Biomaterials for In Situ Tissue Regeneration: A Review
Abstract
1. Introduction
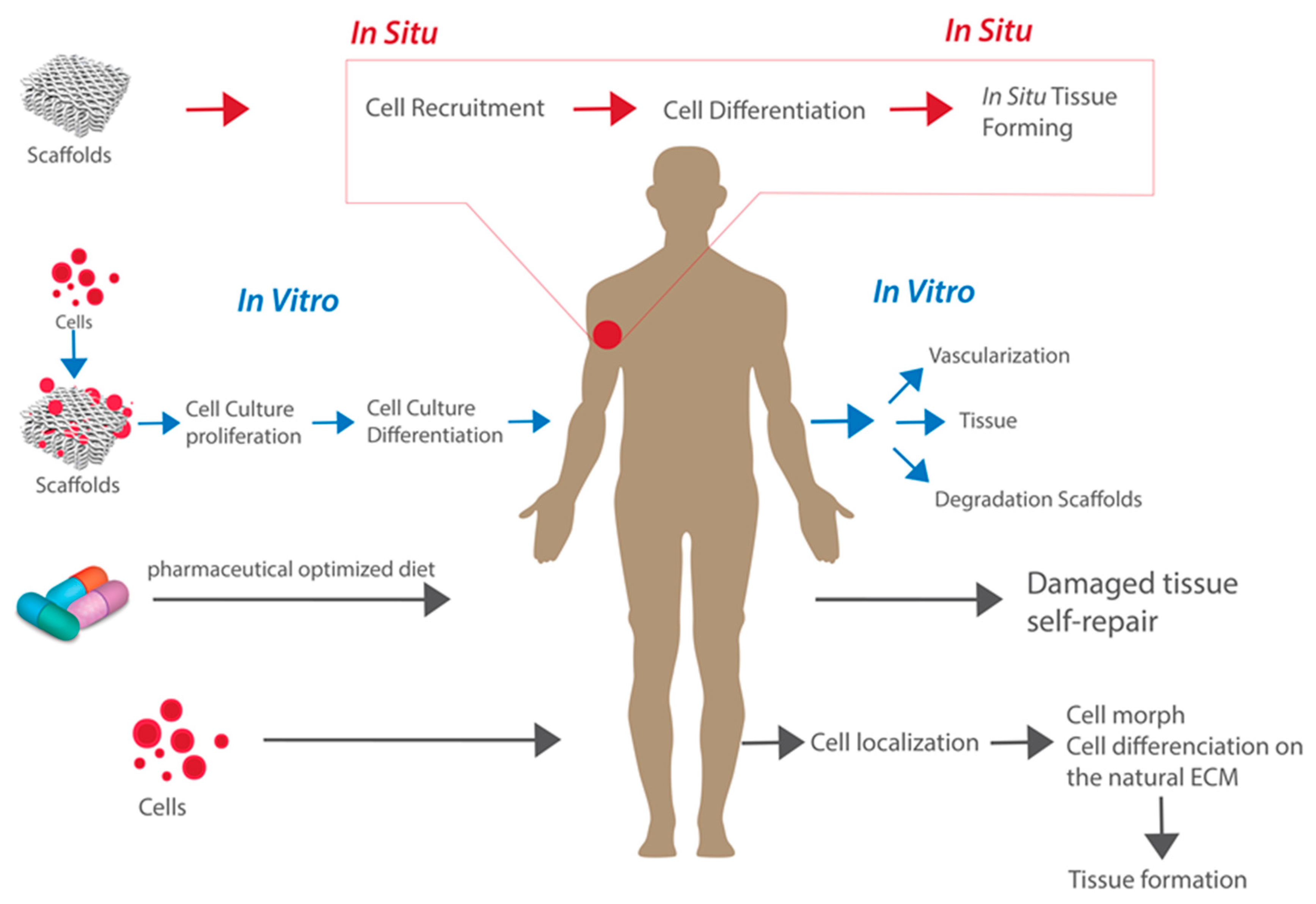
2. From In Vitro to In Situ
3. Biomaterials: Types and Requirements for In Situ Tissue Regeneration
3.1. Biocompatibility and Biodegradability
3.2. Surface Topography and Chemistry
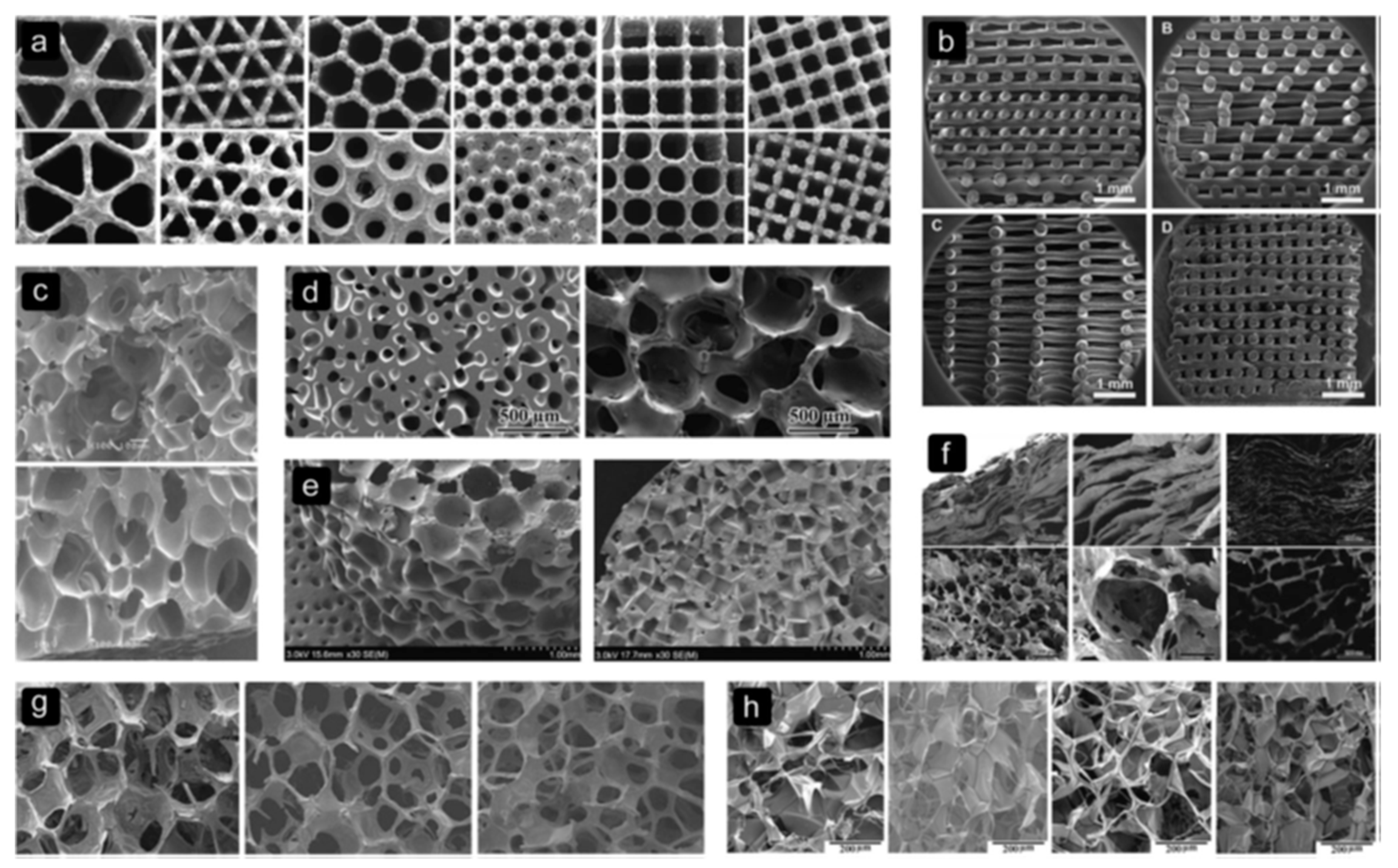
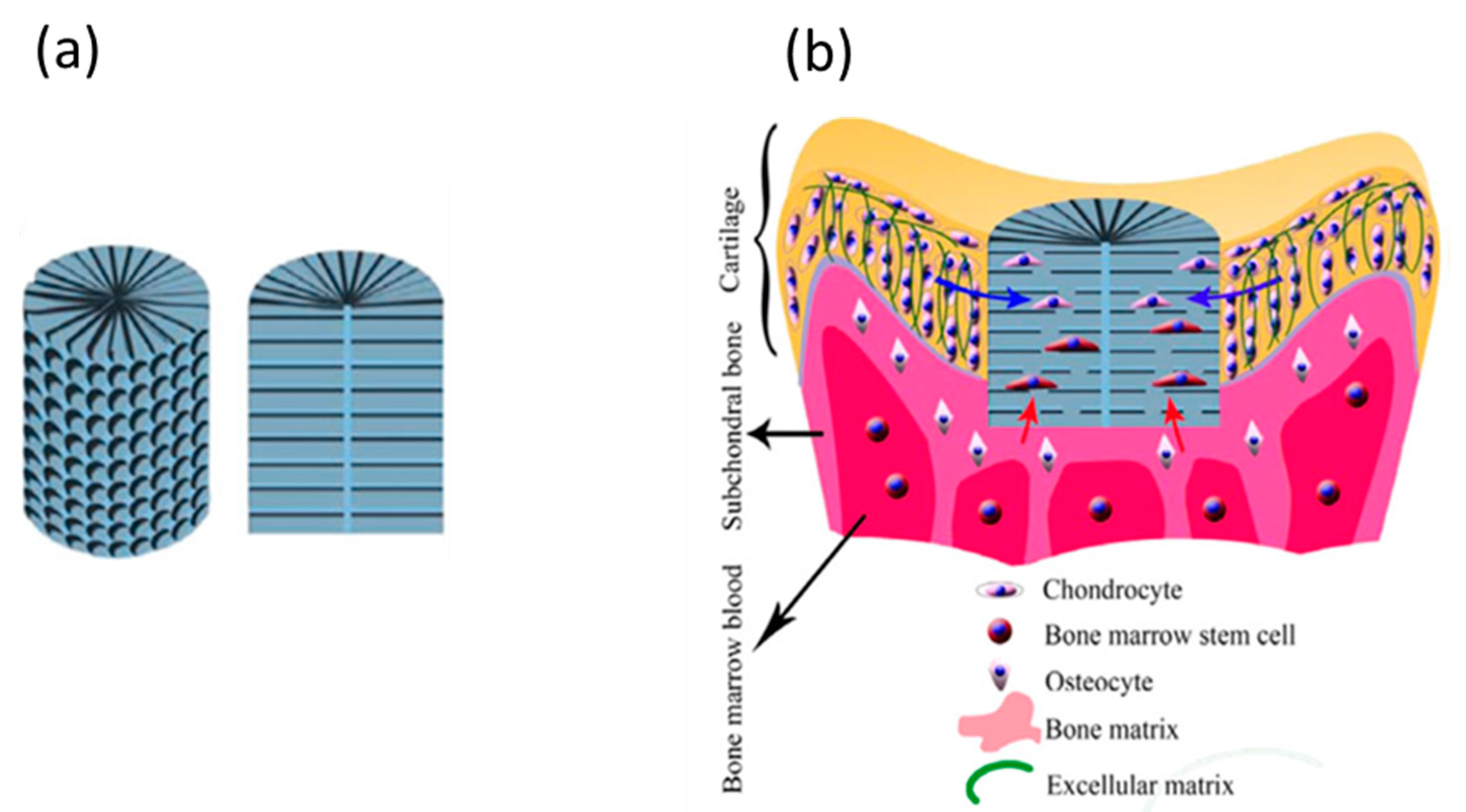
3.3. Scaffold Architecture
3.4. Mechanical Properties
3.5. Handling Properties
4. Biomaterials
4.1. Synthetic Polymers
4.2. Natural Polymers
4.3. ECM
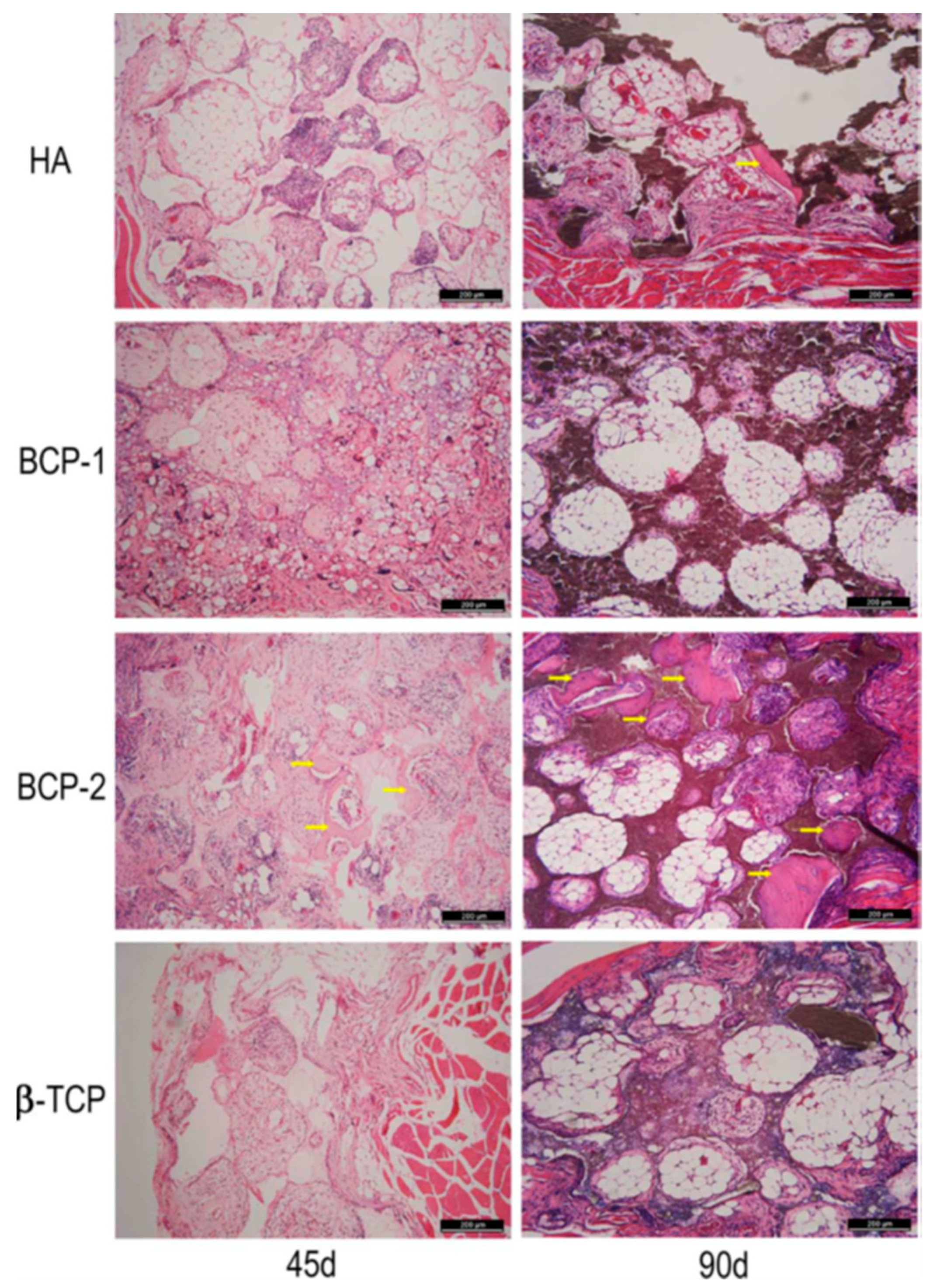
5. Bioceramics
6. Future Prospects
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kung, J.W.C.; Currie, I.S.; Forbes, S.J.; Ross, J.A. Liver Development, Regeneration, and Carcinogenesis. J. Biomed. Biotechnol. 2010, 2010, 8. [Google Scholar] [CrossRef]
- Atala, A. Tissue engineering and regenerative medicine: Concepts for clinical application. Rejuvenation Res. 2004, 7, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Skalak, R.; Fox, C.F. Tissue Engineering; Alan, R., Ed.; Liss. Inc.: New York, NY, USA, 1988. [Google Scholar]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. The Lancet 1999, 354, 32–34. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Salick, M.R.; Cordie, T.M.; Peng, X.-F.; Turng, L.-S. Morphology, mechanical properties, and mineralization of rigid thermoplastic polyurethane/hydroxyapatite scaffolds for bone tissue applications: Effects of fabrication approaches and hydroxyapatite size. J. Mater. Sci. 2014, 49, 2324–2337. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Ebrahimi, M. 13-Biomimetic principle for development of nanocomposite biomaterials in tissue engineering. In Applications of Nanocomposite Materials in Orthopedics Inamuddin; Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, Cambridge, CA, USA, 2019; pp. 287–306. [Google Scholar]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775. [Google Scholar] [CrossRef]
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D Printing of Bioceramic Scaffolds-Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef]
- Miar, S.; Shafiee, A.; Guda, T.; Narayan, R. Additive Manufacturing for Tissue Engineering. In 3D Printing and Biofabrication; Ovsianikov, A., Yoo, J., Mironov, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–54. [Google Scholar]
- Shick, T.M.; Abdul Kadir, A.Z.; Ngadiman, N.H.A.; Ma’aram, A. A review of biomaterials scaffold fabrication in additive manufacturing for tissue engineering. J. Bioact. Compat. Polym. 2019. [Google Scholar] [CrossRef]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, Y.B.; Hinton, T.J.; Feinberg, A.W.; Alsberg, E. Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 2019, 12, 61–70. [Google Scholar] [CrossRef]
- Newsom, J.P.; Payne, K.A.; Krebs, M.D. Microgels: Modular, tunable constructs for tissue regeneration. Acta Biomater. 2019, 88, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.A.; Papantoniou, I.; Mendes, L.F.; Hall, G.N.; Bosmans, K.; Tam, W.L.; Teixeira, L.M.; Moos, M., Jr.; Geris, L.; Luyten, F.P. Limb derived cells as a paradigm for engineering self-assembling skeletal tissues. J. Tissue Eng. Regen. Med. 2018, 12, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Xue, Y.; Hartley, R.; Sant, V.; Eles, J.R.; Cui, X.T.; Stolz, D.B.; Sant, S. Hierarchically aligned fibrous hydrogel films through microfluidic self-assembly of graphene and polysaccharides. Biotechnol. Bioeng. 2018, 115, 2654–2667. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Akiyama, Y.; Yamato, M.; Shimizu, T.; Okano, T. Design of Temperature-Responsive Cell Culture Surfaces for Cell Sheet-Based Regenerative Therapy and 3D Tissue Fabrication. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer Singapore: Singapore, 2018; pp. 371–393. [Google Scholar]
- Nagarajan, N.; Dupret-Bories, A.; Karabulut, E.; Zorlutuna, P.; Vrana, N.E. Enabling personalized implant and controllable biosystem development through 3D printing. Biotechnol. Adv. 2018, 36, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Whitely, M.; Cereceres, S.; Dhavalikar, P.; Salhadar, K.; Wilems, T.; Smith, B.; Mikos, A.; Cosgriff-Hernandez, E. Improved in situ seeding of 3D printed scaffolds using cell-releasing hydrogels. Biomaterials 2018, 185, 194–204. [Google Scholar] [CrossRef]
- Ko, I.K.; Lee, S.J.; Atala, A.; Yoo, J.J. In situ tissue regeneration through host stem cell recruitment. Exp. Amp. Mol. Med. 2013, 45, 57. [Google Scholar] [CrossRef]
- Ju, Y.M.; Atala, A.; Yoo, J.J.; Lee, S.J. In situ regeneration of skeletal muscle tissue through host cell recruitment. Acta Biomater. 2014, 10, 4332–4339. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Kluin, J.; Talacua, H.; Smits, A.I.P.M.; Emmert, M.Y.; Brugmans, M.C.P.; Fioretta, E.S.; Dijkman, P.E.; Söntjens, S.H.M.; Duijvelshoff, R.; Dekker, S.; et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant – From material design to 12 months follow-up in sheep. Biomaterials 2017, 125, 101–117. [Google Scholar] [CrossRef]
- Augustine, R.; Dalvi, Y.B.; Dan, P.; George, N.; Helle, D.; Varghese, R.; Thomas, S.; Menu, P.; Sandhyarani, N. Nanoceria Can Act as the Cues for Angiogenesis in Tissue-Engineering Scaffolds: Toward Next-Generation in Situ Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 4338–4353. [Google Scholar] [CrossRef]
- Cipitria, A.; Boettcher, K.; Schoenhals, S.; Garske, D.S.; Schmidt-Bleek, K.; Ellinghaus, A.; Dienelt, A.; Peters, A.; Mehta, M.; Madl, C.M.; et al. In-situ tissue regeneration through SDF-1α driven cell recruitment and stiffness-mediated bone regeneration in a critical-sized segmental femoral defect. Acta Biomater. 2017, 60, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoo, J.J.; Atala, A. Fundamentals of In Situ Tissue Regeneration. In Situ Tissue Regeneration; Elsevier B.V.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Li, Q.; Ma, L.; Gao, C. Biomaterials for in situ tissue regeneration: Development and perspectives. J. Mater. Chem. B. 2015, 3, 8921–8938. [Google Scholar] [CrossRef]
- Kim, B.S.; Baez, C.E.; Atala, A. Biomaterials for tissue engineering. World J. Urol. 2000, 18, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nakasaki, M.; Shih, Y.-R.V.; Varghese, S. Effect of age on biomaterial-mediated in situ bone tissue regeneration. Acta Biomater. 2018, 78, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Morouço, P.G. Biofabrication for osteochondral tissue regeneration: Bioink printability requirements. J. Mater. Sci. Mater. Med. 2019, 30, 20. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, X.; Gao, W.; Fu, X.; Fang, R.H.; Zhang, L.; Zhang, K. Tissue repair and regeneration with endogenous stem cells. Nat. Rev. Mater. 2018, 3, 174–193. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135. [Google Scholar] [CrossRef]
- Crowder, S.W.; Leonardo, V.; Whittaker, T.; Papathanasiou, P.; Stevens, M.M. Material Cues as Potent Regulators of Epigenetics and Stem Cell Function. Cell Stem Cell 2016, 18, 39–52. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Sengupta, D.; Waldman, S.D.; Li, S. From In Vitro to In Situ Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.K.; Gobin, A.S.; Tsai, A.T.; Schmedlen, R.H.; West, J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: Synthetic ECM analogs for tissue engineering. Biomaterials 2001, 22, 3045–3051. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B. 2017, 5, 6175–6192. [Google Scholar] [CrossRef]
- Martins, A.M.; Alves, C.M.; Reis, R.L.; Mikos, A.G.; Kasper, F.K. Toward Osteogenic Differentiation of Marrow Stromal Cells and In Vitro Production of Mineralized Extracellular Matrix onto Natural Scaffolds. In Biological Interactions on Materials Surfaces: Understanding and Controlling Protein, Cell, and Tissue Responses; Puleo, D.A., Bizios, R., Eds.; Springer US: New York, NY, USA, 2009; pp. 263–281. [Google Scholar]
- Amin Yavari, S.; van der Stok, J.; Chai, Y.C.; Wauthle, R.; Tahmasebi Birgani, Z.; Habibovic, P.; Mulier, M.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Bone regeneration performance of surface-treated porous titanium. Biomaterials 2014, 35, 6172–6181. [Google Scholar] [CrossRef]
- Ravi, S.; Chaikof, E.L. Biomaterials for vascular tissue engineering. Regen. Med. 2009, 5, 107–120. [Google Scholar] [CrossRef]
- Thevenot, P.T.; Nair, A.M.; Shen, J.; Lotfi, P.; Ko, C.-Y.; Tang, L. The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 2010, 31, 3997–4008. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [PubMed]
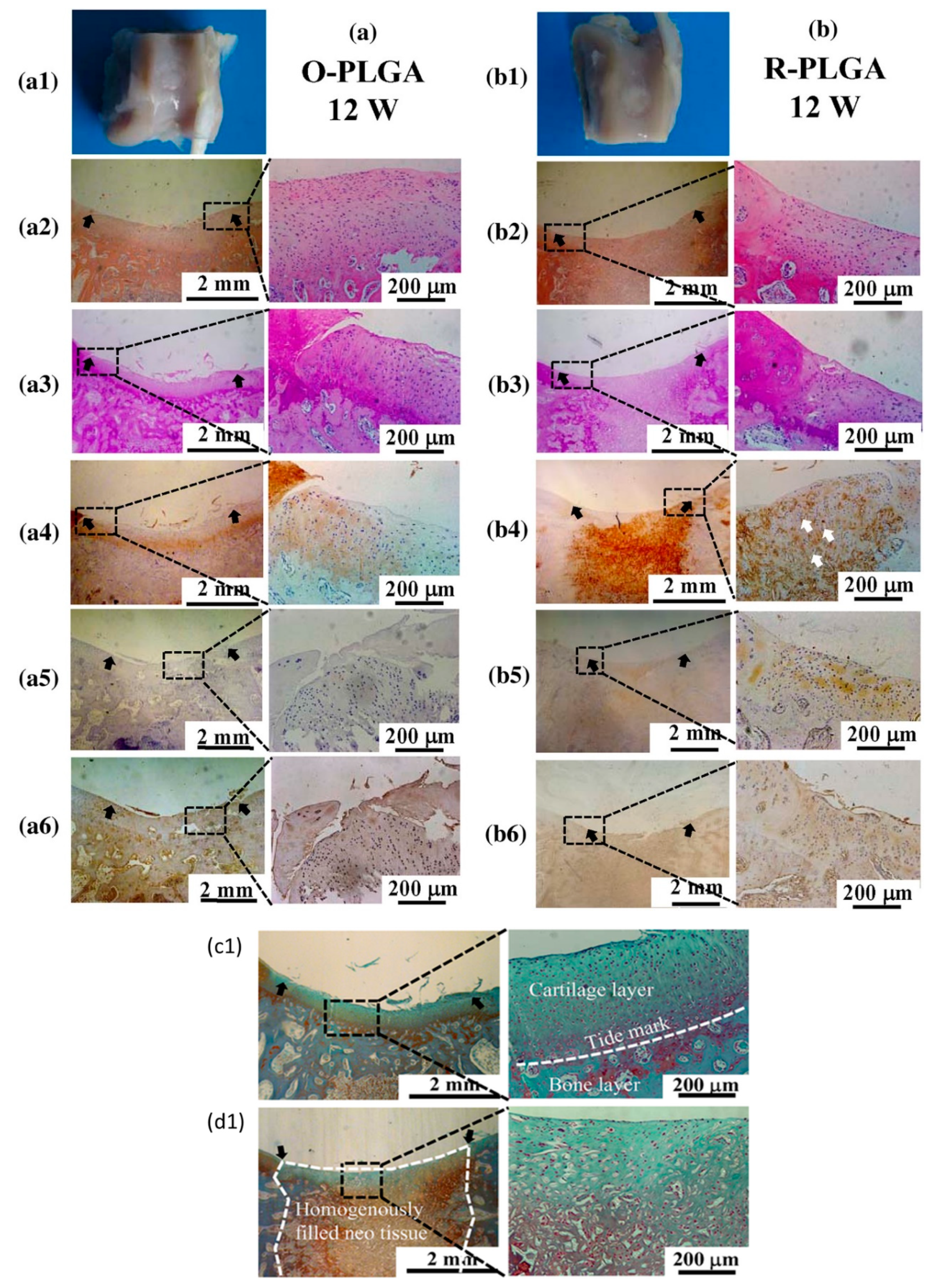
- Dai, Y.; Shen, T.; Ma, L.; Wang, D.; Gao, C. Regeneration of osteochondral defects in vivo by a cell-free cylindrical poly(lactide-co-glycolide) scaffold with a radially oriented microstructure. J. Tissue Eng. Regen. Med. 2018, 12, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Kothari, A.; Katti, D.S. Pore orientation mediated control of mechanical behavior of scaffolds and its application in cartilage-mimetic scaffold design. J. Mech. Behav. Biomed. Mater. 2015, 51, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F. Preparation and in vitro evaluation of bioactive glass (13–93) scaffolds with oriented microstructures for repair and regeneration of load-bearing bones. J. Biomed. Mater. Res. Part. A 2010, 93A, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, M.S.; Lee, J.W.; Kim, Y.H.; Kim, S.-H.; Kim, S.H. Cartilage regeneration with highly-elastic three-dimensional scaffolds prepared from biodegradable poly(l-lactide-co-ɛ-caprolactone). Biomaterials 2008, 29, 4630–4636. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Grayson, W.L.; Castaneda, A.; Rockwood, D.N.; Gil, E.S.; Kaplan, D.L.; Vunjak-Novakovic, G. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials 2011, 32, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Zhu, Y.; Friis, T.; Xiao, Y. Structure–property relationships of silk-modified mesoporous bioglass scaffolds. Biomaterials 2010, 31, 3429–3438. [Google Scholar] [CrossRef]
- Nauman, E.A.; Fong, K.E.; Keaveny, T.M. Dependence of Intertrabecular Permeability on Flow Direction and Anatomic Site. Ann. Biomed. Eng. 1999, 27, 517–524. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Slaughter, B.B.V.; Slaughter, B.B.V.; Khurshid, S.S.; Khurshid, S.S.; Fisher, O.Z.; Fisher, O.Z.; Khademhosseini, A.; Khademhosseini, A.; Peppas, N.A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 4, 3307–3329. [Google Scholar] [CrossRef]
- Chen, B.; Jones, R.R.; Mi, S.; Foster, J.; Alcock, S.G.; Hamley, I.W.; Connon, C.J. The mechanical properties of amniotic membrane influence its effect as a biomaterial for ocular surface repair. Soft Matter 2012, 8, 8379–8387. [Google Scholar] [CrossRef]
- Wei, G.; Jin, Q.; Giannobile, W.V.; Ma, P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials 2007, 28, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Montenegro, R.; Koh, H.S.; Shoichet, M.S. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 2005, 26, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A Perspective on the Clinical Translation of Scaffolds for Tissue Engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.N.; Stapleton, L.M.; Farry, J.M.; Lucian, H.J.; Paulsen, M.J.; Eskandari, A.; Hironaka, C.E.; Thakore, A.D.; Wang, H.; Yu, A.C.; et al. A Biocompatible Therapeutic Catheter-Deliverable Hydrogel for In Situ Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1801147. [Google Scholar] [CrossRef]
- Momeni, F.; Mehdi, M.; Hassani, N.S.; Liu, X.; Ni, J. A review of 4D printing. Mater. Des. 2017, 122, 42–79. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2019, 64, 91–126. [Google Scholar] [CrossRef]
- Murdock, M.H.; Badylak, S.F. Biomaterials-based In Situ Tissue Engineering. Curr. Opin. Biomed. Eng. 2017, 1, 4–7. [Google Scholar] [CrossRef]
- Magnusson, J.P.; Saeed, A.O.; Fernández-Trillo, F.; Alexander, C. Synthetic polymers for biopharmaceutical delivery. Polym. Chem. 2011, 2, 48–59. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Talacua, H.; Smits, A.I.P.M.; Muylaert, D.E.P.; van Rijswijk, J.W.; Vink, A.; Verhaar, M.C.; Driessen-Mol, A.; van Herwerden, L.A.; Bouten, C.V.C.; Kluin, J.; et al. In Situ Tissue Engineering of Functional Small-Diameter Blood Vessels by Host Circulating Cells Only. Tissue Eng. Part A 2015, 21, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.C.; Krieghoff, J.; Mikos, A.G. Synthetic Polymers, 3rd ed.; Academic Press: Boston, MA, USA, 2019. [Google Scholar]
- Hakkarainen, M.; Albertsson, A.-C. Degradation Products of Aliphatic and Aliphatic–Aromatic Polyesters. In Chromatography for Sustainable Polymeric Materials: Renewable, Degradable and Recyclable; Albertsson, A.-C., Hakkarainen, M., Eds.; Springer: Heidelberg/Berlin, Germany, 2008; pp. 85–116. [Google Scholar]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; You, Z.; Gao, J.; Fan, X.; Wang, Y. A functional polyester carrying free hydroxyl groups promotes the mineralization of osteoblast and human mesenchymal stem cell extracellular matrix. Acta Biomater. 2014, 10, 2814–2823. [Google Scholar] [CrossRef]
- Gómez, G.; Korkiakoski, S.; González, M.M.; Länsman, S.; Ellä, V.; Salo, T.; Kellomäki, M.; Ashammakhi, N.; Arnaud, E. Effect of FGF and polylactide scaffolds on calvarial bone healing with growth factor on biodegradable polymer scaffolds. J. Craniofacial Surg. 2006, 17, 935–942. [Google Scholar] [CrossRef]
- Mabilleau, G.; Aguado, E.; Stancu, I.C.; Cincu, C.; Baslé, M.F.; Chappard, D. Effects of FGF-2 release from a hydrogel polymer on bone mass and microarchitecture. Biomaterials 2008, 29, 1593–1600. [Google Scholar] [CrossRef]
- Fujimoto, K.L.; Guan, J.; Oshima, H.; Sakai, T.; Wagner, W.R. In Vivo Evaluation of a Porous, Elastic, Biodegradable Patch for Reconstructive Cardiac Procedures. Ann. Thorac. Surg. 2007, 83, 648–654. [Google Scholar] [CrossRef]
- Erggelet, C.; Endres, M.; Neumann, K.; Morawietz, L.; Ringe, J.; Haberstroh, K.; Sittinger, M.; Kaps, C. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J. Orthop. Res. 2009, 27, 1353–1360. [Google Scholar] [CrossRef]
- Yosuke, M.; Osama, I.I.S.; Mohammad, A.; Athanasios, K.; Christophe, N.; Susana, L.; Boris, W.; Martijn, C.; Yoshinobu, O.; Patrick, W.S. Acute performance of a novel restorative transcatheter aortic valve: Preclinical results. EuroIntervention 2017, 13, 1410–1417. [Google Scholar]
- Herberg, S.; Siedler, M.; Pippig, S.; Schuetz, A.; Dony, C.; Kim, C.K.; Wikesjö, U.M.E. Development of an injectable composite as a carrier for growth factor-enhanced periodontal regeneration. J. Clin. Periodontol. 2008, 35, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Navarro, R.S.; Yang, O.; Liu, Z.; Luo, J.; Qiu, P.; Chen, E.Y.; Ma, P.X.; Yang, B. Abstract 15000: In Situ Vascular Reconstruction by Using Heparinized PCL/PLLA Bilayer Nanofibrous Scaffolds in a Rat Model. Circulation 2018, 138, 15000. [Google Scholar]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, 41–56. [Google Scholar] [CrossRef]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 2013, 34, 5571–5580. [Google Scholar] [CrossRef]
- Elia, R.; Fuegy, P.W.; VanDelden, A.; Firpo, M.A.; Prestwich, G.D.; Peattie, R.A. Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials 2010, 31, 4630–4638. [Google Scholar] [CrossRef]
- Bhakta, G.; Rai, B.; Lim, Z.X.H.; Hui, J.H.; Stein, G.S.; van Wijnen, A.J.; Nurcombe, V.; Prestwich, G.D.; Cool, S.M. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 2012, 33, 6113–6122. [Google Scholar] [CrossRef]
- Dinjaski, N.; Kaplan, D.L. Recombinant protein blends: Silk beyond natural design. Curr. Opin. Biotechnol. 2016, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatric Res. 2008, 63, 492. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, Q.; Zhao, Y.; Chen, W.; Chen, B.; Xiao, Z.; Lin, H.; Nie, L.; Wang, D.; Dai, J. Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials 2011, 32, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, L.; Liu, Y.; Zheng, A.; Jiao, D.; Zeng, D.; Wang, X.; Kaplan, D.L.; Jiang, X. Functionalization of Silk Fibroin Electrospun Scaffolds via BMSC Affinity Peptide Grafting through Oxidative Self-Polymerization of Dopamine for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 8878–8895. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, K.; Huang, X.; Liu, J.; Liu, H.; Boccaccini, A.R.; Wan, Y.; Guo, X.; Shao, Z. Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 2019, 91, 60–71. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Rath, S.N.; Susanto, E.; Haupt, L.M.; Hutmacher, D.W.; Nurcombe, V.; Cool, S.M. Sustained release and osteogenic potential of heparan sulfate-doped fibrin glue scaffolds within a rat cranial model. J. Mol. Histol. 2007, 38, 425–433. [Google Scholar] [CrossRef]
- Kodama, N.; Nagata, M.; Tabata, Y.; Ozeki, M.; Ninomiya, T.; Takagi, R. A local bone anabolic effect of rhFGF2-impregnated gelatin hydrogel by promoting cell proliferation and coordinating osteoblastic differentiation. Bone 2009, 44, 699–707. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, K.; Lin, S.; Xu, Z.; Sun, Y.; Shi, P.; Li, G.; Bian, L. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials 2016, 101, 217–228. [Google Scholar] [CrossRef]
- Lee, S.J.; Wang, H.J.; Kim, T.H.; Choi, J.S.; Kulkarni, G.; Jackson, J.D.; Atala, A.; Yoo, J.J. In Situ Tissue Regeneration of Renal Tissue Induced by Collagen Hydrogel Injection. Stem Cells Transl. Med. 2018, 7, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Landa, N.; Miller, L.; Feinberg, M.S.; Holbova, R.; Shachar, M.; Freeman, I.; Cohen, S.; Leor, J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008, 117, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Imai, S.; Fujimiya, M.; Isoya, E.; Ando, K.; Mimura, T.; Matsusue, Y. Exogenous collagen-enhanced recruitment of mesenchymal stem cells during rabbit articular cartilage repair. Acta Orthop. 2007, 78, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liu, G.; Ma, L.; Wang, D.; Gao, C. Cell-free macro-porous fibrin scaffolds for in situ inductive regeneration of full-thickness cartilage defects. J. Mater. Chem. B 2016, 4, 4410–4419. [Google Scholar] [CrossRef]
- Ma, F.; Ge, Y.; Liu, N.; Pang, X.; Shen, X.; Tang, B. In situ fabrication of a composite hydrogel with tunable mechanical properties for cartilage tissue engineering. J. Mater. Chem. B 2019, 7, 2463–2473. [Google Scholar] [CrossRef]
- Kin, S.; Hagiwara, A.; Nakase, Y.; Kuriu, Y.; Nakashima, S.; Yoshikawa, T.; Sakakura, C.; Otsuji, E.; Nakamura, T.; Yamagishi, H. Regeneration of skeletal muscle using in situ tissue engineering on an acellular collagen sponge scaffold in a rabbit model. Asaio J. 2007, 53, 506–513. [Google Scholar] [CrossRef]
- Brouwer, K.M.; Daamen, W.F.; van Lochem, N.; Reijnen, D.; Wijnen, R.M.H.; van Kuppevelt, T.H. Construction and in vivo evaluation of a dual layered collagenous scaffold with a radial pore structure for repair of the diaphragm. Acta Biomater. 2013, 9, 6844–6851. [Google Scholar] [CrossRef]
- Nakahara, T.; Nakamura, T.; Kobayashi, E.; Inoue, M.; Shigeno, K.; Tabata, Y.; Eto, K.; Shimizu, Y. Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: Effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 2003, 9, 153–162. [Google Scholar] [CrossRef]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kim, H.; Kwak, G.; Yoon, H.Y.; Jo, S.D.; Lee, J.E.; Cho, D.; Kwon, I.C.; Kim, S.H. Development of Biocompatible HA Hydrogels Embedded with a New Synthetic Peptide Promoting Cellular Migration for Advanced Wound Care Management. Adv. Sci. 2018, 5, 1800852. [Google Scholar] [CrossRef]
- Chung, Y.I.; Ahn, K.M.; Jeon, S.H.; Lee, S.Y.; Lee, J.H.; Tae, G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J. Control. Release 2007, 121, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feng, C.; Quan, J.; Wang, Z.; Wei, W.; Zang, S.; Kang, S.; Hui, G.; Chen, X.; Wang, Q. In situ controlled release of stromal cell-derived factor-1α and antimiR-138 for on-demand cranial bone regeneration. Carbohydr. Polym. 2018, 182, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jin, Y.; Park, H.-J.; Yang, K.; Lee, M.S.; Yang, H.S.; Cho, S.-W. In Situ Bone Tissue Engineering With an Endogenous Stem Cell Mobilizer and Osteoinductive Nanofibrous Polymeric Scaffolds. Biotechnol. J. 2017, 12, 1700062. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C.; et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Perdisa, F.; Filardo, G.; Sessa, A.; Busacca, M.; Zaffagnini, S.; Marcacci, M.; Kon, E. One-Step Treatment for Patellar Cartilage Defects With a Cell-Free Osteochondral Scaffold: A Prospective Clinical and MRI Evaluation. Am. J. Sports Med. 2017, 45, 1581–1588. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, Z.; Ma, L.; Wang, D.; Gao, C. Cell-Free HA-MA/PLGA Scaffolds with Radially Oriented Pores for In Situ Inductive Regeneration of Full Thickness Cartilage Defects. Macromol. Biosci. 2016, 16, 1632–1642. [Google Scholar] [CrossRef]
- Capulli, A.K.; Emmert, M.Y.; Pasqualini, F.S.; Kehl, D.; Caliskan, E.; Lind, J.U.; Sheehy, S.P.; Park, S.J.; Ahn, S.; Weber, B.; et al. JetValve: Rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials 2017, 133, 229–241. [Google Scholar] [CrossRef]
- Hori, Y.; Nakamura, T.; Matsumoto, K.; Kurokawa, Y.; Satomi, S.; Shimizu, Y. Experimental study on In Situ tissue engineering of the stomach by an acellular collagen sponge scaffold graft. Asaio J. 2001, 47, 206–210. [Google Scholar] [CrossRef]
- Abbushi, A.; Endres, M.; Cabraja, M.; Kroppenstedt, S.N.; Thomale, U.W.; Sittinger, M.; Hegewald, A.A.; Morawietz, L.; Lemke, A.J.; Bansemer, V.G.t.; et al. Regeneration of intervertebral disc tissue by resorbable cell-free polyglycolic acid-based implants in a rabbit model of disc degeneration. Spine 2008, 33, 1527–1532. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Reprint of: Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2015, 23, 17–26. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Agmon, G.; Christman, K.L. Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr. Opin. Solid State Mater. Sci. 2016, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Cramer, M.C.; Badylak, S.F. Extracellular Matrix Bioscaffolds for Building Gastrointestinal Tissue. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. Npj. Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Nakamoto, T.; Kawazoe, N.; Chen, G. Influence of stepwise chondrogenesis-mimicking 3D extracellular matrix on chondrogenic differentiation of mesenchymal stem cells. Biomaterials 2015, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Benders, K.E.M.; Weeren, P.R.v.; Badylak, S.F.; Saris, D.B.F.; Dhert, W.J.A.; Malda, J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef]
- Lih, E.; Park, K.W.; Chun, S.Y.; Kim, H.; Kwon, T.G.; Joung, Y.K.; Han, D.K. Biomimetic Porous PLGA Scaffolds Incorporating Decellularized Extracellular Matrix for Kidney Tissue Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 21145–21154. [Google Scholar] [CrossRef]
- Woo, J.S.; Fishbein, M.C.; Reemtsen, B. Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovasc. Pathol. 2016, 25, 12–17. [Google Scholar] [CrossRef]
- Dai, J.; Qiao, W.; Shi, J.; Liu, C.; Hu, X.; Dong, N. Modifying decellularized aortic valve scaffolds with stromal cell-derived factor-1α loaded proteolytically degradable hydrogel for recellularization and remodeling. Acta Biomater. 2019, 88, 280–292. [Google Scholar] [CrossRef]
- Levato, R.; Planell, J.A.; Mateos-Timoneda, M.A.; Engel, E. Role of ECM/peptide coatings on SDF-1α triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomater. 2015, 18, 59–67. [Google Scholar] [CrossRef]
- Muylaert, D.E.P.; van Almen, G.C.; Talacua, H.; Fledderus, J.O.; Kluin, J.; Hendrikse, S.I.S.; van Dongen, J.L.J.; Sijbesma, E.; Bosman, A.W.; Mes, T.; et al. Early in-situ cellularization of a supramolecular vascular graft is modified by synthetic stromal cell-derived factor-1α derived peptides. Biomaterials 2016, 76, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; DeWard, A.; Londono, R.; Saldin, L.T.; Castleton, A.A.; Carey, L.; Nieponice, A.; Lagasse, E.; Badylak, S.F. Tissue-Specific Effects of Esophageal Extracellular Matrix. Tissue Eng. Part. A 2015, 21, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Katerinaki, E.; Zanetto, U.; Sterne, G.D. Histological appearance of Stratticetm tissue matrix used in breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.; McQuillan, D.; Sandor, M.; Wan, H.; Lombardi, J.; Bachrach, N.; Harper, J.; Xu, H. Retention of structural and biochemical integrity in a biological mesh supports tissue remodeling in a primate abdominal wall model. Regen. Med. 2009, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-W.; Zhang, Y.; Luo, J.-C.; Zhang, D.; Xiong, B.-J.; Yang, J.-Q.; Xie, H.-Q.; Lv, Q. Hydrogel derived from decellularized porcine adipose tissue as a promising biomaterial for soft tissue augmentation. J. Biomed. Mater. Res. Part. A 2017, 105, 1756–1764. [Google Scholar] [CrossRef]
- Kitamura, M.; Hirano, S.; Kanemaru, S.-i.; Kitani, Y.; Ohno, S.; Kojima, T.; Nakamura, T.; Ito, J.; Rosen, C.A.; Gilbert, T.W. Glottic regeneration with a tissue-engineering technique, using acellular extracellular matrix scaffold in a canine model. J. Tissue Eng. Regen. Med. 2016, 10, 825–832. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, J.; Zhu, Z.; Xu, J.; Yi, Y.; Li, Y.; Fan, H.; Bai, S.; Yang, J.; Tang, Y.; et al. Surface biofunctionalization of the decellularized porcine aortic valve with VEGF-loaded nanoparticles for accelerating endothelialization. Mater. Sci. Eng. 2019, 97, 632–643. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive Materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 548–581. [Google Scholar] [CrossRef]
- Nyberg, E.; Holmes, C.; Witham, T.; Grayson, W.L. Growth factor-eluting technologies for bone tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Changchun, Z.; Xiangfeng, L.; Junqiu, C.; Hongsong, F.; Xingdong, Z. Bioactive Ceramics and Metals for Regenerative Engineering. In Regenerative Engineering; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Tanner, K.E. 3-Hard tissue applications of biocomposites. In Biomedical Composites, 2nd ed.; Ambrosio, L., Ed.; Woodhead Publishing: Swaston, Cambridge, UK, 2017. [Google Scholar]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Leite, Á.J.; Sarker, B.; Zehnder, T.; Silva, R.; Mano, J.F.; Boccaccini, A.R. Bioplotting of a bioactive alginate dialdehyde-gelatin composite hydrogel containing bioactive glass nanoparticles. Biofabrication 2016, 8, 035005. [Google Scholar] [CrossRef] [PubMed]
- Meka, S.R.K.; Jain, S.; Chatterjee, K. Strontium eluting nanofibers augment stem cell osteogenesis for bone tissue regeneration. Colloids Surf. B Biointerfaces 2016, 146, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Meka, S.R.K.; Agarwal, V.; Chatterjee, K. In situ preparation of multicomponent polymer composite nanofibrous scaffolds with enhanced osteogenic and angiogenic activities. Mater. Sci. Eng. 2019, 94, 565–579. [Google Scholar] [CrossRef]
- Song, G.; Habibovic, P.; Bao, C.; Hu, J.; van Blitterswijk, C.A.; Yuan, H.; Chen, W.; Xu, H.H.K. The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials 2013, 34, 2167–2176. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhu, X.; Yuan, T.; Tan, Y.; Fan, Y.; Zhang, X. Effect of phase composition on protein adsorption and osteoinduction of porous calcium phosphate ceramics in mice. J. Biomed. Mater. Res. Part. A 2014, 102, 4234–4243. [Google Scholar] [CrossRef]
- Li, X.; Song, T.; Chen, X.; Wang, M.; Yang, X.; Xiao, Y.; Zhang, X. Osteoinductivity of Porous Biphasic Calcium Phosphate Ceramic Spheres with Nanocrystalline and Their Efficacy in Guiding Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3722–3736. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C 2011, 31, 1245–1256. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Chen, C. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Trbakovic, A.; Hedenqvist, P.; Mellgren, T.; Ley, C.; Hilborn, J.; Ossipov, D.; Ekman, S.; Johansson, C.B.; Jensen-Waern, M.; Thor, A. A new synthetic granular calcium phosphate compound induces new bone in a sinus lift rabbit model. J. Dent. 2018, 70, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Schlickewei, C.; Klatte, T.O.; Wildermuth, Y.; Laaff, G.; Rueger, J.M.; Ruesing, J.; Chernousova, S.; Lehmann, W.; Epple, M. A bioactive nano-calcium phosphate paste for in-situ transfection of BMP-7 and VEGF-A in a rabbit critical-size bone defect: Results of an in vivo study. J. Mater. Sci. Mater. Med. 2019, 30, 15. [Google Scholar] [CrossRef]
- Oliveira, H.; Catros, S.; Boiziau, C.; Siadous, R.; Marti-Munoz, J.; Bareille, R.; Rey, S.; Castano, O.; Planell, J.; Amédée, J.; et al. The proangiogenic potential of a novel calcium releasing biomaterial: Impact on cell recruitment. Acta Biomater. 2016, 29, 435–445. [Google Scholar] [CrossRef]
- Shao, H.; Sun, M.; Zhang, F.; Liu, A.; He, Y.; Fu, J.; Yang, X.; Wang, H.; Gou, Z. Custom Repair of Mandibular Bone Defects with 3D Printed Bioceramic Scaffolds. J. Dent. Res. 2018, 97, 68–76. [Google Scholar] [CrossRef]
- Fu, J.; Zhuang, C.; Qiu, J.; Ke, X.; Yang, X.; Jin, Z.; Zhang, L.; Yang, G.; Xie, L.; Xu, S.; et al. Core–Shell Biphasic Microspheres with Tunable Density of Shell Micropores Providing Tailorable Bone Regeneration. Tissue Eng. Part. A 2018, 25, 588–602. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Carrabba, M.; Madeddu, P. Current Strategies for the Manufacture of Small Size Tissue Engineering Vascular Grafts. Front. Bioeng. Biotechnol. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Application Tissue | Biomaterial * | Animal Model | Reference |
|---|---|---|---|
| Bone | PLA | Rat calvarial bone defect | Gómez et al. [75] |
| P(HEMA) | Rabbit femoral defect | Mabilleau et al. [76] | |
| PEUU | Rat myocardial infraction model | Fujimoto et al. [77] | |
| Cartilage | PGA | Sheep cartilage defect | Erggelet et al. [78] |
| PLGA | Rabbit articular osteochondral defect | Dai et al. [48] | |
| Heart Valve | Polycarbonate bis-urea (PC-BU) | Sheep pulmonary valve | Kluin et al. [23] |
| Polyester-urethane | Sheep aortic valve | Yosuke et al. [79] | |
| Periodontal Tissue | PLGA | Canine periodontal | Herberg et al. [80] |
| Blood Vessels | PLLA/PCL | Rat abdominal aorta | Jiang et al. [81] |
| Application Tissue | Biomaterials | Animal Model | Reference |
|---|---|---|---|
| Bone | Silk fibroin | Rat critical size calvarial bone defect | Wu et al. [93] |
| Chitosan/silk-Fibrin | Rat calvarial bone defect model | Wu et al. [95] | |
| Fibrin | Rat cranial defect | Woodruff et al. [96] | |
| Gelatin | Mouse maxillae | Kodama et al. [97] | |
| Gelatin | Rat calvarial bone defect model | Feng et al. [98] | |
| Kidney | Collagen | Renal ischemia/reperfusion rat model | Lee et al. [99] |
| Heart and vessel | Alginate | Rat myocardial infraction model | Landa et al. [100] |
| Cartilage | Collagen | Rabbit articular cartilage | Kubo et al. [101] |
| Fibrin | New Zealand white rabbit full thickness cartilage defect | Dai et al. [102] | |
| Alginate | Rabbit cartilage defect | Ma et al. [103] | |
| Muscle | Collagen | Rabbit muscle | Kin et al. [104] |
| Gelatin | Rat muscle | Ju et al. [21] | |
| Collagen | Rat diaphragm defect | Brouwer et al. [105] | |
| Periodontal tissue | Collagen | Canine periodontal | Nakahara et al. [106] |
| Skin | Chitosan | Porcine burned skin | Boucard et al. [107] |
| Hyaluronic acid-HA | Mouse cutaneous wound model | Wang et al. [108] |
| Application Tissue | Biomaterial * | Animal Model | Reference |
|---|---|---|---|
| Bone | Fibrin/PLGA | Rat calvarial bone defect | Chung et al. [109] |
| Chitosan/β-sodium glycerol phosphate (CS/GP) | Rat critical size calvarial bone defect | Wu et al. [110] | |
| PCL-PDA-HAp | Mouse critical size calvarial bone defect | Lee et al. [111] | |
| Cartilage | HA-GelMa | Sheep model | Di Bella et al. [112] |
| Collagen-HAp | Human osteochondral defect | Perdisa et al. [113] | |
| HA-MA/PLGA | Rabbit full thickness cartilage defect | Dai et al. [114] | |
| Blood Vessels | PCL/fibrin | Rat model aorta | Talacua et al. [67] |
| Heart Valve Leaflets | P4HB/Gelatin | Ovine model pulmonary valve | Capulli et al. [115] |
| Stomach | Collagen/PGA | Canine stomach | Hori et al. [116] |
| Spine | PGA/HA | Rabbit disc defect | Abbushi et al. [117] |
| Application Tissue | Biomaterials | Animal Model | Reference |
|---|---|---|---|
| Esophagus | Urinary Bladder Matrix—UBM | Rat Abdominal Esophagus | Keane et al. [130] |
| Expander/implant breast reconstruction, (a commercial material: Strattice) tissue reconstructive matrix (LifeCell, Branchburg, NJ, USA) | Porcine acellular dermal matrix (PADM) | In humans | Katerinakiet al. [131] |
| Abdominal wall (a commercial material: Strattice) tissue reconstructive matrix (LifeCell, Branchburg, NJ, USA) | porcine-derived tissue matrix | Primates | Connoret al. [132] |
| Kidney | PLGA/dECM of porcine kidney tissue | Mouse | Lih et al. [125] |
| Skin | Porcine subcutaneous adipose tissue | Mouse subcutaneous model | Tan et al. [133] |
| Hemilarynx | Porcine UBM | Canine model | Kitamura et al. [134] |
| Heart Valve | PEG/decellularized porcine aortic valve | Rat subdermal model | Dai et al. [127] |
| PCL/decellularized porcine aortic valve | Rat subcutaneous model | Zhou et al. [135] |
| Application Tissue | Biomaterials * | Animal Model | Reference |
|---|---|---|---|
| Sinus Mucosa | CP | Rabbit sinus lift model | Trbakovic et al. [155] |
| Bone | BCP-N | Rabbit mandible critical size defect model | Li et al. [149] |
| DNA-loaded nano-calcium phosphate | New Zealand white rabbits, critical size bone defect model | Schlickewei et al. [156] | |
| PLA/CP | Mouse subcutaneous model | Oliveira et al. [157] | |
| Calcium-silicate | Rabbit mandibular alveolar bone defect model | Shao et al. [158] | |
| Alginate/* CSi-Sr4 and CaP | New Zealand rabbits, distal femur detect | Fu et al. [159] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. https://doi.org/10.3390/biom9110750
Abdulghani S, Mitchell GR. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules. 2019; 9(11):750. https://doi.org/10.3390/biom9110750
Chicago/Turabian StyleAbdulghani, Saba, and Geoffrey R. Mitchell. 2019. "Biomaterials for In Situ Tissue Regeneration: A Review" Biomolecules 9, no. 11: 750. https://doi.org/10.3390/biom9110750
APA StyleAbdulghani, S., & Mitchell, G. R. (2019). Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules, 9(11), 750. https://doi.org/10.3390/biom9110750






