Lipid Membrane Adsorption Determines Photodynamic Efficiency of β-Imidazolyl-Substituted Porphyrins
Abstract
1. Introduction
2. Materials and Methods
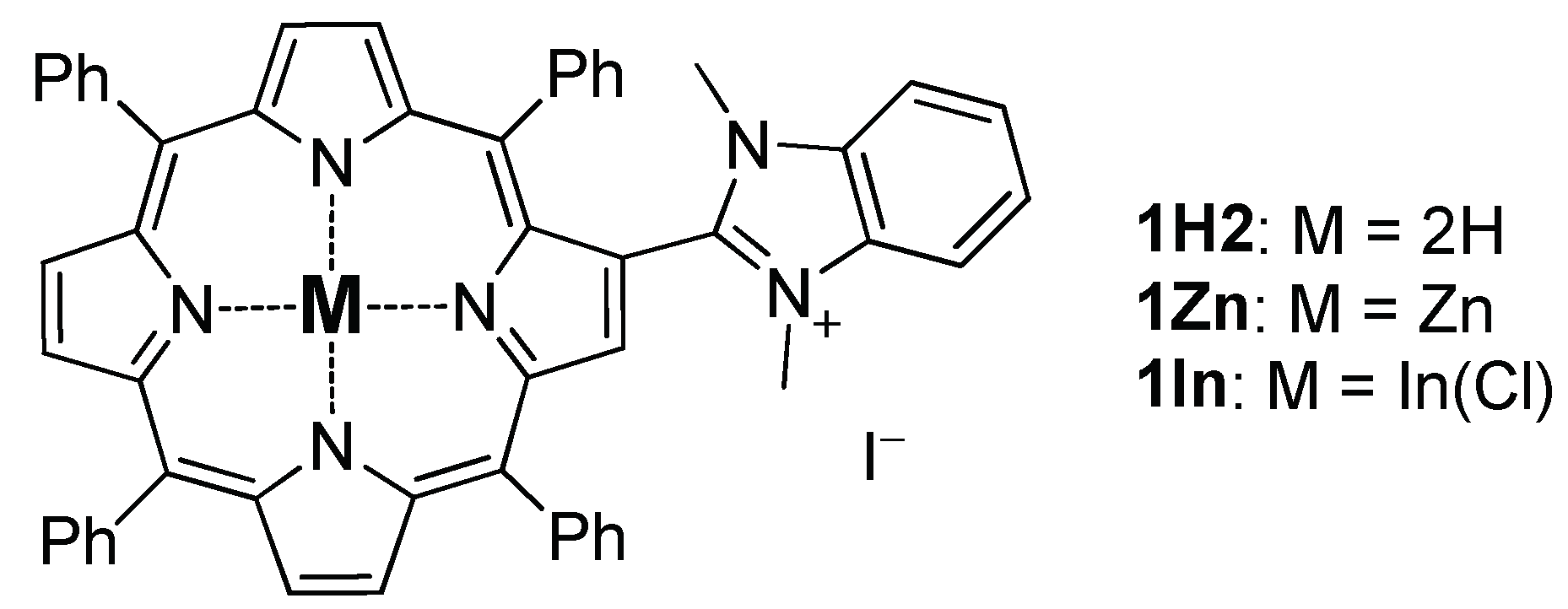
2.1. Synthesis of the Porphyrins
2.1.1. Zinc(II) 5,10,15,20-Tetraphenyl-2-(N, N-Dimethylbenzimidazol-2-Yl)-Porphyrin
2.1.2. Chlorooindium(III) 5,10,15,20-Tetraphenyl-2-(N, N-Dimethylbenzimidazolium-2-Yl)-Porphyrin Iodide
2.2. Experiments at BLM
3. Results and Discussion
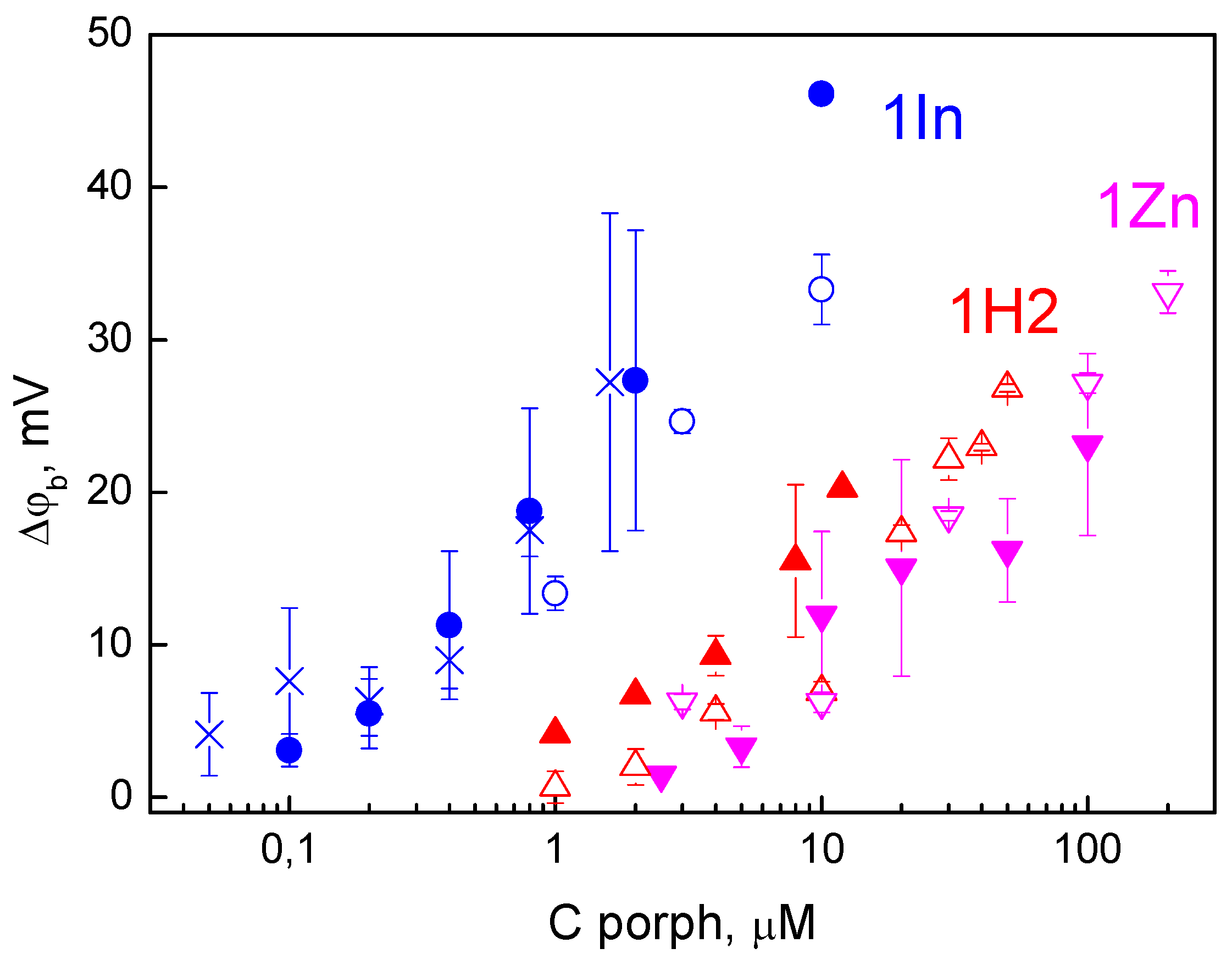
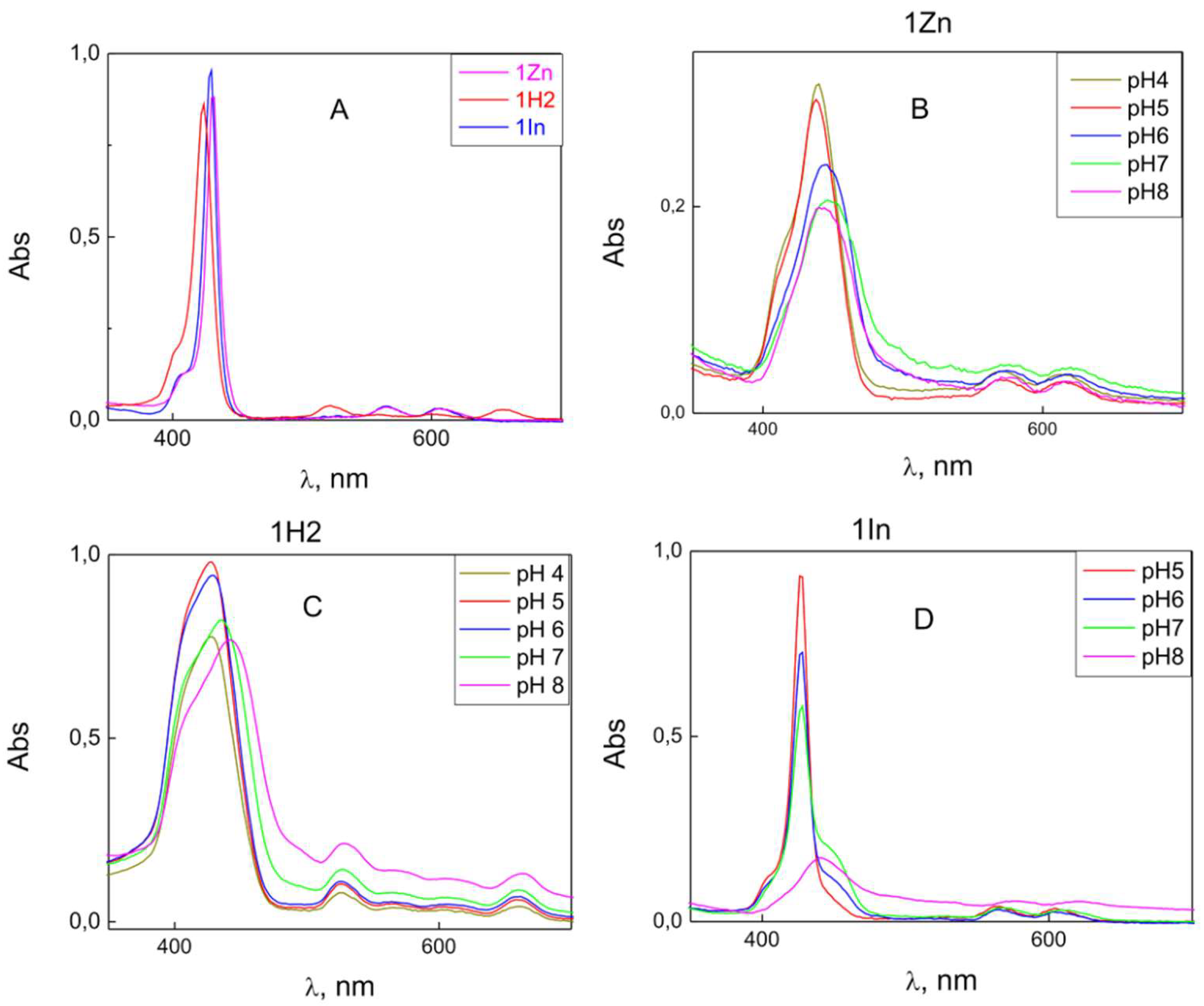
3.1. Adsorption on the Membrane
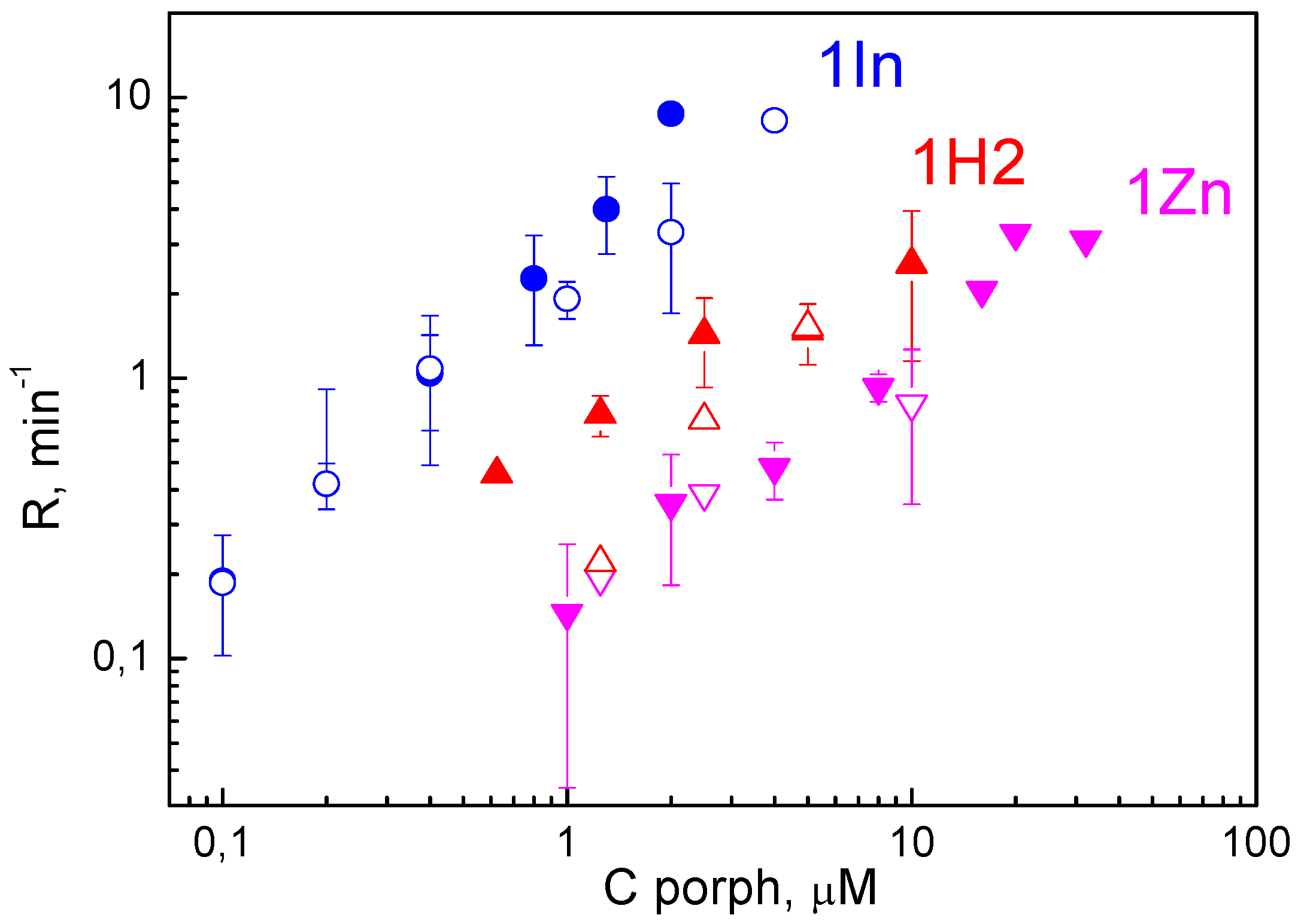
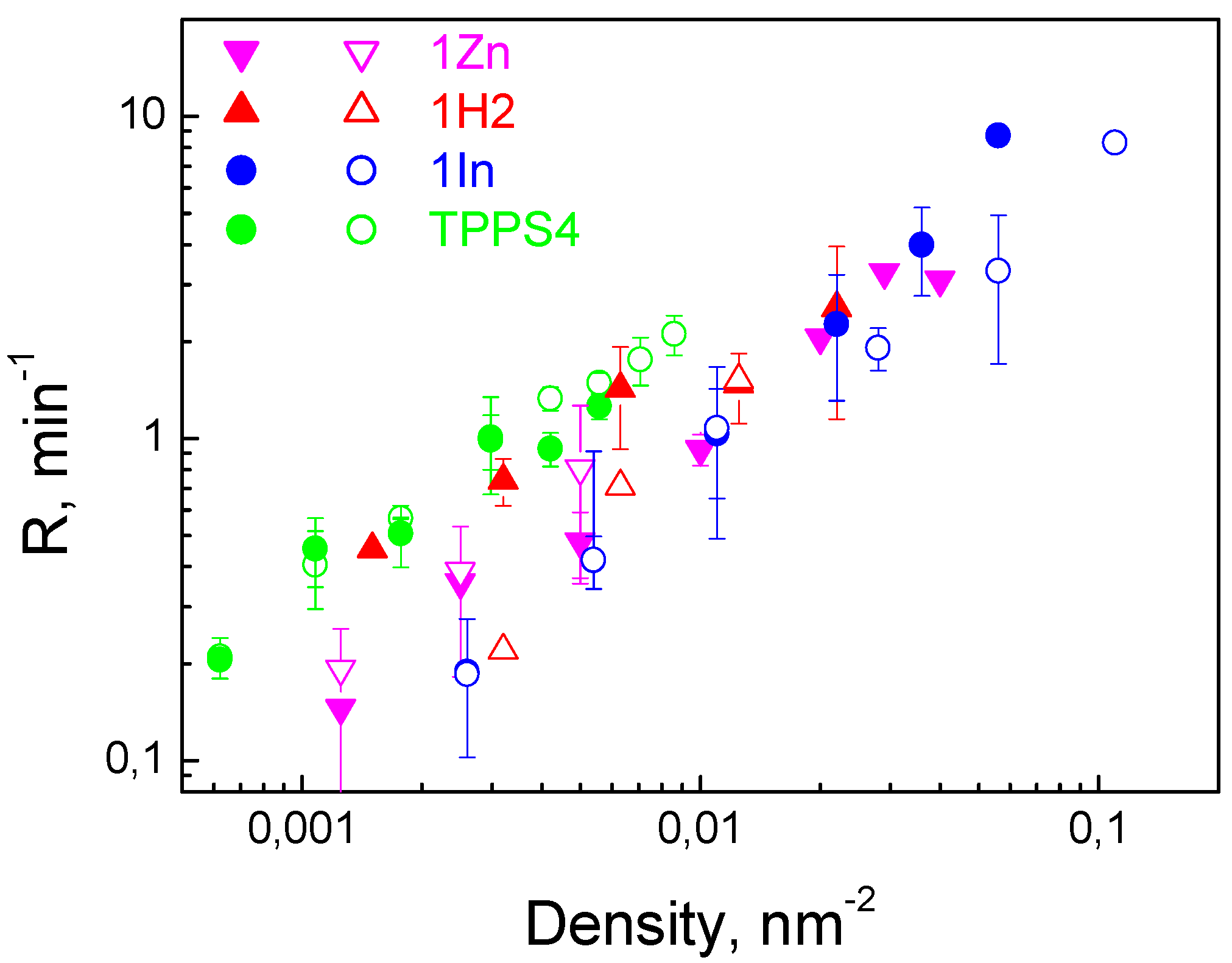
3.2. Photodynamic Efficiency
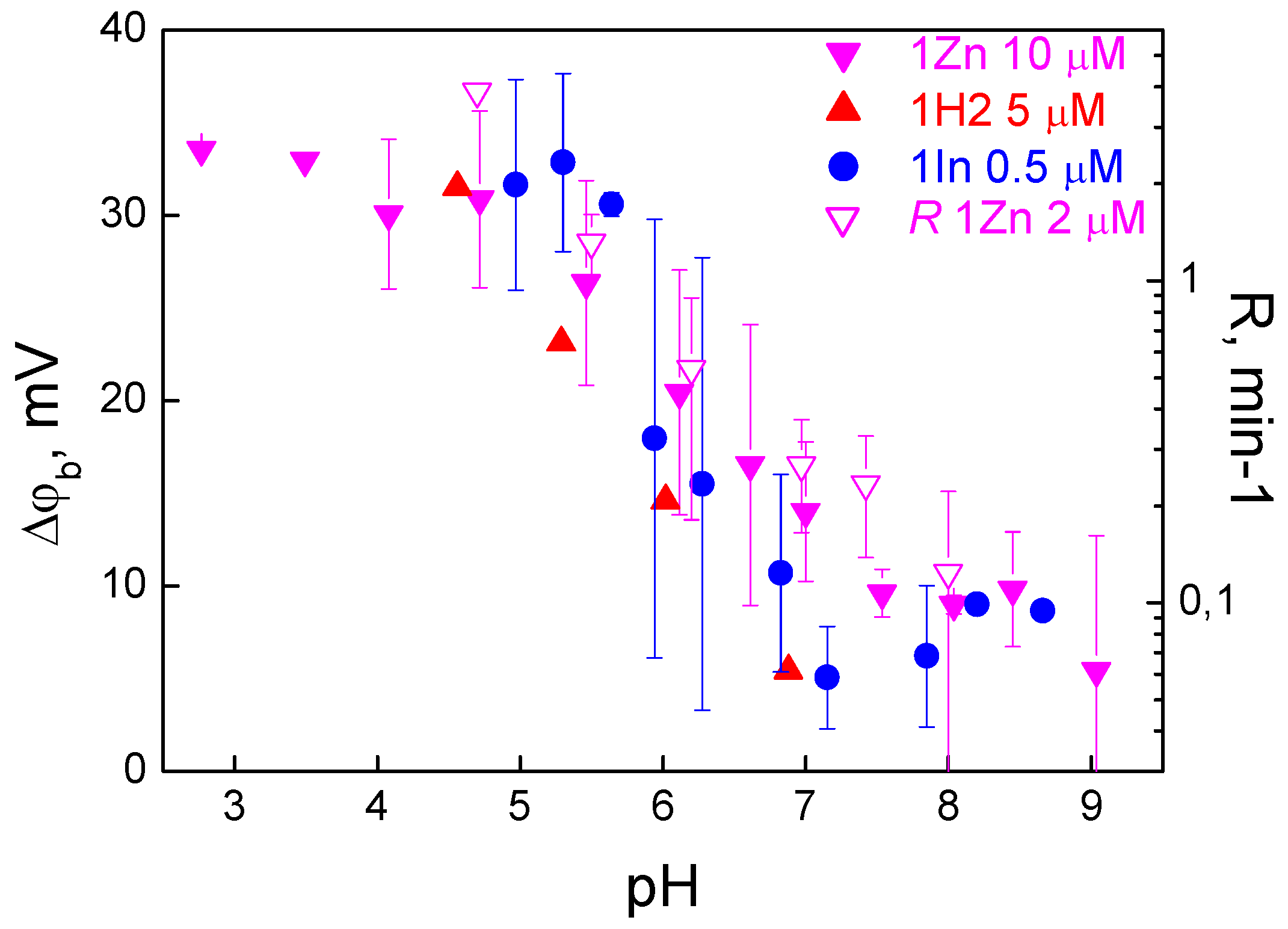
3.3. Effect of pH on the Adsorption and Photodynamic Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Nyokong, T.; Ahsen, V. Photosensitizers in Medicine, Environment, and Security; Springer: Berlin, Germany, 2012; pp. 1–654. [Google Scholar]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Jiang, L.; Gan, C.R.; Gao, J.; Loh, X.J. A Perspective on the Trends and Challenges Facing Porphyrin-Based Anti-Microbial Materials. Small 2016, 12, 3609–3644. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyziol, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Jori, G.; Brown, S.B. Photosensitized inactivation of microorganisms. Photochem. Photobiol. Sci. 2004, 3, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef]
- Caminos, D.A.; Spesia, M.B.; Pons, P.; Durantini, E.N. Mechanisms of Escherichia coli photodynamic inactivation by an amphiphilic tricationic porphyrin and 5,10,15,20-tetra(4-N,N,N-trimethylammoniumphenyl) porphyrin. Photochem. Photobiol. Sci. 2008, 7, 1071–1078. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Merchat, M.; Spikes, J.D.; Bertoloni, G.; Jori, G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J. Photochem. Photobiol. B 1996, 35, 149–157. [Google Scholar] [CrossRef]
- Mirsky, V.M.; Stozhkova, I.N.; Szito, T.V. Photosensitized damage of bilayer lipid membrane in presence of haematoporphyrin dimethylether. J. Photochem. Photobiol. 1990, 8, 315–324. [Google Scholar] [CrossRef]
- Stozhkova, I.N.; Cherny, V.V.; Sokolov, V.S.; Ermakov, Y. Adsorption of haematoporphyrins on the planar bilayer membrane. Membr. Cell Biol. 1997, 11, 381–399. [Google Scholar] [PubMed]
- Rokitskaya, T.I.; Block, M.; Antonenko, Y.N.; Kotova, E.A.; Pohl, P. Photosensitizer binding to lipid bilayers as a precondition for the photoinactivation of membrane channels. Biophys. J. 2000, 78, 2572–2580. [Google Scholar] [CrossRef]
- Sokolov, V.S.; Block, M.; Stozhkova, I.N.; Pohl, P. Membrane Photopotential Generation by Interfacial Differences in the Turnover of a Photodynamic Reaction. Biophys. J. 2000, 79, 2121–2131. [Google Scholar] [CrossRef]
- Pashkovskaya, A.A.; Sokolenko, E.A.; Sokolov, V.S.; Kotova, E.A.; Antonenko, Y.N. Photodynamic activity and binding of sulfonated metallophthalocyanines to phospholipid membranes: Contribution of metal-phosphate coordination. Biochim. Biophys. Acta 2007, 1768, 2459–2465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pashkovskaya, A.A.; Maizlish, V.E.; Shaposhnikov, G.P.; Kotova, E.A.; Antonenko, Y.N. Role of electrostatics in the binding of charged metallophthalocyanines to neutral and charged phospholipid membranes. Biochim. Biophys. Acta 2008, 1778, 541–548. [Google Scholar] [CrossRef]
- Sokolov, V.S.; Pohl, P. Membrane transport of singlet oxygen monitored by dipole potential measurements. Biophys. J. 2009, 96, 77–85. [Google Scholar] [CrossRef][Green Version]
- Sokolov, V.S.; Gavrilchik, A.N.; Kulagina, A.O.; Meshkov, I.N.; Pohl, P.; Gorbunova, Y.G. Voltage-sensitive styryl dyes as singlet oxygen targets on the surface of bilayer lipid membrane. J. Photochem. Photobiol. B 2016, 161, 162–169. [Google Scholar] [CrossRef]
- Konstantinova, A.N.; Sokolov, V.S.; Jimenez-Munguia, I.; Finogenova, O.A.; Ermakov, Y.A.; Gorbunova, Y.G. Adsorption and photodynamic efficiency of meso-tetrakis(p-sulfonatophenyl)porphyrin on the surface of bilayer lipid membranes. J. Photochem. Photobiol. B 2018, 189, 74–80. [Google Scholar] [CrossRef]
- Sokolov, V.S.; Batishchev, O.V.; Akimov, S.A.; Galimzyanov, T.R.; Konstantinova, A.N.; Malingriaux, E.; Gorbunova, Y.G.; Knyazev, D.G.; Pohl, P. Residence time of singlet oxygen in membranes. Sci. Rep. 2018, 8, 14000. [Google Scholar] [CrossRef]
- Kubat, P.; Lang, K.; Kral, V.; Anzenbacher, P. Preprogramming of Porphyrin?Nucleic Acid Assemblies via Variation of the Alkyl/Aryl Substituents of Phosphonium Tetratolylporphyrins. J. Phys. Chem. B 2002, 106, 6784–6792. [Google Scholar] [CrossRef]
- Chirvony, V.S.; Galievsky, V.A.; Kruk, N.N.; Dzhagarov, B.M.; Turpin, P.-Y. Photophysics of cationic 5,10,15,20-tetrakis-(4-N-methylpyridyl) porphyrin bound to DNA, [poly(dA-dT)]2 and [poly(dG-dC)]2: On a possible charge transfer process between guanine and porphyrin in its excited singlet state. J. Photochem. Photobiol. B Biol. 1997, 40, 154–162. [Google Scholar] [CrossRef]
- Hirakawa, K.; Kawanishi, S.; Hirano, T.; Segawa, S. Guanine-specific DNA oxidation photosensitized by the tetraphenylporphyrin phosphorus(V) complex via singlet oxygen generation and electron transfer. J. Photochem. Photobiol. B Biol. 2007, 87, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Meshkov, I.N.; Bulach, V.; Gorbunova, Y.G.; Gostev, F.E.; Nadtochenko, V.A.; Tsivadze, A.Y.; Hosseini, M.W. Tuning photochemical properties of phosphorus(V) porphyrins photosensitizers. Chem. Commun. 2017, 53, 9918–9921. [Google Scholar] [CrossRef] [PubMed]
- Angeli, N.G.; Lagorio, M.G.; Roman, E.A.S.; Dicelio, L.E. Meso-substituted cationic porphyrins of biological interest. Photophysical and physicochemical properties in solution and bound to liposomes. Photochem. Photobiol. 2000, 72, 49–56. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Elsevier/Butterworth-Heinemann: Oxford, UK, 2009; pp. 1–760. [Google Scholar]
- Moura, N.M.M.; Esteves, M.; Vieira, C.; Rocha, G.M.S.R.O.; Faustino, M.A.F.; Almeida, A.; Cavaleiro, J.A.S.; Lodeiroc, C.; Neves, M.G.P.M.S. Novel β-functionalized mono-charged porphyrinic derivatives: Synthesis and photoinactivation of Escherichia coli. Dyes Pigment. 2019, 160, 361–371. [Google Scholar] [CrossRef]
- Abdulaeva, I.A.; Birin, K.P.; Sinelshchikova, A.A.; Grigoriev, M.S.; Lyssenko, K.A.; Gorbunova, Y.G.; Tsivadze, A.Y.; Bessmertnykh-Lemeune, A. Imidazoporphyrins as supramolecular tectons: Synthesis and selfassembling of zinc 2-(4-pyridyl)-1H-imidazo[4,5-b]porphyrinate. CrystEngComm 2019, 21, 1488–1498. [Google Scholar] [CrossRef]
- Raptopoulou, C.; Daphnomili, D.; Karamalides, A.; di Vaira, M.; Terzis, A.; Coutsolelos, A.G. Perhalogenated porphyrinic derivatives with indium and thallium: The X-ray structures of (b-Cl4TPP)Tl(Cl), (b-Cl4TPP)In(Cl) and (TpFTPP)Tl(Cl). Polyhedron 2004, 23, 1777–1784. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Methods for the formation of single bimolecular lipid membranes in aqueous solution. J. Phys. Chem. 1963, 67, 534–535. [Google Scholar] [CrossRef]
- Sokolov, V.S.; Kuz’min, V.G. Measurement of the difference in the surface potentials of bilayer membranes from the second harmonic of the capacitance current. Biophysics 1980, 25, 174–177. [Google Scholar]
- Jimenez-Munguia, I.; Volynsky, P.E.; Batischev, O.V.; Akimov, S.A.; Korshunova, G.A.; Smirnova, E.A.; Knorre, D.A.; Sokolov, S.S.; Severin, F.F. Effects of Sterols on the Interaction of SDS, Benzalkonium Chloride, and A Novel Compound, Kor105, with Membranes. Biomolecules 2019, 9, 627. [Google Scholar] [CrossRef]
- McLaughlin, S. Electrostatic Potentials at Membrane-Solution Interfaces; Academic Press, A Subsidiary of Harcourt Brace Jovanovich: New York, NY, USA; San Francisco, CA, USA; London, UK, 1977; pp. 71–144. [Google Scholar]
- Ermakov, Y.A.; Sokolov, V.S. Boundary potentials of bilayer lipid membranes: Methods and interpretations. In Planar Lipid Bilayers (BLMs) and Their Applications; Tien, H.T., Ottova-Leitmannova, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 109–141. [Google Scholar]
- Sokolov, V.S.; Mirsky, V.M. Electrostatic potentials of bilayer lipid membranes: Basic research and analytical applications. In Ultrathin Electrochemical Chemo- and Biosensors: Technology and Performance; Mirsky, V.M., Ed.; Springer: Berlin, Germany, 2004; pp. 255–291. [Google Scholar]
- Merchat, M.; Bertolini, G.; Giacomini, P.; Villanueva, A.; Jori, G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Cherny, V.V.; Simonova, M.V.; Sokolov, V.S.; Markin, V.S. Transport of the neutral form of amphiphilic drugs through a planar bilayer lipid meembrane: The role of the pH gradient. Bioelectrochem. Bioenerg. 1990, 23, 17–26. [Google Scholar] [CrossRef]
- Stozhkova, I.N.; Cherny, V.V.; Sokolov, V.S. Haematoporphyrin dimethyl ether transport across bilayer lipid membrane. Membr. Cell Biol. 1995, 9, 215–222. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Munguía, I.; Fedorov, A.K.; Abdulaeva, I.A.; Birin, K.P.; Ermakov, Y.A.; Batishchev, O.V.; Gorbunova, Y.G.; Sokolov, V.S. Lipid Membrane Adsorption Determines Photodynamic Efficiency of β-Imidazolyl-Substituted Porphyrins. Biomolecules 2019, 9, 853. https://doi.org/10.3390/biom9120853
Jiménez-Munguía I, Fedorov AK, Abdulaeva IA, Birin KP, Ermakov YA, Batishchev OV, Gorbunova YG, Sokolov VS. Lipid Membrane Adsorption Determines Photodynamic Efficiency of β-Imidazolyl-Substituted Porphyrins. Biomolecules. 2019; 9(12):853. https://doi.org/10.3390/biom9120853
Chicago/Turabian StyleJiménez-Munguía, Irene, Arseniy K. Fedorov, Inna A. Abdulaeva, Kirill P. Birin, Yury A. Ermakov, Oleg V. Batishchev, Yulia G. Gorbunova, and Valerij S. Sokolov. 2019. "Lipid Membrane Adsorption Determines Photodynamic Efficiency of β-Imidazolyl-Substituted Porphyrins" Biomolecules 9, no. 12: 853. https://doi.org/10.3390/biom9120853
APA StyleJiménez-Munguía, I., Fedorov, A. K., Abdulaeva, I. A., Birin, K. P., Ermakov, Y. A., Batishchev, O. V., Gorbunova, Y. G., & Sokolov, V. S. (2019). Lipid Membrane Adsorption Determines Photodynamic Efficiency of β-Imidazolyl-Substituted Porphyrins. Biomolecules, 9(12), 853. https://doi.org/10.3390/biom9120853







