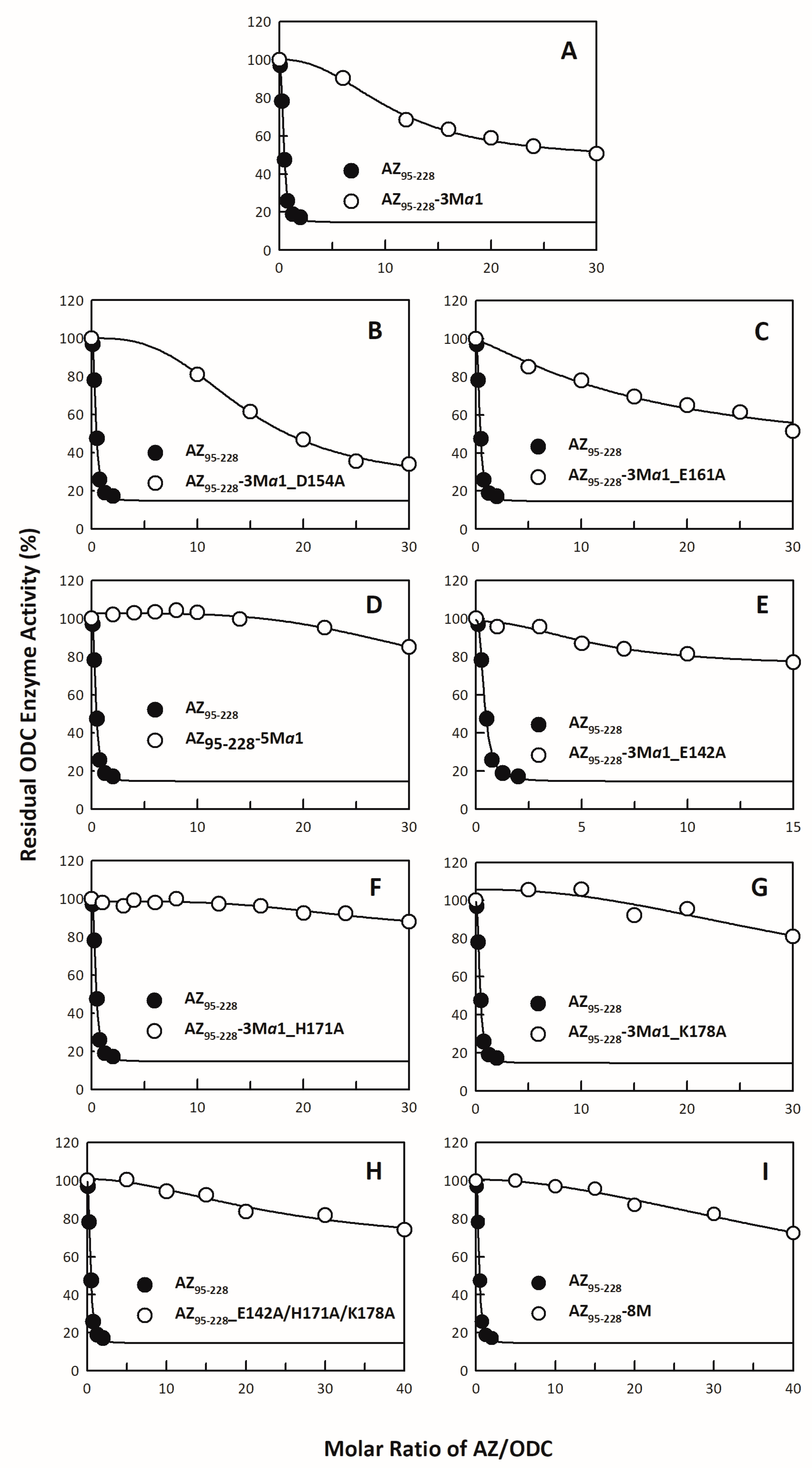
3.1. Inhibitory Effect of the AZ95–228 Mutants on ODC
3.2. Binding Affinity of the AZ95–228 Mutants toward ODC
3.3. Identification the Essential Elements Determining the ODC-Degradative Activity of AZ
3.4. Identification of the Crucial Factors that Govern the Differential Binding and Inhibition of AZ Isoforms toward ODC
3.5. Structural Elements of AZ Responsible for Binding, Inhibition, and Degradation of ODC
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kahana, C. The Antizyme family for regulating polyamines. J. Biol. Chem. 2018, 293, 18730–18735. [Google Scholar] [CrossRef] [PubMed]
- Coffino, P. Regulation of cellular polyamines by antizyme. Nat. Rev. Mol Cell Biol 2001, 2, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Regulation of Ornithine Decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef] [PubMed]
- Bercovich, Z.; Snapir, Z.; Keren-Paz, A.; Kahana, C. Antizyme affects cell proliferation and viability solely through regulating cellular polyamines. J. Biol. Chem. 2011, 286, 33778–33783. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, M.; Paasinen, A.; Andersson, L.C.; Holtta, E. Ornithine decarboxylase activity is critical for cell transformation. Nature 1992, 360, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Moshier, J.A.; Dosescu, J.; Skunca, M.; Luk, G.D. Transformation of NIH/3T3 Cells by Ornithine Decarboxylase Overexpression. Cancer Res. 1993, 53, 2618–2622. [Google Scholar] [PubMed]
- Gerner, E.W.; Meyskens, F.L. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Bercovich, Z.; Rosenberg-Hasson, Y.; Ciechanover, A.; Kahana, C. Degradation of ornithine decarboxylase in reticulocyte lysate is ATP-dependent but ubiquitin-independent. J. Biol. Chem. 1989, 264, 15949–15952. [Google Scholar]
- Ichiba, T.; Matsufuji, S.; Miyazaki, Y.; Murakami, Y.; Tanaka, K.; Ichihara, A.; Hayashi, S. Functional Regions of Ornithine Decarboxylase Antizyme. Biochem. Biophys. Res. Commun. 1994, 200, 1721–1727. [Google Scholar] [CrossRef]
- Liu, G.Y.; Liao, Y.F.; Hsu, P.C.; Chang, W.H.; Hsieh, M.C.; Lin, C.Y.; Hour, T.C.; Kao, M.C.; Tsay, G.J.; Hung, H.C. Antizyme, a natural ornithine decarboxylase inhibitor, induces apoptosis of haematopoietic cells through mitochondrial membrane depolarization and caspases’ cascade. Apoptosis 2006, 11, 1773–1788. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, P.C.; Hung, Y.C.; Liao, Y.F.; Liu, C.C.; Hour, C.T.; Kao, M.C.; Tsay, G.J.; Hung, H.C.; Liu, G.Y. Ornithine decarboxylase prevents methotrexate-induced apoptosis by reducing intracellular reactive oxygen species production. Apoptosis 2005, 10, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Hour, T.C.; Liao, Y.F.; Hung, Y.C.; Liu, C.C.; Chang, W.H.; Kao, M.C.; Tsay, G.J.; Hung, H.C.; Liu, G.Y. Increasing ornithine decarboxylase activity is another way of prolactin preventing methotrexate-induced apoptosis: Crosstalk between ODC and BCL-2. Apoptosis 2006, 11, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Matsufuji, S.; Kameji, T.; Hayashi, S.-i.; Igarashi, K.; Tamura, T.; Tanaka, K.; Ichihara, A. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature 1992, 360, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Coffino, P. Degradation of ornithine decarboxylase: Exposure of the C-terminal target by a polyamine-inducible inhibitory protein. Mol. Cell Biol. 1993, 13, 2377–2383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takeuchi, J.; Chen, H.; Hoyt, M.A.; Coffino, P. Structural elements of the ubiquitin-independent proteasome degron of ornithine decarboxylase. Biochem. J. 2008, 410, 401–407. [Google Scholar] [CrossRef]
- Coleman, C.S.; Stanley, B.A.; Viswanath, R.; Pegg, A.E. Rapid exchange of subunits of mammalian ornithine decarboxylase. J. Biol. Chem. 1994, 269, 3155–3158. [Google Scholar]
- Su, K.-L.; Liao, Y.-F.; Hung, H.-C.; Liu, G.-Y. Critical Factors Determining Dimerization of Human Antizyme Inhibitor. J. Biol. Chem. 2009, 284, 26768–26777. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Liu, Y.-L.; Su, J.-Y.; Liu, G.-Y.; Hung, H.-C. Critical Factors Governing the Difference in Antizyme-Binding Affinities between Human Ornithine Decarboxylase and Antizyme Inhibitor. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Olsen, R.R.; Zetter, B.R. Evidence of a Role for Antizyme and Antizyme Inhibitor as Regulators of Human Cancer. Mol. Cancer Res. 2011, 9, 1285–1293. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Hsu, D.-H.; Huang, C.-L.; Liu, Y.-L.; Liu, G.-Y.; Hung, H.-C. Determinants of the Differential Antizyme-Binding Affinity of Ornithine Decarboxylase. PLoS ONE 2011, 6, e26835. [Google Scholar] [CrossRef]
- Zhang, M.; Pickart, C.M.; Coffino, P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. Embo J. 2003, 22, 1488–1496. [Google Scholar] [CrossRef]
- Newman, R.M.; Mobascher, A.; Mangold, U.; Koike, C.; Diah, S.; Schmidt, M.; Finley, D.; Zetter, B.R. Antizyme targets cyclin D1 for degradation. A novel mechanism for cell growth repression. J. Biol Chem 2004, 279, 41504–41511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Lee, C.-Y.; Lin, C.-L.; Chen, H.-Y.; Liu, G.-Y.; Hung, H.-C.; Liu, Y.-C.; Lee, C.-Y.; Lin, C.-L.; Chen, H.-Y.; et al. Multifaceted interactions and regulation between antizyme and its interacting proteins cyclin D1, ornithine decarboxylase and antizyme inhibitor. Oncotarget 2015, 6, 23917–23929. [Google Scholar] [CrossRef] [PubMed]
- Erales, J.; Coffino, P. Ubiquitin-independent proteasomal degradation. Biochim. Et Biophys. Acta (Bba)-Mol. Cell Res. 2014, 1843, 216–221. [Google Scholar] [CrossRef]
- Lim, S.K.; Gopalan, G. Antizyme1 mediates AURKAIP1-dependent degradation of Aurora-A. Oncogene 2007, 26, 6593–6603. [Google Scholar] [CrossRef]
- Gruendler, C.; Lin, Y.; Farley, J.; Wang, T. Proteasomal Degradation of Smad1 Induced by Bone Morphogenetic Proteins. J. Biol. Chem. 2001, 276, 46533–46543. [Google Scholar] [CrossRef]
- Murakami, Y.; Matsufuji, S.; Miyazaki, Y.; Hayashi, S. Forced expression of antizyme abolishes ornithine decarboxylase activity, suppresses cellular levels of polyamines and inhibits cell growth. Biochem. J. 1994, 304, 183–187. [Google Scholar] [CrossRef]
- Beenukumar, R.R.; Gödderz, D.; Palanimurugan, R.; Dohmen, R.J. Polyamines directly promote antizyme-mediated degradation of ornithine decarboxylase by the proteasome. Microb. Cell 2015, 2, 197–207. [Google Scholar] [CrossRef]
- Iwata, S.; Sato, Y.; Asada, M.; Takagi, M.; Tsujimoto, A.; Inaba, T.; Yamada, T.; Sakamoto, S.; Yata, J.-i.; Shimogori, T.; et al. Anti-tumor activity of antizyme which targets the ornithine decarboxylase (ODC) required for cell growth and transformation. Oncogene 1999, 18, 165–172. [Google Scholar] [CrossRef]
- Fong, L.Y.Y.; Feith, D.J.; Pegg, A.E. Antizyme Overexpression in Transgenic Mice Reduces Cell Proliferation, Increases Apoptosis, and Reduces N-Nitrosomethylbenzylamine-induced Forestomach Carcinogenesis. Cancer Res. 2003, 63, 3945–3954. [Google Scholar]
- Feith, D.J.; Origanti, S.; Shoop, P.L.; Sass-Kuhn, S.; Shantz, L.M. Tumor suppressor activity of ODC antizyme in MEK-driven skin tumorigenesis. Carcinogenesis 2006, 27, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, S.; Matsufuji, T.; Miyazaki, Y.; Murakami, Y.; Atkins, J.F.; Gesteland, R.F.; Hayashi, S.-i. Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell 1995, 80, 51–60. [Google Scholar] [CrossRef]
- Rom, E.; Kahana, C. Polyamines regulate the expression of ornithine decarboxylase antizyme in vitro by inducing ribosomal frame-shifting. PNAS 1994, 91, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Palanimurugan, R.; Scheel, H.; Hofmann, K.; Jürgen Dohmen, R. Polyamines regulate their synthesis by inducing expression and blocking degradation of ODC antizyme. Embo. J. 2004, 23, 4857–4867. [Google Scholar] [CrossRef]
- Ivanov, I.P.; Gesteland, R.F.; Atkins, J.F. Antizyme expression: A subversion of triplet decoding, which is remarkably conserved by evolution, is a sensor for an autoregulatory circuit. Nucleic. Acids Res. 2000, 28, 3185–3196. [Google Scholar] [CrossRef]
- Kurian, L.; Palanimurugan, R.; Gödderz, D.; Dohmen, R.J. Polyamine sensing by nascent ornithine decarboxylase antizyme stimulates decoding of its mRNA. Nature 2011, 477, 490–494. [Google Scholar] [CrossRef]
- Hayashi, S.-i.; Murakami, Y.; Matsufuji, S. Ornithine decarboxylase antizyme: A novel type of regulatory protein. Trends Biochem. Sci. 1996, 21, 27–30. [Google Scholar] [CrossRef]
- Kahana, C. Protein degradation, the main hub in the regulation of cellular polyamines. Biochem. J. 2016, 473, 4551–4558. [Google Scholar] [CrossRef]
- Li, X.; Coffino, P. Distinct domains of antizyme required for binding and proteolysis of ornithine decarboxylase. Mol. Cell Biol. 1994, 14, 87–92. [Google Scholar] [CrossRef][Green Version]
- Hsieh, J.-Y.; Yang, J.-Y.; Lin, C.-L.; Liu, G.-Y.; Hung, H.-C. Minimal Antizyme Peptide Fully Functioning in the Binding and Inhibition of Ornithine Decarboxylase and Antizyme Inhibitor. PLoS ONE 2011, 6, e24366. [Google Scholar] [CrossRef][Green Version]
- Wu, H.-Y.; Chen, S.-F.; Hsieh, J.-Y.; Chou, F.; Wang, Y.-H.; Lin, W.-T.; Lee, P.-Y.; Yu, Y.-J.; Lin, L.-Y.; Lin, T.-S.; et al. Structural basis of antizyme-mediated regulation of polyamine homeostasis. PNAS 2015, 112, 11229–11234. [Google Scholar] [CrossRef]
- Ivanov, I.P.; Gesteland, R.F.; Atkins, J.F. A Second Mammalian Antizyme: Conservation of Programmed Ribosomal Frameshifting. Genomics 1998, 52, 119–129. [Google Scholar] [CrossRef]
- Zhu, C.; Lang, D.W.; Coffino, P. Antizyme2 Is a Negative Regulator of Ornithine Decarboxylase and Polyamine Transport. J. Biol. Chem. 1999, 274, 26425–26430. [Google Scholar] [CrossRef]
- Chen, H.; MacDonald, A.; Coffino, P. Structural Elements of Antizymes 1 and 2 Are Required for Proteasomal Degradation of Ornithine Decarboxylase. J. Biol. Chem. 2002, 277, 45957–45961. [Google Scholar] [CrossRef]
- Ivanov, I.P.; Rohrwasser, A.; Terreros, D.A.; Gesteland, R.F.; Atkins, J.F. Discovery of a spermatogenesis stage-specific ornithine decarboxylase antizyme: Antizyme 3. PNAS 2000, 97, 4808–4813. [Google Scholar] [CrossRef]
- Snapir, Z.; Keren-Paz, A.; Bercovich, Z.; Kahana, C. Antizyme 3 inhibits polyamine uptake and ornithine decarboxylase (ODC) activity, but does not stimulate ODC degradation. Biochem. J. 2009, 419, 99. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System; Version 2.0; Schrödinger, LLC: Manheim, Germany, 2004.
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Schuck, P.; Perugini, M.A.; Gonzales, N.R.; Howlett, G.J.; Schubert, D. Size-Distribution Analysis of Proteins by Analytical Ultracentrifugation: Strategies and Application to Model Systems. Biophys. J. 2002, 82, 1096–1111. [Google Scholar] [CrossRef]
- Schuck, P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 2003, 320, 104–124. [Google Scholar] [CrossRef]
- Brown, P.H.; Balbo, A.; Schuck, P. Characterizing Protein-Protein Interactions by Sedimentation Velocity Analytical Ultracentrifugation. Curr. Protoc. Immunol. 2008, 81, 18.15.11–18.15.39. [Google Scholar] [CrossRef]
- Dam, J.; Schuck, P. Sedimentation Velocity Analysis of Heterogeneous Protein-Protein Interactions: Sedimentation Coefficient Distributions c(s) and Asymptotic Boundary Profiles from Gilbert-Jenkins Theory. Biophys. J. 2005, 89, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
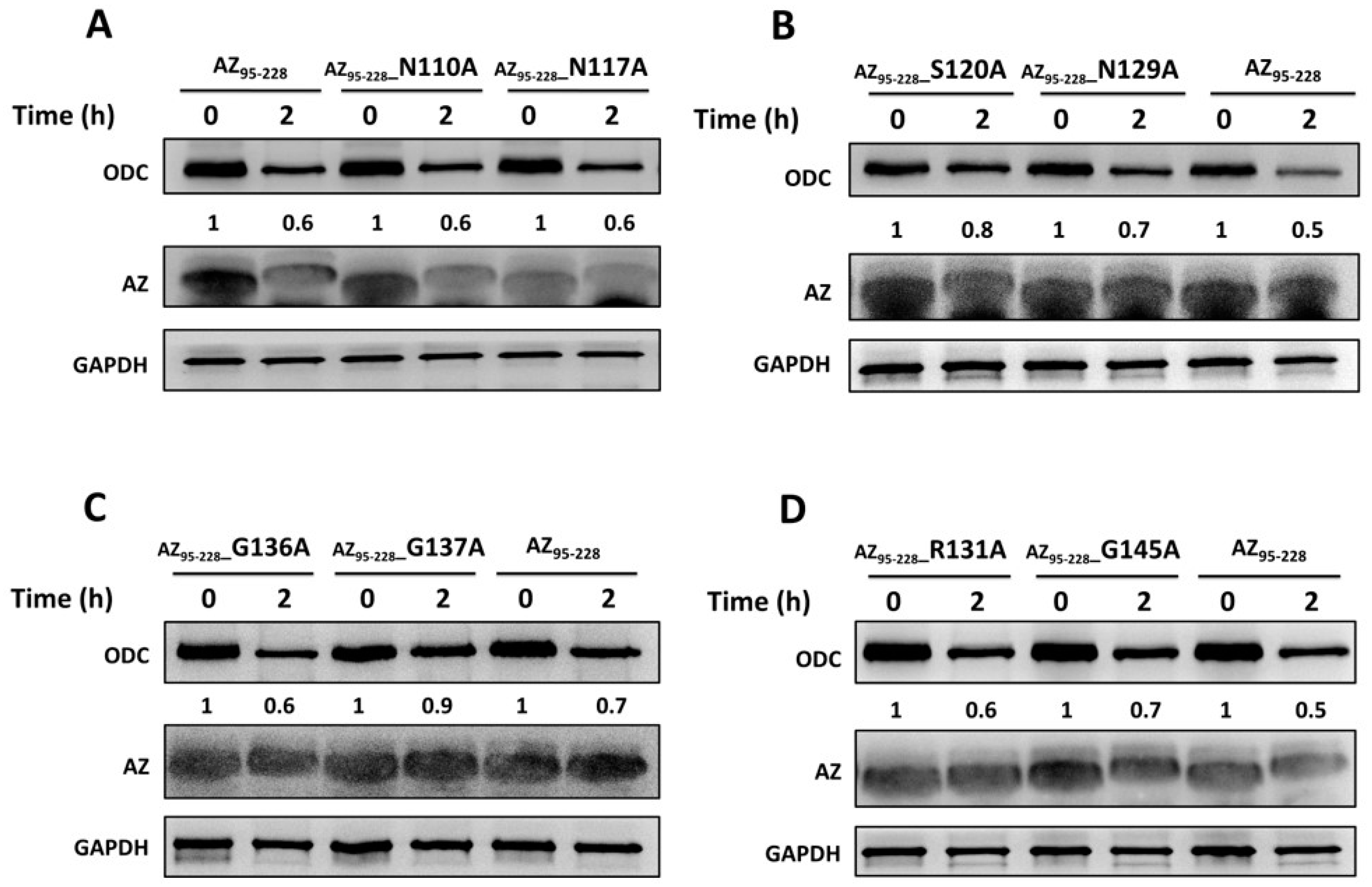
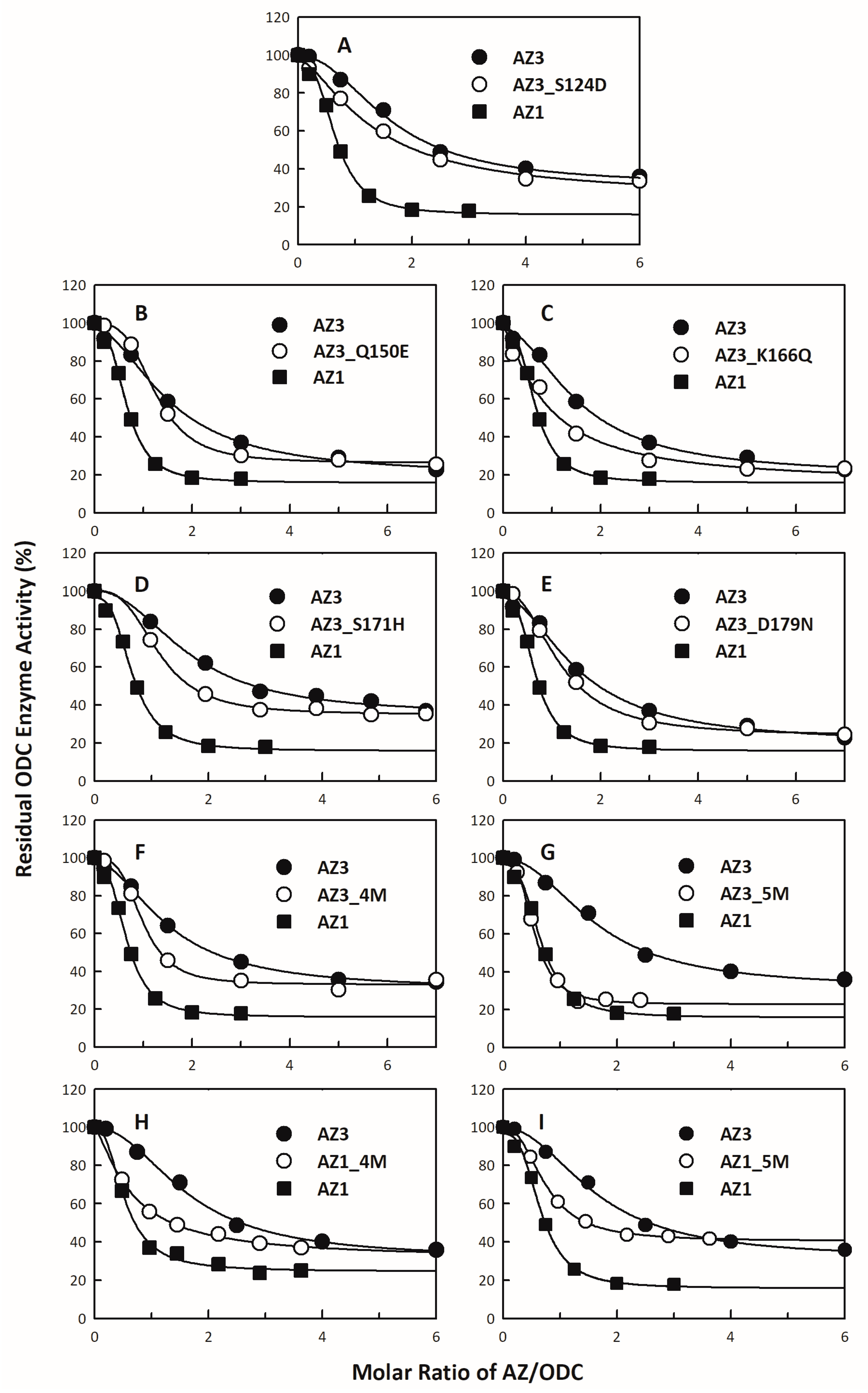
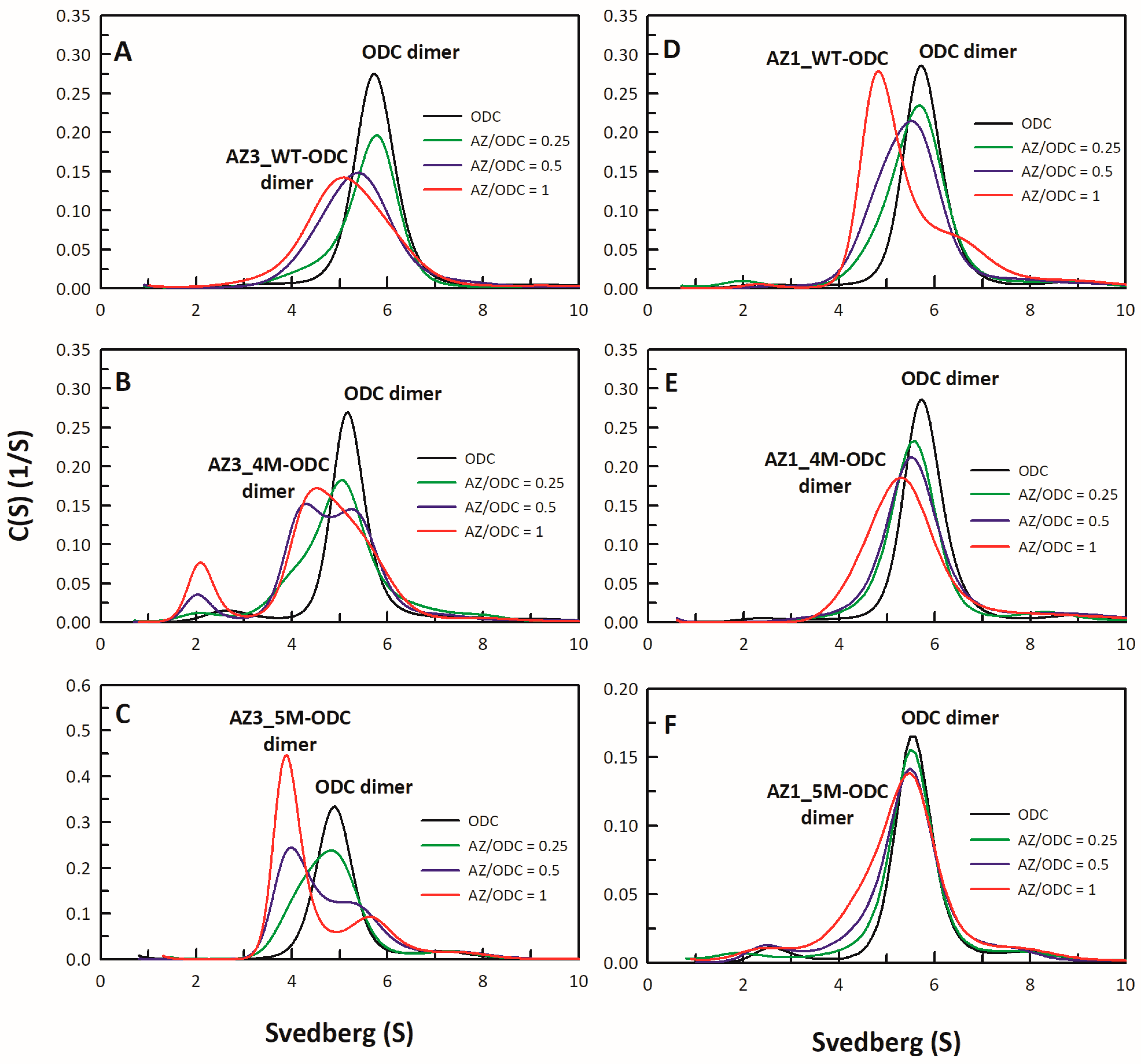
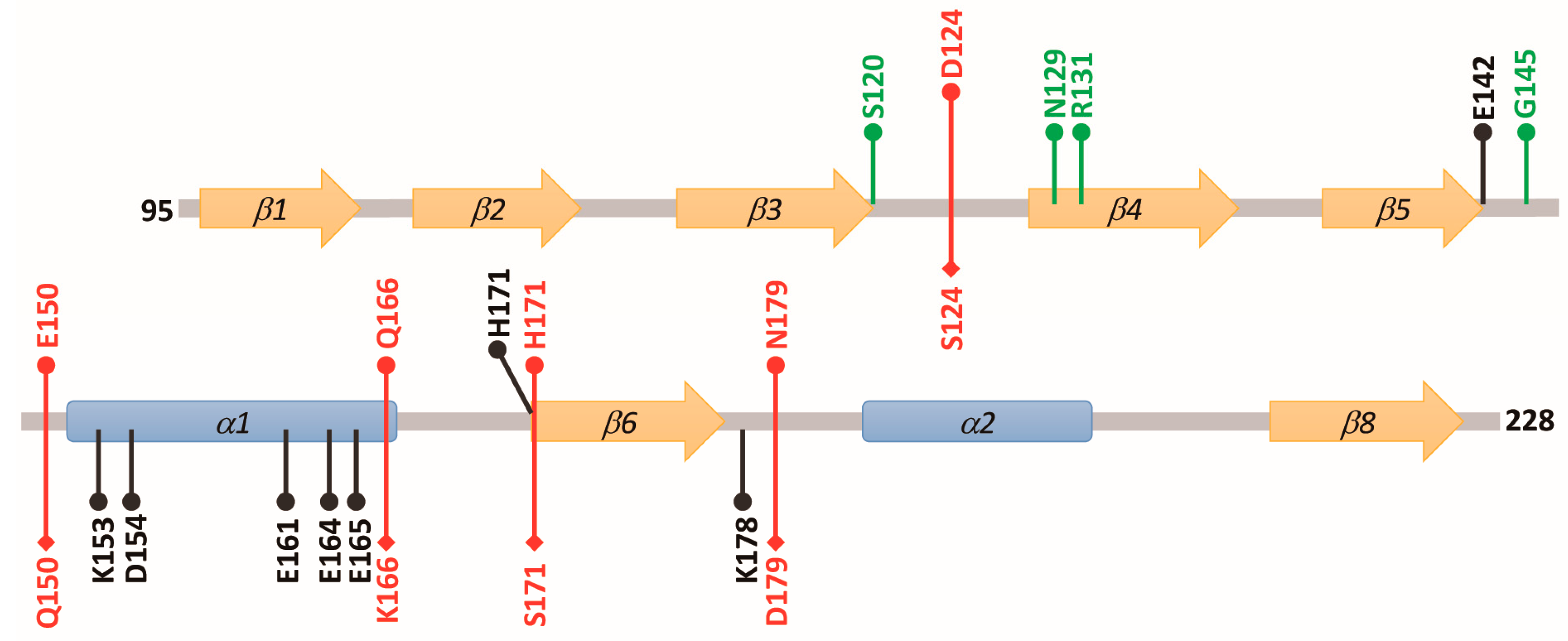
| 1 AZ Variants | Location | 2 IC50 (μM) | 3 Fold Change (IC50,mutant/IC50,WT) |
|---|---|---|---|
| AZ95–228 | C-terminal domain | 0.16 ± 0.01 | 1 |
| AZ95–228_E142A | β5 | 0.31 ± 0.02 | 1.93 |
| AZ95–228_K153A | α1 | 0.40 ± 0.10 | 2.5 |
| AZ95–228_D154A | α1 | 0.27 ± 0.06 | 1.69 |
| AZ95–228_E161A | α1 | 0.25 ± 0.06 | 1.56 |
| AZ95–228_E164A | α1 | 0.30 ± 0.06 | 1.88 |
| AZ95–228_E165A | α1 | 0.30 ± 0.07 | 1.88 |
| AZ95–228_H171A | β6 | 0.27 ± 0.09 | 1.69 |
| AZ95–228_K178A | Loop between β6 and α2 | 0.31 ± 0.05 | 1.94 |
| AZ95–228-3Mα1 | α1 | 4.13 ± 0.34 | 25.82 |
| AZ95–228-3Mα1_D154A | α1 | 5.61 ± 0.35 | 35.08 |
| AZ95–228-3Mα1_E161A | α1 | 6.31 ± 5.95 | 39.45 |
| AZ95–228-5Mα1 | α1 | No Inhibition | - |
| AZ95–228-3Mα1_E142A | α1/β5 | No Inhibition | - |
| AZ95–228-3Mα1_H171A | α1/β6 | No Inhibition | - |
| AZ95–228-3Mα1_K178A | α1/loop_β6-α2 | No Inhibition | - |
| AZ95–228_E142A/H171A/K178A | β5/β6/loop_β6-α2 | No Inhibition | - |
| AZ95–228-8M | β5/α1/β6/loop_β6-α2 | No Inhibition | - |
| AZ95–228-ODC dimer | 1Kd,AZ-ODC (μM) | 2 Fold Change (Kd,mutant/Kd,WT) |
|---|---|---|
| AZ95–228-ODC | 1.15 ± 0.01 | 1 |
| AZ95–228_E142A-ODC | 2.20 ± 0.02 | 1.9 |
| AZ95–228_K153A-ODC | 1.21 ± 0.01 | 1.05 |
| AZ95–228_D154A-ODC | 1.70 ± 0.02 | 1.5 |
| AZ95–228_E161A-ODC | 1.11 ± 0.01 | 0.97 |
| AZ95–228_E164A-ODC | 1.68 ± 0.01 | 1.46 |
| AZ95–228_E165A-ODC | 1.73 ± 0.02 | 1.5 |
| AZ95–228_H171A-ODC | 2.03 ± 0.02 | 1.77 |
| AZ95–228_K178A-ODC | 3.03 ± 0.02 | 2.63 |
| AZ95–228-3Mα1-ODC | 6.58 ± 0.02 | 5.72 |
| AZ95–228-3Mα1_D154A | 3.39 ± 0.03 | 2.95 |
| AZ95–228-3Mα1_E161A-ODC | 4.97 ± 0.06 | 4.32 |
| AZ95–228-5Mα1-ODC | 6.81 ± 0.06 | 5.92 |
| AZ95–228-3Mα1_E142A-ODC | 8.86 ± 0.05 | 7.7 |
| AZ95–228-3Mα1_H171A-ODC | 8.55 ± 0.08 | 7.43 |
| AZ95–228-3Mα1_K178A-ODC | 7.67 ± 0.06 | 6.67 |
| AZ95–228_E142A/H171A/K178A-ODC | 11.49 ± 0.6 | 10 |
| AZ95–228-8M-ODC | 12.44 ± 0.7 | 10.8 |
| AZ3 | 1 IC50 (μM) | 2 Fold Change (IC50,mutant/IC50,WT) |
|---|---|---|
| AZ3_WT | 0.61 ± 0.07 | 1 |
| AZ3_A98D | 0.67 ± 0.12 | 1.1 |
| AZ3_G99D | 0.78 ± 0.08 | 1.28 |
| AZ3_N100R | 0.66 ± 0.07 | 1.08 |
| AZ3_T106E | 1.21 ± 0.28 | 2 |
| AZ3_D112K | 0.55 ± 0.01 | 0.9 |
| AZ3_S124D | 0.50 ± 0.06 | 0.82 |
| AZ3_T126K | 0.70 ± 0.10 | 1.15 |
| AZ3_S127R | 1.02 ± 0.41 | 1.67 |
| AZ3_H129N | 0.67 ± 0.03 | 1.1 |
| AZ3_D136G | 0.72 ± 0.08 | 1.18 |
| AZ3_R137G | 1.23 ± 0.25 | 2.02 |
| AZ3_R138S | 0.81 ± 0.20 | 1.33 |
| AZ3_Y145G | 0.98 ± 0.05 | 1.61 |
| AZ3_D149P | 2.05 ± 0.43 | 3.36 |
| AZ3_Q150E | 0.48 ± 0.01 | 0.79 |
| AZ3_K166Q | 0.42 ± 0.05 | 0.69 |
| AZ3_N168R | 1.38 ± 0.57 | 2.26 |
| AZ3_S171H | 0.43 ± 0.01 | 0.7 |
| AZ3_N175C | 0.65 ± 0.04 | 1.07 |
| AZ3_Q177H | 1.34 ± 0.21 | 2.19 |
| AZ3_N178K | 0.67± 0.04 | 1.1 |
| AZ3_D179N | 0.45 ± 0.01 | 0.73 |
| AZ Protein | 1 IC50 (μM) | 2Kd,AZ-ODC (μM) |
|---|---|---|
| AZ1_WT | 0.23 ± 0.03 | 0.22 ± 0.01 |
| AZ3_WT | 0.61 ± 0.07 | 1.52 ± 0.01 |
| AZ1_4M (D124S/E150Q/Q166K/N179D) | 0.55 ± 0.02 | 0.95 ± 0.03 |
| AZ3_4M (S124D/Q150E/K166Q/D179N) | 0.25 ± 0.01 | 0.50 ± 0.01 |
| AZ1_5M (D124S/E150Q/Q166K/H171S/N179D) | 0.49 ± 0.02 | 1.78 ± 0.02 |
| AZ3_5M (S124D/Q150E/K166Q/S171H/D179N) | 0.29 ± 0.01 | 0.14 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, J.-Y.; Liu, Y.-C.; Cheng, I.-T.; Lee, C.-J.; Wang, Y.-H.; Fang, Y.-S.; Liu, Y.-L.; Liu, G.-Y.; Hung, H.-C. Critical Factors in Human Antizymes that Determine the Differential Binding, Inhibition, and Degradation of Human Ornithine Decarboxylase. Biomolecules 2019, 9, 864. https://doi.org/10.3390/biom9120864
Hsieh J-Y, Liu Y-C, Cheng I-T, Lee C-J, Wang Y-H, Fang Y-S, Liu Y-L, Liu G-Y, Hung H-C. Critical Factors in Human Antizymes that Determine the Differential Binding, Inhibition, and Degradation of Human Ornithine Decarboxylase. Biomolecules. 2019; 9(12):864. https://doi.org/10.3390/biom9120864
Chicago/Turabian StyleHsieh, Ju-Yi, Yen-Chin Liu, I-Ting Cheng, Chu-Ju Lee, Yu-Hsuan Wang, Yi-Shiuan Fang, Yi-Liang Liu, Guang-Yaw Liu, and Hui-Chih Hung. 2019. "Critical Factors in Human Antizymes that Determine the Differential Binding, Inhibition, and Degradation of Human Ornithine Decarboxylase" Biomolecules 9, no. 12: 864. https://doi.org/10.3390/biom9120864
APA StyleHsieh, J.-Y., Liu, Y.-C., Cheng, I.-T., Lee, C.-J., Wang, Y.-H., Fang, Y.-S., Liu, Y.-L., Liu, G.-Y., & Hung, H.-C. (2019). Critical Factors in Human Antizymes that Determine the Differential Binding, Inhibition, and Degradation of Human Ornithine Decarboxylase. Biomolecules, 9(12), 864. https://doi.org/10.3390/biom9120864